Abstract
Plastic pollution is an ever-escalating issue with detrimental effects on both the environment and human health. Plastic breaks down into smaller pieces, and depending on the size they are called macroplastics, microplastics (MPs), and nanoplastics (NPs). Some of these particles can easily enter the food chain causing toxicity to many plants and animals. The extensive use of synthetic polymers such as polyethylene (PE), polyvinyl chloride (PVC), polystyrene (PS), and polyethylene terephthalate (PET) poses substantial environmental concerns due to their degradation-resistant characteristics. One of the ways microorganisms address this issue is by producing enzymes. This review examines the recent advancements in enzymatic degradation of both commercial-grade and pure polymers, including the effectiveness of enzymes such as laccases, proteases, cutinases, PETase, and MHETase, and the governing mechanisms of degradation across various plastic categories. Bioinformatic tools such as multi-omics, molecular docking, and enzyme mining are particularly useful in identifying unconventional biocatalysts and plastic-degrading microbes in a culture-independent manner. Furthermore, techniques to enhance the catalytic efficiency of plastic degrading enzymes (PDEs) using modern approaches such as protein engineering, mutations, chimeric fusion, etc. have also been reviewed. This review accentuates the pivotal role of enzymatic and microbial degradation in mitigating plastic pollution, the associated challenges, and suitable prospects to achieve closed-loop plastic recycling in the future.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Over the years, plastic has become an integral part of everyday human life. From packaging to transportation to healthcare, plastic has proven to be an indispensable component, which has pros and cons. Plastic waste accumulation is one of the biggest challenges in the 21st century. Although consumers are becoming more aware of this issue, the production of single-use plastics continues to increase, leading to environmental and economic problems caused by plastic waste accumulation [1]. According to a statistical report by Plastics Europe, the global yield of fossil-based plastics escalated to an astounding 400.3 million tonnes in 2022 [2]. India, the third largest consumer of plastics, used approximately 21 million tonnes of plastic in 2021 [3]. If unorganized plastic waste disposal and inadequate recycling techniques continue, it is estimated that annual emissions may increase by up to 53 million tons per year by 2030 [4]. Countries worldwide accumulate waste indiscriminately due to the steep cost of waste disposal. The most common techniques for discarding plastic waste are landfilling, and incineration. Landfilling in India requires 120 ha of land per year and only 21% of the landfills are properly managed, leading to greenhouse gas emissions, obnoxious gas production, and fire hazards [5]. In contrast, developed countries including Japan, France, Germany, and the United States use thermal waste-to-energy plants to recover energy from plastic wastes. However, developing countries are lagging due to the colossal investment required, and fear of mishandling toxic byproducts (including dioxins and furans) [6]. A report stated that the combustion of plastic packaging resulted in the discharge of approximately 16 million metric tons of CO2 emissions [7].
Plastic materials steadily break down to generate tiny particles due to environmental factors such as weathering, solar radiation, mechanical forces, and microorganisms. This leads to the formation of microplastics (MPs) (ranging from 5 mm to 0.1 µm) and nanoplastics (NPs) (ranging from 0.1 µm to 100 nm) [8]. These emerging pollutants are a nuisance to the environment and humans. MPs and NPs are now distributed in freshwater sources, oceans, drinking water [9], soil [10], atmosphere, food, as well as in the human body [11]. Due to their small sizes, both are easily mistaken for food and are devoured by or attached to aquatic organisms (rotifers, fish, shrimp, algae, zooplankton, etc.) causing them to potentially travel into humans through the food chain [12, 13]. When ingested, MPs cause severe health complications in the human body such as tissue damage, abnormal lipid metabolism, inflammation, and even cancer [14] Thus, the removal of plastic particles of all sizes from the environment is the need of the hour. Conventional plastic upcycling strategies such as using waste plastic in road construction is a viable option but the release of MPs due to physical abrasions and environmental weathering could cause secondary pollution. Industrial-scale thermochemical degradation methods such as solvolysis, chemolysis, and pyrolysis have been reported for mitigating plastic pollution. However, the generation of toxic byproducts and the energy-intensive nature of these strategies make them unsuitable [15, 16], leading to the search for more sustainable alternatives. Biological degradation involving microbes such as Ideonella sakiensis, capable of thriving on and assimilating PET using two enzymes: MHETase and PETase in 2016 was a milestone in the study of enzymatic plastic degradation [17]. Since then, numerous bacteria, algae, and yeasts have been reported to produce plastic-degrading enzymes (PDE) including protease [18], esterase, hydrolase [19], oxidase [20], cutinase [21, 22] and lipase [17, 23,24,25,26,27].
If the sustainable development goals set forth by the United Nations are to be reached, a circular economy framework for plastics has to be addressed. A circular economy focuses on all phases of the value chain and considers the best possible outcome to reduce the impact of producing virgin petrochemical-based plastic polymers [28]. In this regard, enzymatic closed-loop recycling technologies provide a promising solution to managing plastic waste [29]. In this review, the types of plastics, their environmental impacts on various environmental settings, and the sources of these types of plastics at the industrial level. The details of the enzymes reported to degrade multiple plastics, including their interactions, mechanism of action, and related factors have been thoroughly reviewed. Additionally, the recent advancements, challenges, and future prospects of enzymatic plastic degradation have also been discussed.
Types of plastics
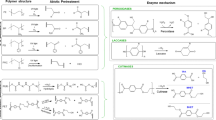
Industrial-level plastic production is focused on manufacturing various thermoplastic materials due to their requirements for many commodities. Among them, the major 6 types of plastics are low-density polyethylene (LDPE), high-density polyethylene (HDPE), polystyrene (PS), polypropylene (PP), polyethylene terephthalate (PET), and polyvinyl chloride (PVC)- some of the commonly used plastic polymers, as discussed (Fig. 1).
Polyethylene, present in two forms—LDPE and HDPE—is one of the most widely produced plastics. HDPE is hard and brittle, making it useful for construction purposes (e.g., pipes and window frames) and household items (e.g., toys and utensils). On the other hand, LDPE is used the most for plastic bag manufacturing, packing materials, containers, etc. [30, 31]. PP is another type of plastic used in the preparation of disposable straws and bottle caps. Among all the plastics, it is inexpensive, fire resistant, and has the lowest density. In addition, it covers approximately 16% of the total plastic industry [32]. The production of approximately 7 million metric tonnes of PET in 2022 is largely due to its frequent use in single-use plastic bottles [33]. On the contrary, PVC consists of the greatest proportion of plasticizers, which can reach 50%. Its mechanical strength makes it the base material for the production of household items such as window frames, shower curtains, pipes, and floor coverings [31, 34]. Overall, the classification of plastic polymers is vital for understanding their diverse properties and recyclability. The categorization into different resin codes helps in effective waste management and recycling efforts. As we move forward, a balanced approach should be introduced where the polymers belonging to the 7th plastic category i.e., other plastics should be further classified as recyclable, biodegradable or non-biodegradable for efficient waste management.
