Abstract
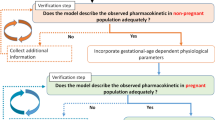
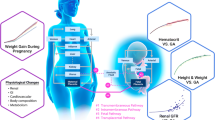
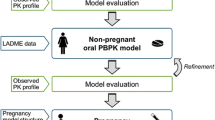
During pregnancy, a drug’s pharmacokinetics may be altered and hence anticipation of potential systemic exposure changes is highly desirable. Physiologically based pharmacokinetics (PBPK) models have recently been used to influence clinical trial design or to facilitate regulatory interactions. Ideally, whole-body PBPK models can be used to predict a drug’s systemic exposure in pregnant women based on major physiological changes which can impact drug clearance (i.e., in the kidney and liver) and distribution (i.e., adipose and fetoplacental unit). We described a simple and readily implementable multitissue/organ whole-body PBPK model with key pregnancy-related physiological parameters to characterize the PK of reference drugs (metformin, digoxin, midazolam, and emtricitabine) in pregnant women compared with the PK in nonpregnant or postpartum (PP) women. Physiological data related to changes in maternal body weight, tissue volume, cardiac output, renal function, blood flows, and cytochrome P450 activity were collected from the literature and incorporated into the structural PBPK model that describes HV or PP women PK data. Subsequently, the changes in exposure (area under the curve (AUC) and maximum concentration (C max)) in pregnant women were simulated. Model-simulated PK profiles were overall in agreement with observed data. The prediction fold error for C max and AUC ratio (pregnant vs. nonpregnant) was less than 1.3-fold, indicating that the pregnant PBPK model is useful. The utilization of this simplified model in drug development may aid in designing clinical studies to identify potential exposure changes in pregnant women a priori for compounds which are mainly eliminated renally or metabolized by CYP3A4.




Similar content being viewed by others
References
Bonati M, Bortolus R, Marchetti F, Romero M, Tognoni G. Drug use in pregnancy: an overview of epidemiological (drug utilization) studies. Eur J Clin Pharmacol. 1990;38(4):325–8.
De Vigan C, De Walle HE, Cordier S, Goujard J, Knill-Jones R, Ayme S, et al. Therapeutic drug use during pregnancy: a comparison in four European countries. OECM Working Group. Occupational Exposures and Congenital Anomalies. J Clin Epidemiol. 1999;52(10):977–82.
Lacroix I, Damase-Michel C, Lapeyre-Mestre M, Montastruc JL. Prescription of drugs during pregnancy in France. Lancet. 2000;356(9243):1735–6.
Knoppert D. Safety and efficacy of drugs in pregnancy. J Popul Ther Clin Pharmacol. 2011;18(3):e506–12.
Chen HS, Gross JF. Physiologically based pharmacokinetic models for anticancer drugs. Cancer Chemother Pharmacol. 1979;2(2):85–94.
Himmelstein KJ, Lutz RJ. A review of the applications of physiologically based pharmacokinetic modeling. J Pharmacokinet Biopharm. 1979;7(2):127–45.
Nestorov I. Whole body pharmacokinetic models. Clin Pharmacokinet. 2003;42(10):883–908.
Grass GM, Sinko PJ. Physiologically-based pharmacokinetic simulation modelling. Adv Drug Deliv Rev. 2002;54(3):433–51.
Parrott N, Lave T. Applications of physiologically based absorption models in drug discovery and development. Mol Pharm. 2008;5(5):760–75.
Rowland M, Peck C, Tucker G. Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol. 2011;51:45–73.
Hill CC, Pickinpaugh J. Physiologic changes in pregnancy. Surg Clin N Am. 2008;88(2):391–401. vii.
Feghali MN, Mattison DR. Clinical therapeutics in pregnancy. J Biomed Biotechnol. 2011;2011:783528.
Gaohua L, Abduljalil K, Jamei M, Johnson TN, Rostami-Hodjegan A. A pregnancy physiologically based pharmacokinetic (p-PBPK) model for disposition of drugs metabolized by CYP1A2, CYP2D6 and CYP3A4. Br J Clin Pharmacol. 2012;74(5):873–85.