Plastic disposal, recycling, and degradation methods
Landfilling and incineration are the most sought-after established methods for plastic disposal. Landfilling not only comes with a massive land commitment but plastics due to their non-biodegradable nature cause further problems [35, 36]. This can lead to a land shortage in densely populated countries and the plastics in landfills can act as a source of secondary environmental pollutants such as complex hydrocarbons, endocrine disrupting compounds, and hydrogen sulfides released in the form of leachates and gases leading to air, water and soil pollution [35, 36].
To overcome the limitation of space, incineration is promoted, which has the added advantage of simultaneous energy generation [37]. However, incineration can lead to the generation of harmful compounds such as greenhouse gases and carbon-oxygen free radicals along with polycyclic aromatic hydrocarbon (PAHs) and polychlorinated biphenyls (PCBs). There is a significant trade-off since the incineration of plastics leads to the formation of numerous harmful compounds, most of which are released into the atmosphere [38, 39]. A study by Vlasopoulos et al., compared two techniques: landfilling with energy recovery and incineration for energy recovery, and it was concluded that landfilling is the worst choice due to its environmental impacts. Besides, incineration does not completely eradicate plastic waste, as MPs persist in the bottom ash [40]. Nonetheless, the energy content of plastic is comparable to that of heating oil but exploiting it as a secondary fuel needs to be monitored stringently under the Hazardous Waste Incineration Directive [15, 41].
Plastic waste presents a great opportunity if its economic value can be maintained after recycling. This can ideally be created by breaking down plastic polymers into their monomers or oligomers for further upcycling [42]. Several modern plastic recycling techniques have been able to utilize this concept to some extent on a commercial scale. Modern plastic recycling techniques can be divided into two categories based on physical and chemical technology. Physical or mechanical plastic recycling methods rely on physical segregation, sorting, washing, and grinding of plastic waste. In the construction industry, recycled plastic is used as a replacement for the virgin construction materials required in concrete and mortar [15]. For example, a study on the life cycle analysis of road pavement systems using recycled polyethylene terephthalate (PET) plastic bottles and carbonated materials revealed critical insights. This demonstrated that asphalt mixtures derived from these two components can be produced at significantly lower temperatures. Furthermore, the results of Monte Carlo simulations revealed that this method could result in a substantial reduction in environmental impacts, with climate change emissions reduced by 40-60% compared to those of traditional road pavement systems [43]. The drawback of mechanical plastic recycling is that it is mostly associated with downcycling combined with degraded plastic properties due to the mixing of various plastic polymers [15].
On the other hand, the industrial chemical methods of plastic recycling are promising due to energy recovery and the production of economically important products such as monomers and feedstocks. Chemical methods include solvolysis, chemolysis, and pyrolysis for amalgamated plastics. However, the variability of plastic waste makes it an inconsistent process yet to be economically viable. In addition, from an environmental point of view, these techniques are energy intensive and require toxic and nonbiodegradable solvents (e.g., in solvolysis) and produce lethal gaseous products (e.g., carbon monoxide, hydrogen cyanide, and hydrogen chloride) [15, 16]. Therefore, scientists have turned to the biodegradation of plastic waste as a green solution. Although mechanical and chemical methods of plastic degradation have been adopted in industrial settings, enzymatic degradation is a relatively newer concept.
Bacteria such as Escherichia coli, Micrococcus spp., Pseudomonas spp., Staphylococcus spp., and Corynebacterium spp., could degrade plastics between 45-56% weight reduction [44] and some species of fungi (Geotrichum candidum, Fusarium oxysporum, Alternaria alternata, and Trichoderma sp.) could degrade between 17-95% of the molecular weight of PE [45, 46]. Pseudomonas aeruginosa DSM 50071 [47] and Bacillus paralicheniformis G1 [48] played a crucial role in polystyrene degradation. Thermophilic microbes Aneurinibacillus aneurinilyticus btDSCE01, Brevibacillus agri btDSCE02, Brevibacillus sp. btDSCE03 and Brevibacillus brevis btDSCE04 are capable of degrading polyethylene (HDPE, LDPE) and polypropylene (PP) [49]. Similarly, Pseudomonas aeruginosa LICME WZH-4 and Pseudomonas aeruginosa WGH-6 reported weight reduction of polypropylene up to 17% but bacterial biomass increased suggesting the potential of the bacterium to utilize the PP as substrate [50]. These are a few among thousands of other microorganisms with the potential to produce enzymes for enzymatic plastic degradation. Table 1 summarizes a list of microbes capable of producing a wide variety of enzymes that can play a crucial role in plastic degradation. These compounds are mainly classified into two types based on production: intracellular enzymes (such as polyethylene glycol dehydrogenase and alkane hydrolase [51]) and extracellular enzymes (ex. depolymerase and hydrolase [52]). Tao et. al. discovered an extracellular enzyme producer, Rhodococcus strain A34, derived from naturally weathered plastic waste (Table 1). Proteomic studies revealed that strain secretes multiple extracellular enzymes including multicopper oxidase, lipase, and six esterases capable of breaking down PE up to 1% after 1 month of incubation [53]. These two enzymes also have different mechanisms of action. Intracellular enzymatic degradation, for example, induces the breakdown of stored endogenous carbon for polymer hydrolysis by accumulating various microorganisms. While extracellular enzymes use an external carbon source for the energy to break down plastic polymers [54]. Overall, studies about extracellular enzymes are extensive due to their wide applicability [55].
Microbial and enzymatic plastic biodegradation
Under natural conditions, microorganisms colonize the surface of plastics and form a biofilm. The colonizing species are found to differ from the surrounding environment significantly. Plastic particles act as a sturdy matrix on which various microorganisms colonize and this phenomenon gradually impacts the integrity of the polymer (Fig. 2). The complex interaction between the plastic and the microbes colonizing it is called the plastisphere; its role in facilitating petro-derived plastic degradation is of critical interest. Although colonization does not necessarily equate to the sinking of plastic particles or their degradation, microbial colonization facilitates the enzymatic breakdown of plastic [67,68,69]. Enzymatic catalysis of plastic waste is an innovative and sustainable way of reaching environmental goals [29].
Insights into the enzymatic degradation of commercial plastic products
Mechanical and enzymatic recycling and dissolution of plastics is currently a topic of immense interest [70]. Recently, a group of Austrian scientists achieved closed-loop recycling of post-consumer a variety of yoghurt cups composed of PP and PS. They mechanically sorted and shredded yoghurt cups before thermos-forming them into new cups. Surprisingly, the characterization parameters of the recycled cups indicated their likeness to the virgin grade value of that plastic [71]. Moreover, the future of closed-loop plastic waste recycling can benefit by introducing enzymatic catalysis. Already many publications have made immense contributions to enzymatic PET closed-loop recycling or simply PET enzymatic hydrolysis at a large scale [72,73,74], however the same cannot be said for other recalcitrant plastics. For example, a study discussed the enzymatic recycling of a commercial textile (wool/polyester blend) using protease. Results showed a massive 73% weight reduction for the 45/55% wool/polyester textile [75]. However, it was a lab-scale experiment, and further studies need to be performed to standardize the enzymatic recycling of commercial plastic products.