Abduljalil K, Furness P, Johnson TN, Rostami-Hodjegan A, Soltani H. Anatomical, physiological and metabolic changes with gestational age during normal pregnancy: a database for parameters required in physiologically based pharmacokinetic modelling. Clin Pharmacokinet. 2012;51(6):365–96.
Anderson GD. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet. 2005;44(10):989–1008.
Dawes M, Chowienczyk PJ. Drugs in pregnancy. Pharmacokinetics in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2001;15(6):819–26.
(CDER) CfDEaR. Pharmacokinetics in pregnancy—study design, data analysis, and impact on dosing and labeling. 2004. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm072133.pdf. Accessed 19 Feb 2013
Eyal S, Easterling TR, Carr D, Umans JG, Miodovnik M, Hankins GD, et al. Pharmacokinetics of metformin during pregnancy. Drug Metab Dispos. 2010;38(5):833–40.
Hebert MF, Easterling TR, Kirby B, Carr DB, Buchanan ML, Rutherford T, et al. Effects of pregnancy on CYP3A and P-glycoprotein activities as measured by disposition of midazolam and digoxin: a University of Washington specialized center of research study. Clin Pharmacol Ther. 2008;84(2):248–53.
Stek AM, Best BM, Luo W, Capparelli E, Burchett S, Hu C, et al. Effect of pregnancy on emtricitabine pharmacokinetics. HIV Med. 2012;13(4):226–35.
Yu LX, Amidon GL. Characterization of small intestinal transit time distribution in humans. Int J Pharm. 1998;171(2):157–63.
Xia BF, Heimbach T, Lin TH, He HD, Wang YF, Tan E. Novel physiologically based pharmacokinetic modeling of patupilone for human pharmacokinetic predictions. Cancer Chemoth Pharm. 2012;69(6):1567–82.
Poulin P, Jones RD, Jones HM, Gibson CR, Rowland M, Chien JY, et al. PHRMA CPCDC initiative on predictive models of human pharmacokinetics, part 5: prediction of plasma concentration–time profiles in human by using the physiologically-based pharmacokinetic modeling approach. J Pharm Sci. 2011. doi:10.1002/jps.22550.
Loebstein R, Lalkin A, Koren G. Pharmacokinetic changes during pregnancy and their clinical relevance. Clin Pharmacokinet. 1997;33(5):328–43.
O'Hare MF, Leahey W, Murnaghan GA, McDevitt DG. Pharmacokinetics of sotalol during pregnancy. Eur J Clin Pharmacol. 1983;24(4):521–4.
Philipson A. Pharmacokinetics of ampicillin during pregnancy. J Infect Dis. 1977;136(3):370–6.
Philipson A, Stiernstedt G, Ehrnebo M. Comparison of the pharmacokinetics of cephradine and cefazolin in pregnant and non-pregnant women. Clin Pharmacokinet. 1987;12(2):136–44.
Sachdeva P, Patel BG, Patel BK. Drug use in pregnancy; a point to ponder! Indian J Pharm Sci. 2009;71(1):1–7.
Dean M, Stock B, Patterson RJ, Levy G. Serum protein binding of drugs during and after pregnancy in humans. Clin Pharmacol Ther. 1980;28(2):253–61.
Loccisano AE, Longnecker MP, Campbell Jr JL, Andersen ME, Clewell 3rd HJ. Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxic Environ Health. 2013;76(1):25–57.
Pitkin RM. Nutritional support in obstetrics and gynecology. Clin Obstet Gynecol. 1976;19(3):489–513.
Okereke NC, Huston-Presley L, Amini SB, Kalhan S, Catalano PM. Longitudinal changes in energy expenditure and body composition in obese women with normal and impaired glucose tolerance. Am J Physiol. 2004;287(3):E472–9.
Sato J, Sawada Y, Iga T, Hanano M. Effect of quinidine on digoxin distribution and elimination in guinea pigs. J Pharm Sci. 1983;72(10):1137–41.
Tracy TS, Venkataramanan R, Glover DD, Caritis SN. Temporal changes in drug metabolism (CYP1A2, CYP2D6 and CYP3A activity) during pregnancy. Am J Obstet Gynecol. 2005;192(2):633–9.