Plastic wastes in the environment are majorly commercially produced and contain a mixture of plasticizers, heat stabilizers antioxidants, and pigments, apart from the basic polymer backbone. Therefore, attention must be drawn towards polymer and additive degradation/ recyclability [52]. A recent study showed the influence of additives in enzymatic degradation by comparing pristine and commercial HDPE. It was observed that a laccase enzyme from Trametes versicolor could reduce only 3% of the weight of the commercial HDPE (containing additives). In contrast, the pure HDPE substrate was seen to have a 33% weight reduction under the same conditions. This highlights the need for pretreatments in the removal of additives from post-consumer plastic waste [76].
Studies related to PET-recycling enzymes have matured in the past decade to such an extent that a French company named Carbios has announced the opening of an industry that can recycle tons of plastic per year based on enzymatic PET degradation [29]. However, since PET represents a minor portion of the total unrecycled plastic (approx. 7%), research should focus more on other recalcitrant wastes, such as PU, PE, and PS [77].
Mechanism of microbial and enzymatic plastic degradation
The mechanism of microbial degradation can be classified into four steps: attachment/colonization, bio-fragmentation, assimilation, and mineralization (Fig. 2) [78]. Ongoing research on the colonization of microbes indicates that biofilm formation significantly alters the hydrodynamics of plastic particles. Extracellular polymeric substances secreted by microbes to form biofilms cause the surface of the plastic to become sticky. This adhesive property promotes hetero-aggregation and further growth of microbial populations.
Microbes, in turn, produce (intracellular/extracellular) enzymes that stimulate the chemical breakdown of polymeric chains into smaller elements. Enzymatic decomposition occurs in two stages: Firstly, enzyme adsorption onto the polymer and then bond hydrolyzation/ peroxidation [67, 79]. The biological degradation of plastics generally involves breaking down long polymers into short oligomers, dimers, and monomers. These bacteria are broken down to facilitate microbial uptake by passing through their membranes, ultimately leading to their mineralization in cells. Finally, the monomers are converted to CO2 and H2O under aerobic conditions and to CO2, H2O, and CH4 under anaerobic conditions [27]. The two types of microbial enzymes capable of polymer breakdown have different mechanisms. Extracellular enzymes, for example, depolymerase and hydrolase enzymes, induce hydrolytic cleavage in polymers. In addition, free radical formation can also cause a change in the number of bonds between monomers. The action of hydrolytic enzymes such as depolymerase, oxidase, and peroxidase causes an increase in the hydrophilicity of the polymer, thus increasing its rate of biodeterioration [79]. The adherence of enzymes to polymeric materials is a crucial step that is mediated by anchoring peptides on the surface of these enzymes (Fig. 3). These anchor peptides are believed to link the active site of the enzymes to the polymeric surface [80]. In a proof-of-concept experiment, immobilized cutinases along with specific anchor peptides were incubated with polyester-polyurethane to observe up to a 6.62-fold increase in degradation kinetics [81].
There are 24 known PET hydrolytic enzymes to date. Among them, only 4 are mesophilic, while the rest are thermophilic [82]. The widely studied PDEs, MHETase and PETase, are secreted by Ideonella sakaiensis 201-F6 and degrade PET and some hydrolyzable plastics. These enzymes are notable not only due to their polymer degradation capabilities but also due to their metabolic capabilities at relatively low temperatures (30 \(^\circ{\rm C} -37^\circ{\rm C} )\). These two enzymes act synergistically to increase PET breakdown by as much as six times. PETase activity produces the dimer BHET and monomer MHET. MHETase then acts on MHET to convert it to TPA and EG. Therefore, identifying synergistic enzymes from multiple microbes with a two-enzyme system is important for developing efficient enzymatic biodegradation techniques [52]. Along with enzyme discovery, a better understanding of the reaction course is necessary. While looking for the key steps in PET breakdown, it has been suggested that hydrolyzation of internal bonds by endolytic enzymes plays a significant role in the total degradation rate. This is because endolytic enzymes promote the formation of soluble PET fragments with two or three aromatic rings. In contrast, enzymes with low endolytic activity have been shown to have a distinctive lag phase as well as moderate efficacy, probably due to the formation of low soluble aromatic fragments [83].
Factors affecting microbial and enzymatic plastic degradation
Plastics are highly resistant to degradation under natural environmental conditions. This is due to a variety of factors, including their hydrophobicity, the presence of functional groups that are not easily broken down by enzymes, and their chemically inert covalent backbones. Consequently, the chemical structure of plastic is a critical determinant of its biodegradability. For instance, plastics can be classified as hydrolysable or non-hydrolysable. Hydrolysable plastics such as PET, PUR, and polyamide contain ester or amide groups that can be easily broken down by enzymes. On the other hand, non-hydrolysable polymers such as PE, PVC, and PP are difficult to degrade by enzymes. It has been observed that non-hydrolysable polymers have a backbone similar to that of lignin. Hence, enzymes that are efficient at degrading lignin can also degrade non-hydrolysable polymers [84, 85]. Additionally, similar major factors influencing plastic decomposition are discussed below.
Abiotic factors
The environmental conditions surrounding plastics play a crucial role in the process of plastic breakdown, and biodegradation is influenced by the interaction of abiotic and biological factors. Since a major portion of the plastic litter end up in water bodies, several studies focus on the degradation of plastics in natural and stimulated aquatic (marine water and freshwater) environments [86,87,88]. Plastic particles floating in these environments (photic zone) are subjected to shear stress due to wave action, solar UV radiation, heating, and microbial colonization [89], while microbial enzymatic action is the primary influential factor in the aphotic zone [67, 84]. A study on the effects of UV light on MP degradation showed that the wavelength of the light, the additives, as well as the composition of the particle, impact surface oxidation levels due to UV exposure [90]. In an experiment by Wu et. al., they studied the effect of long-term photoaging on floating PP plastics. It was observed that after 68 days of UV irradiation, the size of the particles decreased as much as 99%, giving rise to MP and NP generation. They identified the cause to be due to sustained photoaging which leads to the generation of reactive oxygen species [91]. This study highlights the critical role of UV radiation duration and intensity in the fragmentation of PP within environmental contexts. The rate of photooxidation of plastics varies significantly with the environmental location, leading to differential fragmentation rates. This variation is influenced by the specific UV exposure conditions prevalent in distinct geographic areas. Based on this idea UV pretreatment of plastic before enzymatic degradation has been suggested for enhanced degradation of plastics [92].
Crystallinity
Plastic polymers contain varying degrees of crystalline and amorphous regions. The latter offers flexibility. An increase in crystallinity reduces the movement of the backbone, therefore limiting the availability of the polymer chains for enzymatic attacks [79]. The degree of crystallinity (Xc) of a polymer refers to the proportion of the polymer in a crystalline state as opposed to an amorphous state. Crystalline regions are structured and ordered with polymer chains aligned in a repeating pattern. In contrast, the amorphous regions are disorganized and lack a regular structure. Xc influences a material’s physical properties, including density, thermal stability, and chemical and enzymatic degradation resistance. It is measured using methods including X-ray diffraction and differential scanning calorimetry [93,94,95]. There is a strong correlation between the enzymatic degradation process and the Xc of the polymer; higher crystallinity is associated with a slower rate of enzymatic reaction [96]. For instance, a major difference between LDPE and HPDE is their Xc. HDPE is more crystalline than LDPE and hence more resistant to enzymatic degradation [78]. Conversely, as a semicrystalline polymer, PET has better biodegradability [96]. A recent study on enzymatic PET hydrolysis showed how an industrially relevant PET-degrading enzyme, LCCICCG performed with varying levels of Xc. A pronounced lag phase was visible for crystalline PET (Xc> 20%) substrates which extended up to several days. Nonetheless, soluble products were detected after longer incubations with the enzyme. In some cases, the reaction rate post-lag phase reached levels (factor of 2-3) comparable to the initial reaction rates of amorphous substrate decomposition [97].
Molecular weight
The resistance of plastics to degradation is attributed to their long-chain structure. As the length of the polymer increases, the number of bonds the enzyme must break increases. Besides, it also impairs the ability of microorganisms to uptake polymers since high molecular weight polymers have low permeability across the cell membrane. To utilize plastics as a carbon source, they must first be processed and fragmented by enzymes, which break down long-chain polymers into smaller monomers and oligomers (Fig. 2). These smaller fragments can then be taken up by the cell and assimilated into a carbon source followed by metabolism. [78, 98, 99].
Furthermore, molecular weight is an important criterion for monitoring enzymatic plastic breakdown. For example, Nikolaivits et. al., monitored the degradation of multiple plastics by a polyesterase enzyme from the Antarctic bacteria Moraxella sp. using molecular weight loss. Other researchers have also noted changes in the molecular weight using gel permeation chromatography (Table 1). Apart from molecular weight studies have shown that plastic size, surface area, surface texture, buoyancy, etc., also influence the enzymatic degradation rate of plastics [67].
Recent trends in the study of microbial and enzymatic plastic degradation
The study of enzymatic plastic degradation has garnered tremendous attention from the scientific community, driven by the global imperative to address plastic pollution. The recent trends in this field reflect a growing emphasis on innovative, sustainable solutions to the global plastic pollution crisis. Researchers are exploring the use of naturally occurring microbes and enzymes to offer a promising alternative to traditional plastic waste management methods.
Study of the plastisphere for analyzing inhabitant microbial community on plastic debris
One of the recent and innovative approaches to discovering potential microbial enzymes involved in plastic degradation is studying the plastisphere. The term ‘plastisphere’ was introduced more than a decade ago and has since been the central topic of discussion. It comprises the microbial inhabitants of plastic debris and the environment containing the microbial community [100]. The study of the plastisphere is crucial because it selectively attracts microbes capable of utilizing it as a substrate or carbon source for growth [101,102,103]. Du et al. divided plastisphere formation into three stages: pioneer colonization, secondary colonization, and maturation. Although many studies have focused on isolating strains from polluted sites rich in various types of plastics, the percentage of microorganisms that can be cultured under laboratory conditions is less than 1% [104]. Hence, metagenomic and in silico studies are valuable tools since they are not dependent on culture techniques (Fig. 4).
Researchers have utilized various tools for the study of the plastisphere. Various sets of molecular techniques, bioinformatic tools, and in vitro techniques can all be employed in combination for the analysis (Fig. 4). For example, Frey et al. discovered a novel esterase enzyme from the plastisphere in alpine soils by utilizing DNA shotgun metagenomics, screening the metagenomes to detect plastic-degrading genes, and identifying the genera expressing these genes. Using heterologous expression followed by functional validation proved to be an effective way of identifying a highly active enzyme for plastic decomposition [105]. Another study by Wright et al. was performed using an existing marine plastisphere, where it was found that the communities were significantly different from those of the native aquatic source. Using metagenomic approaches, the study of microbial community succession on PET revealed a sizeable number of enzymes involved in its degradation. Further metabolomic and proteogenomic studies performed using strains isolated from the plastisphere indicated the presence of two potent PET-degrading strains, one of which might use a novel pathway for PET degradation [106]. Further studies incorporating in-silico, molecular, and/or laboratory techniques are required for advancement in this field.
Pretreatments of plastic to overcome recalcitrance and increase susceptibility to microbes or enzymes
Pretreatment of plastics has received the most attention in the last 3 years. Pretreatment refers to any process capable of modifying the physical, chemical, or thermochemical nature of a polymer and increasing its susceptibility to enzymatic degradation. Ciuffi et al. classified plastic pretreatments broadly into 4 categories: oxidative, chemical, mechanical, and thermal/thermochemical [99]. Oxidative pretreatments can be induced by natural means, including photooxidative degradation with UV light, or by the use of oxidizing chemicals. Among these methods, ultraviolet (UV) irradiation is a widely used pretreatment method for enhancing plastic degradation. This technique induces surface oxidation, which is quantified by the carbonyl index and facilitates the formation of cracks on the plastic surface [90]. It also initiates the degradation of plastic polymers with a carbon-carbon (C-C) backbone, leading to fragmentation and a reduction in the molecular weight of the polymer. These modifications collectively contribute to the increased susceptibility of plastic to enzymatic degradation. Other oxidizing agents used in studies include \(\gamma\)-irradiation and peroxide [79]. Recently, enzymatic pretreatment for the biocatalytic oxidation of plastics has also been explored. It was observed that a laccase enzyme has the potential to reduce the hydrophobicity of PE and polycarbonate plastics. GPC has shown a promising reduction in the molecular weights of test polymers subjected to enzymatic and microbial biofilm treatment [107]. Furthermore, chemical pretreatments on plastics have also shown promising results. In one study, the hydrolyzable bonds in PET were made more accessible to the enzyme by treating it with NaOH. Alkaline treatment at room temperature reduced the crystallinity from 33.70% to 27.68% after 24 h of incubation, which in turn increased enzymatic degradation of up to one order of magnitude [108].
Mechanical forces can also expedite enzymatic plastic degradation by making the surface of the polymer more bioavailable for enzymatic action. For example, a study was performed using an ultrahigh-speed twin-screw extruder to inflict physical damage to PET as a test plastic. Pretreatment with a low mechanical shear of 200 rpm caused more than 70% weight loss after 72 hr of incubation with a cutinase enzyme [109]. Finally, both thermal and thermochemical processes involve the use of heat to increase the biodegradation rate of recalcitrant plastics. Thermal and thermochemical treatments aid in fragmentation, generating oligomers that are easily accessible to the enzyme. For example, polyether PU foam (PUR), a particularly recalcitrant polymer, showed better enzymatic degradation by a lipase from Candida rugosa after thermochemical pretreatment by hydrothermal liquefaction. The results determined by FTIR, LC-MS, and HPLC-UV-Vis analysis showed the possibility of the recovery of precursors for a circular economy [110].