Davison JM, Dunlop W. Renal hemodynamics and tubular function normal human pregnancy. Kidney Int. 1980;18(2):152–61.
Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256(4 Pt 2):H1060–5.
Flo K, Wilsgaard T, Vårtun Å, Acharya G. A longitudinal study of the relationship between maternal cardiac output measured by impedance cardiography and uterine artery blood flow in the second half of pregnancy. BJOG An Int J Obstet Gynaecol. 2010;117(7):837–44.
Graham GG, Punt J, Arora M, Day RO, Doogue MP, Duong JK, et al. Clinical pharmacokinetics of metformin. Clin Pharmacokinet. 2011;50(2):81–98.
Wang ZJ, Yin OQ, Tomlinson B, Chow MS. OCT2 polymorphisms and in-vivo renal functional consequence: studies with metformin and cimetidine. Pharmacogenet Genomics. 2008;18(7):637–45.
Kimura N, Masuda S, Tanihara Y, Ueo H, Okuda M, Katsura T, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20(5):379–86.
Tanihara Y, Masuda S, Sato T, Katsura T, Ogawa O, Inui K. Substrate specificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-organic cation antiporters. Biochem Pharmacol. 2007;74(2):359–71.
Zhou M, Xia L, Wang J. Metformin transport by a newly cloned proton-stimulated organic cation transporter (plasma membrane monoamine transporter) expressed in human intestine. Drug Metab Dispos. 2007;35(10):1956–62.
Wu CY, Benet LZ. Predicting drug disposition via application of BCS: transport/absorption/ elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22(1):11–23.
Rodgers T, Leahy D, Rowland M. Tissue distribution of basic drugs: accounting for enantiomeric, compound and regional differences amongst beta-blocking drugs in rat. J Pharm Sci. 2005;94(6):1237–48.
Schinkel AH, Wagenaar E, van Deemter L, Mol CA, Borst P. Absence of the mdr1a P-glycoprotein in mice affects tissue distribution and pharmacokinetics of dexamethasone, digoxin, and cyclosporin A. J Clin Invest. 1995;96(4):1698–705.
Smit JW, Schinkel AH, Muller M, Weert B, Meijer DK. Contribution of the murine mdr1a P-glycoprotein to hepatobiliary and intestinal elimination of cationic drugs as measured in mice with an mdr1a gene disruption. Hepatology. 1998;27(4):1056–63.
Lacarelle B, Rahmani R, de Sousa G, Durand A, Placidi M, Cano JP. Metabolism of digoxin, digoxigenin digitoxosides and digoxigenin in human hepatocytes and liver microsomes. Fundam Clin Pharmacol. 1991;5(7):567–82.
Binnion PF. A comparison of the bioavailability of digoxin in capsule, tablet, and solution taken orally with intravenous digoxin. J Clin Pharmacol. 1976;16(10 Pt 1):461–7.
Marcus FI, Dickerson J, Pippin S, Stafford M, Bressler R. Digoxin bioavailability: formulations and rates of infusions. Clin Pharmacol Ther. 1976;20(3):253–9.
Jogestrand T, Ericsson F. Skeletal muscle digoxin binding in patients with renal failure. Brit J Clin Pharmacol. 1983;16(1):109–11.
Gorski JC, Hall SD, Jones DR, VandenBranden M, Wrighton SA. Regioselective biotransformation of midazolam by members of the human cytochrome P450 3A (CYP3A) subfamily. Biochem Pharmacol. 1994;47(9):1643–53.
Thummel KE, Shen DD, Podoll TD, Kunze KL, Trager WF, Bacchi CE, et al. Use of midazolam as a human cytochrome P450 3A probe: II. Characterization of inter- and intraindividual hepatic CYP3A variability after liver transplantation. J Pharmacol Exp Ther. 1994;271(1):557–66.
Kronbach T, Mathys D, Umeno M, Gonzalez FJ, Meyer UA. Oxidation of midazolam and triazolam by human liver cytochrome P450IIIA4. Mol Pharmacol. 1989;36(1):89–96.