Since biological treatments are slower than chemical and mechanical plastic degradation, pretreatments can be used in conjunction with other techniques to make the process economically and industrially feasible. The promise of a circular economy of plastic and the opportunity to develop closed-loop upcycling technologies is driving increasing research into this domain.
Microbial consortia for synergistic action on plastic for efficient degradation
Axenic microbial cultures are commonly used to test their potential to degrade plastics. However, reports prove the occurrence of various bacteria, fungi, and actinomycetes in environments polluted with plastics. The action of consortia is faster than that of monocultures due to synergistic activity [111]. In a microcosm experiment, plastic-degrading microbial consortia were enriched by the gradual enrichment of LLDPE plastic. After 105 days of incubation, the microbial weight decreased by 2.2-5.5%, and the microbial diversity decreased. Several potent strains were identified molecularly and enzymatically characterized. The participating species demonstrated a degree of enzymatic complementarity, which can be further enhanced for better biodeterioration of plastic materials [112]. Another study was performed to test the ability of the rumen microbial community to degrade several synthetic plastics (PET, polybutylene adipate-co-terephthalate, polyethylene furanoate). HPLC results indicated the presence of the hydrolysis products of both plastics, and SEM provided visual confirmation of plastic degradation. Compared to data for enzymatic degradation from single microorganisms, the polyester-hydrolyzing activity of the rumen consortia was relatively high due to the synergistic activity of various esterases, lipases, and cutinases [113].
Recent bioinformatic studies, specifically metabolic engineering, has promoted the degradation of complex plastic pollutants. On the contrary, microbial consortium does not offer universally enhanced performance. Inhibitory interactions within the consortium can impede their efficacy. For example, a recent experiment on residual mulch film degradation in soil screened and identified plastic-degrading microbes. It was observed that the monocultures of the bacterial species (Burkholderia cepacia, Burkholderia diffusa, Chrysebacterium nepalense, and Burkholderia aenigmatica) showed greater weight loss (1.58-2.44%) than the monocultures (1.34-1.62%) after 90 days of incubation. Hence, they concluded that inhibitory interactions among microbes in the mixed culture [45]. To overcome this problem, Cao et. al., suggest the division of metabolic pathways to minimize cross-reactions, ultimately reducing the metabolic burden. Consequently, the creation of an artificial microbial consortium is often simpler and can be tailored for different target products [114]. Future studies may benefit from employing metabolic engineering and proteomics to map the detailed pathway of polymer degradation by these consortia. This approach would provide a more comprehensive understanding of the products, intermediates, and associated enzymes causing this observed inhibition.
Challenges with microbial and enzymatic plastic degradation
The commercialization of microbial and enzymatic plastic recycling/upcycling is a very difficult path due to several challenges associated with it. The challenges associated with the same are as follows (a) detection and isolation of synthetic polymer-degrading microbes is a tedious, expensive, and time-consuming process (b) enzymatic catalysis of synthetic polymers is generally a slow process (b) enzymes cannot tolerate harsh reaction conditions (d) the variety of plastics usable as substrates are less since it is still limited to polymers containing carbonyl groups in their backbone (including PET, PLA, and PA) (e) since most plastic polymers are not soluble, the enzymatic reaction has to ensue via an interfacial mechanism, requiring improvements in technology to increase the substrate’s contact area with the biocatalyst (f) the result may be inconsistent with changing intrinsic plastic properties (degree of crystallinity, melting temperature, and additives) [29].
Future prospects for sustainable plastic degradation using advanced tools
Despite the disadvantages, the enzymatic technique can compete with the production of virgin petrochemical plastics because of the possibility of closed-loop or open-loop recycling. Plastic waste recycling and valorization involving microbes and enzymes to attain a circular bioeconomy presents an innovative and green approach [77]. Utilization of next-generation bioinformatics and computational tools for mining PDM and PDE, protein engineering for improving enzyme stability, efficacy, and enzyme-substrate binding for better plastic degradation efficacy is the key to reaching sustainable development goals.
Bioinformatics and computational tools for the detection of plastic-degrading microbes and enzymes
In silico studies have emerged as a comprehensive and unconventional way of detecting novel microbes capable of PDE secretion. Through the investigation of metagenomic sequences, enzyme folding patterns, and other computational biology techniques, the detection of potential polymer-degrading microbes and the molecular basis of plastic degradation through enzymes can be determined. Bioinformatic tools such as transcriptomics, metabolomics, metagenomics, and meta-proteomics have shed light on the plausible interactions responsible for plastic breakdown. [67]. In recent developments, machine learning-mediated algorithms have facilitated the enhancement of PET hydrolase in the creation of FAST-PETase. This modified enzyme exhibited notably improved degradation rates toward untreated PET across diverse pH and temperature ranges, demonstrating its viability as a closed-loop PET recycling system within an industrial framework [115]. Moreover, investigations into the structural attributes of enzymes interacting with plastic-specific ligands are crucial for understanding substrate-binding mechanisms and factors contributing to enzyme stabilization. Techniques such as X-ray crystallography and cryo-electron microscopy offer valuable insights into the binding modalities of enzymes with their corresponding plastic substrates [116]. Additionally, genome mining stands out as a robust approach for identifying and synthesizing potent novel enzymes capable of utilizing a wide range of plastics as substrate. Genome mining involves screening for enzymes and natural products based on the comparison of genetic information using tools such as BLAST and profile hidden Markov models/HMMer [117]. In a pioneering experiment, researchers selected eight potential novel polyester-degrading enzymes based on their genomic similarity to known PET-degrading enzymes. The best-performing enzymes were expressed in recombinant cells to augment biofilm formation, thereby expediting the plastic degradation process by a massive margin [77]. Culture-dependent techniques can only determine the metabolic capabilities of <1% of microbes. Metagenomics can help overcome this challenge by elucidating the complete metabolic capacity of microbes, including unculturable species [118]. The synergy between bioinformatics and biochemical investigations is imperative for the discovery and improvement of PDE. Bioinformatic studies can help narrow the search for such enzymes as well as enhance the efficiency of known enzymes. Although the discovery of novel PDE may not fully address the massive problem of plastic accumulation, augmenting their activity using in-silico and biochemical techniques represents a promising way forward. Table 2 provides a summary of recent studies involving utilizing bioinformatics and computational tools such as genomics, sequencing tools, genome mining, AI, and Machine learning for identifying plastic-degrading microbes (PDM) and plastic-degrading enzymes (PDE) for efficient degradation of a wide variety of plastics.
Microbial and enzymatic systems for plastic waste valorization to high-value products
Several mechanical and chemical plastic recycling strategies suggest that the recycled products are of substandard qualities which limits their usage at a large scale [130]. To overcome this challenge, microbial and enzymatic conversion of plastics for the generation of high-value products can be employed. The probable approach could be divided into three major strategies: (i) identification/isolation of PDM and PDE, (ii) breakdown or fragmentation of polymers to monomeric form, and (iii) utilization of monomers for generation of original plastic material (closed loop) or their enzymatic conversion to other high-value products [77, 131].
Purified enzymes or whole microbes can be employed to catalyze the multistep transformation of plastic waste to high-value compounds. Some examples of such studies are (i) utilization of laccase, Mn-peroxidases, peroxygenase, AlkB hydroxylase, and phenol oxidase for the generation of organic acids, and organic amines from polyamides, polyethylene, polypropylene, polystyrene, and polyvinylchloride; (ii) utilization of amidase, cutinases, protease, urease, and urethanase for conversion of polyether and polyester based polyurethanes for generation of adipic acid, 1,4-butanediol, and toluene diamine; (iii) conversion of polyethylene terephthalate to bis(2-hydroxyethyl) terephthalate and 2-hydroxyethyl terephthalate using cutinase, lipase and PET hydrolase in a specially designed bioreactor or one-pot system [77, 131, 132].
Protein engineering for improving the enzymatic properties of PDE
The enzymes are fundamentally proteins whose catalytic performance for degrading plastics can be improved by a protein engineering approach via rational design or directed evolution [133].
Directed evolution
Directed evolution involves the engineering of natural evolution through repeated gene diversification and library screening/selection for an improved novel function of protein [134]. Bell et al., [135] demonstrated the use of high throughput protein engineering of the PET degrading enzyme lsETase of Piscinibacter sakaiensis using high activity at elevated temperatures as selection pressure. The resulting engineered enzyme to HotPETase has Tm = 82.5 °C and can efficiently and more rapidly break down semicrystalline PET as compared to lsPETases. The HotPETase can deconstruct the PET component selectively from a laminated multilateral as it can operate at the glass transition temperature of PET. The improved catalytic and thermotolerant nature of the HotPETase is due to characteristic changes such as the presence of a well-defined conformation of Tryptophan185 and the formation of new disulfide bridges that improved the central-sheet region packing, respectively as compared to native lsPETases [135]. Similarly, another study exploited the directed evolution method to engineer the enzyme PHB depolymerase from Ralstonia pickettii T1 using a cell surface display system. The mutations added aid in a ten-fold increase in the p-nitrophenyl butyrate degradation rate but failed miserably in improving PHB degradation [136]. However, the lack of competent high-throughput screening methods for gene diversification and screening limits the utilization of directed evolution for protein engineering of plastic-degrading enzymes [133, 134]. Due to this, limited studies have been performed utilizing directed evolution. Recently, Apitius et al., [137] developed an ultrahigh-throughput esterase A-based Escherichia coli cell surface display screening system for directed polymer binding peptide evolution. This novel screening system selectively improved the binding properties (12-fold) of displayed adhesion promoters that targeted the binding of whole cellss to polymer surfaces such as PET. However, limited studies to date focus on directed evolution for PDE mutations, and further studies will greatly benefit the field.
Rational protein engineering
The rational protein engineering approach utilizes information on the structure and mechanism of protein through computational simulation and modeling. These structural and mechanistic characteristics of protein are modified to enhance enzyme thermostability, reinforce the binding of substrates to active sites of PDE, improve enzyme-substrate interactions, and tune active sites for refining enzyme functionalities [133].
Enhancing enzyme thermostability and catalytic efficiency by mutation
The enzyme activity and thermostability of enzymes could be improved by structural changes owing to mutations. The polyester hydrolases jmPE13 isolated from Pseudomonas sp. JM16B3 was subjected to mutation of R146S through rational designing [138]. The resulting mutant has decreased flexibility of the C-terminal loop and the loop adjacent to the catalytic center resulting in rigidity of this site. This provided an improved hydrolytic activity of 3-fold and 1.5-fold towards PET and PBAT (Poly (butylene adipate-co-terephthalate)), respectively accompanied by improved enzyme stability [138]. The formation of disulfide bonds, hydrogen bonds, salt bridges, etc. were found to be responsible for the stability. Some of the mechanisms of enhancing enzyme thermostability and catalytic efficiency by mutation are discussed below.
-
(a)
Formation of disulfide bonds and salt bridges by mutation
The formation of disulfide bonds and salt bridges can synergistically improve the enzyme thermostability and catalytic efficiency of PDE. Then et al., suggested that the mutation in esterase (TfCut2) of Thermobifida fusca at their calcium binding sites resulted in the formation of disulfide bonds between D204C and E253C [139]. This leads to an increase in protein melting point and improves plastic hydrolysis activity. In addition, the formation of salt bridges between the positively charged Arg280 residues and negatively charged N246D residue led to improved thermostability of the engineered PETaseN246D [140]. This could be explained by the fact that these disulphide bonds and salt bridges are critical in protein folding which could be critical in providing desired confirmation [133, 140, 141], for imparting thermal stability and crucial to better enzyme-substrate binding for better catalytic efficiency [133]
Hydrogen bond formation by mutation: The introduction of hydrogen bonds at the highly flexible β6–β7 connecting loop region of PETase between S121E and N172 residues enhanced the thermostability due to improvement in the regional rigidity [142]. Further, the mutations at multiple sites can also lead to the formation of new hydrogen bonds that can result in an improvement of melting point by 31°C for the redesigned variant DuraPETase as compared to wild-type PETase from Ideonella sakaiensis [143]. The formation of hydrogen bonds can help in the generation of high-order protein structures that are structurally stable and temperature-resistant.
-
(b)
Increase in proline by mutation
The mutations to increase proline residues in PDE for example mutation of threonine to proline at the 235th position of a cutinase enzyme of Thermobifida alba, can result in improved thermostability and catalytic efficiency [144,145,146]. This is achieved by several mechanisms such as improved tertiary structure stability by hydrogen bond formation and hydrophobic interaction between proline and adjacent residues. Also, the cyclic structure of proline results in a reduction in the conformational entropy that opposes the folding of protein providing it with better structural rigidity [144,145,146], and improving the melting temperature for better hydrolytic efficiency.
-
(c)
Glycosylation
Glycosylation of PDE expressed in eukaryotic microbial cells can result in enhanced stability against thermal protein aggregation through protein thermodynamic stabilization [147]. However, the positioning of the glycosylation site is very critical. For example, if the glycan moiety is placed too close to the enzyme active site it may hamper the substrate accessibility due to steric hindrance even though the mutation may result in high thermostability. The resulting enzyme will still fail to achieve high activity at elevated temperatures. Therefore, the preferred glycosylation sites are positioned at loop regions or hydrophobic patches relatively distant from the active site to achieve high catalytic activity at high temperatures [148,149,150].
Based on the above discussion it must be understood that engineering PDE for enhanced thermostability may even lead to impairment of catalytic efficiency due to the negative impact of resulting mutation on active sites [142]. Therefore, a better understanding of enzyme structure function is very critical to the application of protein engineering on PDE [133]. In those cases, the synergistic role of structural analysis tools such as x-ray crystallography, for mutation analysis [151] and computational tools for the accumulation of beneficial mutation from mutant libraries [152] could be potential tools for achieving a better selection of engineered mutants.