Rodgers T, Leahy D, Rowland M. Physiologically based pharmacokinetic modeling 1: predicting the tissue distribution of moderate-to-strong bases. J Pharm Sci. 2005;94(6):1259–76.
Molina JM, Cox SL. Emtricitabine: a novel nucleoside reverse transcriptase inhibitor. Drugs Today (Barc). 2005;41(4):241–52.
De Buck SS, Sinha VK, Fenu LA, Nijsen MJ, Mackie CE, Gilissen RA. Prediction of human pharmacokinetics using physiologically based modeling: a retrospective analysis of 26 clinically tested drugs. Drug Metab Dispos. 2007;35(10):1766–80.
Luecke RH, Wosilait WD, Pearce BA, Young JF. A physiologically based pharmacokinetic computer model for human pregnancy. Teratology. 1994;49(2):90–103.
Luecke RH, Wosilait WD, Pearce BA, Young JF. A computer model and program for xenobiotic disposition during pregnancy. Comput Methods Programs Biomed. 1997;53(3):201–24.
Corley RA, Mast TJ, Carney EW, Rogers JM, Daston GP. Evaluation of physiologically based models of pregnancy and lactation for their application in children’s health risk assessments. Crit Rev Toxicol. 2003;33(2):137–211.
Davison JM. The effect of pregnancy on kidney function in renal allograft recipients. Kidney Int. 1985;27(1):74–9.
Song IS, Shin HJ, Shim EJ, Jung IS, Kim WY, Shon JH, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008;84(5):559–62.
Nakatani-Freshwater T, Taft DR. Renal excretion of emtricitabine I: effects of organic anion, organic cation, and nucleoside transport inhibitors on emtricitabine excretion. J Pharm Sci. 2008;97(12):5401–10.
Teodori E, Dei S, Martelli C, Scapecchi S, Gualtieri F. The functions and structure of ABC transporters: implications for the design of new inhibitors of Pgp and MRP1 to control multidrug resistance (MDR). Curr Drug Targets. 2006;7(7):893–909.
Ito S. Transplacental treatment of fetal tachycardia: implications of drug transporting proteins in placenta. Semin Perinatol. 2001;25(3):196–201.
Koren G. Pharmacokinetics in pregnancy; clinical significance. J Popul Ther Clin Pharmacol. 2011;18(3):e523–7.
Knutti R, Rothweiler H, Schlatter C. Effect of pregnancy on the pharmacokinetics of caffeine. Eur J Clin Pharmacol. 1981;21(2):121–6.
McGready R, Stepniewska K, Seaton E, Cho T, Cho D, Ginsberg A, et al. Pregnancy and use of oral contraceptives reduces the biotransformation of proguanil to cycloguanil. Eur J Clin Pharmacol. 2003;59(7):553–7.
McGready R, Stepniewska K, Edstein MD, Cho T, Gilveray G, Looareesuwan S, et al. The pharmacokinetics of atovaquone and proguanil in pregnant women with acute falciparum malaria. Eur J Clin Pharmacol. 2003;59(7):545–52.
Dempsey D, Jacob 3rd P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther. 2002;301(2):594–8.
Wadelius M, Darj E, Frenne G, Rane A. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther. 1997;62(4):400–7.
de Haan GJ, Edelbroek P, Segers J, Engelsman M, Lindhout D, Devile-Notschaele M, et al. Gestation-induced changes in lamotrigine pharmacokinetics: a monotherapy study. Neurology. 2004;63(3):571–3.
Stock B, Dean M, Levy G. Serum protein binding of drugs during and after pregnancy in rats. J Pharmacol Exp Ther. 1980;212(2):264–8.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
ESM 1
DOCX 464 kb
Rights and permissions
About this article
Cite this article
Xia, B., Heimbach, T., Gollen, R. et al. A Simplified PBPK Modeling Approach for Prediction of Pharmacokinetics of Four Primarily Renally Excreted and CYP3A Metabolized Compounds During Pregnancy. AAPS J 15, 1012–1024 (2013). https://doi.org/10.1208/s12248-013-9505-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12248-013-9505-3