Improving substrate and enzyme interaction
The improvement of substrate and enzyme interaction could be achieved by improved reinforcement and interaction between substrate and enzyme active site and enzyme surface, respectively. The reinforcement of the binding of the enzyme active site and substrate could be achieved by modification of the size of the enzyme active site or improving active site hydrophobicity [133].
-
(a)
Modification of active site
The modification of the active site involves both, the widening or narrowing of the active site for better substrate binding. Widening of the opening of the active site of Fusarium solani cutinase, Pseudomonas aestusnigri hydrolase, PETase, and MHETase through mutation may increase their hydrolytic efficiency towards PET, polyamide fibres, and PBSA plastics [151, 153,154,155]. However, a larger opening of active sites doesn’t guarantee improved catalytic efficiency due to weaker substrate affinity towards the enzyme active site owing to poor binding between the substrate and enzyme required for efficient hydrolysis [156]. Therefore, Austin et. al., reported that the narrowing of the enzyme’s active sites results in efficient plastic degradation [157]. This narrowing of the active site is the result of a double mutation of S238F and W159H of PETase from Ideonella sakaiensis 201-F6. The double mutation leads to the π-stacking interaction and deep placement of the substrate in the active site cleft enabled by S238F and W159H mutations, respectively, resulting in strong enzyme-substrate binding improving the PET degradation [157]. Similarly, Sevilla et al. [158] demonstrated that mutation of IsPETase from Ideonella sakaiensis showed variation in the PETase activity due to structural changes through rational design. The mutant S238Y mutation has tyrosine as compared to the WT serine residue. The aromatic side chain of tyrosine supports better π-π stacking interaction with the PET substrate leading to a better affinity of the mutated enzyme for PET. This study also reported the narrowing of the PET binding site due to N212 mutation to alanine, moved α-helix closer to the rest of the protein which is otherwise located at a farther distance, and pointed towards the solvent in wild type. Although N212 is not part of the enzymatic site its mutation caused the displacement of the loop 204-201 positioning the catalytic residue D206 for improved efficiency [158]. Thus, the choice of widening and narrowing of the active site depends on the type of enzyme and substrates.
Further, a change in the hydrophobicity of the active site can potentially improve the substrate binding affinity which can further lead to improved PETase efficiency. Furthermore, the active site modification can also lead to tuning of the hydrophobicity of the active site [159]. Thus, the synergistic action of widening/narrowing of enzyme activity and tuning of hydrophobicity can lead to enhanced degradation efficiency. One such example mutation of T. fusca cutinase at Q132A/ T101A resulted in enhanced PETase efficiency, because of widening with improved hydrophobicity of the active sites [159, 160].
However, narrowing, widening, or hydrophobicity of the enzyme active site may impact catalytic efficiency and stability, the substrate's properties, reaction condition, and product accumulation greatly affect the performance of PDE.
Enzyme surface engineering and substrate dynamics
The interaction between enzymes and specific plastic substrates regulates the effectiveness of enzyme-based plastic degradation because of heterogeneous catalysis behavior with insoluble plastic and the soluble PETase enzyme in an aqueous system [131, 161]. The Sabatier principle suggests that when the interaction between substrate and catalyst is of intermediate strength, the optimal catalytic efficiency [162]. This concept applies to heterogenous PDE catalysis where the variation in solubility of PET and PDEs leads to substantial adsorption of enzyme on the PET surface which negatively impacts catalytic efficiency. PET substrate and PDE interaction is controlled by hydrophobic and electrostatic interaction among amino acids of enzyme surface and substrate molecules [97, 163, 164]. Therefore, tailoring the surface hydrophobic and/or electrostatic interaction through surface engineering boosts the binding affinity of enzyme-substrate leading to improved PET degradation [47, 165, 166]. This can be achieved by several approaches such as tailoring surface electrostatics and tuning surface hydrophobicity [133].
-
(a)
Tailoring surface electrostatics
Herrero Acero et. al., [165] demonstrated that surface engineering of cutinase from Thermobifida cellulosilytica by replacement of positively charged Arg29 and Arg19 with electrically neutral asparagine and serine can lead to improved hydrolysis of PET whereas when glutamine (Glu65) is exchanged with negatively charged glutamic acid resulted in complete loss of hydrolysis efficiency. The tuning of the enzyme surface to electrically neutral can lead to a reduction in enzyme-plastic repulsion, thus enhancing degradation efficiency, Similarly, the mutation of R228S of the cutinase (CUT190) from Saccharomonospora viridis AHK190 resulted in providing electrostatic neutrality to the enzyme surface thus improving the enzyme-PET binding leading to improved PET hydrolysis [167].
-
(b)
Tuning enzyme surface hydrophobicity
As opposed to making the enzyme surface electrostatically neutral, tailoring the surface with increased hydrophobicity can enhance binding and improve regulation of enzyme-mediated PET degradation [151, 168]. The mutation of PHB depolymerase (PhaZRpiT1) from Ralstonia pickettii T1 by replacing serine and tyrosine with more hydrophobic cysteine and phenylalanine led to improved adsorption of the mutant onto PHB surface and hence in efficient plastic hydrolysis [169, 170]. The truncation of the N-terminal domain of esterase from Clostridium botulinum resulted in improved accessibility of hydrophobic surface area for PET adsorption which was otherwise covered in the wild-type variant leading to improved enzymatic hydrolysis [171]. Although improving hydrophobicity of the enzyme surface helps in enhancing catalytic activity but introduction of too many hydrophobic residues can lead to intermolecular hydrophobic interactions. These concomitant interactions can cause instability resulting in protein structure disruption or aggregation and impairing catalytic activity [168].
Accessory module and chimeric fusion for improved enzymatic functionalities
Several polymer degrading enzymes are characterized by the presence of an auxiliary binding domain such as carbohydrate-binding modules (CBM) in cellulase [163, 172] and PBMs in polyhydroxyalkanoate (PHA) granule-associated proteins Phasins [173]. This specialized domain plays a critical role in polymer substrate adhesion and increase the proximity of substrates to their enzymes. Plastic degrading enzymes such as PETase lack such accessory modules and, thus, do not inherently facilitate substrate binding [174]. Thus, it has led to the search for accessory binding domains that mimic naturally occurring auxiliary modules specialized in substrate polymer adhesions [175] such as carbohydrate-binding modules (CBMs), polyhydroxyalkanoate binding modules (PBMs), hydrophobins, bioactive and amphiphilic anchor peptides [133, 163]. The integration of these auxiliary modules with PDE leads to improved interaction between enzyme and substrate which further leads to improved degradation efficiency [163].
Carbohydrate-binding modules (CBMs) are accessory modules of cellulases and are compatible with a wide range of natural polymers or synthetic plastics. This can bring these polymers in proximity to the associated enzyme leading to improved hydrolysis [172, 176, 177]. Fusion of cellulose-binding domain of cellobiohydrolase I from Trichoderma reesei onto the C-terminus of engineered IsPETaseEHA from Ideonella sakaiensis showed enhanced enzymatic hydrolysis of PET [178]. A 44.5% and 71.5 % higher hydrolytic activity of IsPETaseEHA_CBM was observed at 30 ℃ and 40 ℃, respectively compared to the parent IsPETaseEHA enzyme [178]. Fusion of CBM from cellobiohydrolase I from Hypocrea jecorina with Thc_Cut1 from T. cellulosilytica. Enhanced adsorption of fusion enzymes was observed onto PET film showing higher binding affinity to PET which was complemented with enhanced activity toward PET [179].
Zhang et al., [180] demonstrated the fusion of Thermobifida fusca cutinase with carbohydrate-binding module (CBM) of cellulase CenA from Cellulomonas fimi. This fusion product resulted in improved hydrolysis of cotton fibres hydrolysis but does not bind to PET. Therefore, Zhang et al., [181] subjected the CMB site of cutinase–CBMCenA fusion protein to site-directed mutagenesis to enhance its activity toward PET. The mutation involved the replacement of tryptophan (Trp14, Trp50, and Trp 68) on the binding module CBMCenA with leucine or tyrosine. The mutant W68L and W68Y exhibited enhanced binding, catalytic activity (1.4-1.5-fold), temperature, and pH stability as compared to native enzyme fusion toward PET fiber. The probable mechanism for improved binding and catalytic activity may be the formation of new hydrogen bonds or hydrophobic interactions toward the PET fibre [181].
Similar to CBMs, the fusion of PBMs of polyhydroxyalkanoate depolymerase from Alcaligenes faecalis with the cutinase from Thermomyces cellullosylitica (Thc_Cut1) resulted in improved adsorption by PET sheet and improved catalytic efficiency [179]. Further, the Thc-Cut1_PBM was subjected to site-directed mutagenesis replacing serine 131 with alanine. The fusion resulted in improved adsorption on a thin film of synthetic polyester Poly (1,4-butylene adipate) and caused its complete degradation within 40 min which otherwise requires approx. 80 min with native Thc_Cut1 [182].
Hydrophobins are polymer-binding biological macromolecules obtained from fungi that are capable of degrading the petroleum-based polymer and can be engineered with the PDE for its improved catalytic efficiency [183]. The fusion of Thc_Cut1 covalently with Trichoderma hydrophobins can lead to a change in active site conformation supporting better substrate enzyme binding leading to a 16-fold increase in PET hydrolysis rate when compared with the native enzyme [184]. The variation in concentration of hydrophobins (HFB4 and HFB7 of Trichoderma), regulated the PET hydrolysis efficiency of cutinase from Humicola insolens. The Thc_Cut showed PETase activity at a very low concentration of HFB4 but has the inhibitory impact of cutinase activity at a high concentration. The HFB7 displayed adsorption isotherm-like behavior [185]. Therefore, the choice of hydrophobins for the generation of plastic-degrading fusion products must be decided rationally.
Bioactive polypeptides such as antimicrobial/anchor peptides having amphipathic properties can be used as binding fusion proteins with PDE. These anchor peptides can strongly bind to the surface of different synthetic polymers thus, are considered as one of the components of chimeric fusion [133, 186, 187]. The fusion of anchor peptide Tachystatin A2 (TA2) with Thermomonospora curvata cutinase (Tcur1278) resulted in the formation of Tcur1278_TA2 chimeric fusion that caused improvement of polyester-PU nanoparticle by 6.6-fold as compared to stand alone Tcur127_WT treatment [81].
To obtain optimized PDE functionalities, important factors that need attention are regulating the impact of product inhibition, creating multifunction PDEs, and enabling the PDE catalytic promiscuity which are sometimes interrelated and intra-dependent. Furthermore, the degradation products/intermediates can regulate the activity of PDE by feedback inhibition. This inhibition can be overcome by initiating structural changes in the substrate binding site or generating multifunctional enzymes [188, 189]. For instance, the modification of the active site of TfCut2 by mutation of G62A resulted in a 5.5-fold decrement of mono(2-hydroxyethyl) terephthalate (MHET) binding constant associated inhibitory products, thus having a positive impact on the PET degradation [190]. Further removal of intermediate products or their degradation can also minimize inhibition. Therefore, a chimeric fusion of ancillary enzyme such as MHETase with the native enzyme PETase can result in simultaneous degradation of MHET which otherwise causes intermediate product inhibition [191, 192].
Further, the fusion of PDE with auxiliary enzymes can be used to develop bifunctional plastic-degrading biocatalysis for efficient depolymerization [22]. The development of fusion protein lipase-cutinase improved PCL hydrolysis which involves the two-step degradation of PCL where lipase cleaves the backbone of PCL and the generated low molecular weight oligomers are further hydrolyzed by cutinase into soluble monomers [22]. Several already-known enzymes can be modified to expand their catalytic horizons known as catalytic promiscuity. This approach can be used to expand the degrading capacity of PDE for a wide range of plastics such as PAs, PEs, and PUs [133]. Biundo et. al., [193] demonstrated an enzyme engineering approach for developing switched reaction specificity in polyesterases (Cutinase, Thc_Cut1). This enzyme engineering approach resulted in promiscuous amidase activity in Thc_Cut1, making it capable of breaking down amide bonds for polymerizing synthetic PAs. The developed promiscuous amidase enzyme was further subjected to mutation of residues that obstruct the interaction of transition state compounds with water molecules. This improved interaction led to improved hydrolytic activity by 6-15 folds [193].
Although protein engineering deals with structural changes through directed evolution and rational designs, understanding the individual chemical kinetics and the optimization of reaction variables can modulate enzyme efficiency and is critical to driving the process cost. Erickson et. al., [194] demonstrated the efficiency of wild type lsPETase from Ideonella sakaiensis with a double mutant DM (W159H/S238F) variant. This suggested that both the wild-type and DM were strongly influenced by reaction temperature and are most efficient toward hydrolysis of moderate crystallinity of substrates. The study by Erickson et. al, [194] also demonstrated that product inhibition or degradation product accumulation impacts enzymatic efficiency at different temperatures. At 30 °C, WT kept hydrolyzing amorphous PET for 168 h, but the catalytic efficiency is low at this temperature for DM. Whereas at 40 °C, the WT product accumulation plateaus at 48 h whereas DM accumulates the highest total product accumulation from 72 h onwards and remains active till 168h. Therefore, regulating multiple structural and process parameters is required for enhanced enzymatic degradation.
Conclusion
Plastic waste management systems in developing countries are significantly underdeveloped, resulting in the emission of high concentrations of greenhouse gases that drive climate change. Beyond traditional mechanical and chemical techniques, it is imperative to consider biological approaches for sustainable solutions. The microbial degradation of recalcitrant plastics has garnered global interest due to the potential of microbial enzymes in combating plastic pollution. Recent advancements, particularly in the enzymatic recycling of PET, have made closed-loop PET recycling a feasible reality. However, more systematic studies need to be developed to deduce efficient technologies for the mitigation of other variants of plastics such as PE, PVC, PUR, and PS. Several conventional strategies such as the discovery of new potent microbes from extreme environments, and microbial diversity analysis associated with waste plastics from different sources can give promising results if combined with computational techniques. Bioinformatic tools, including metagenomics, transcriptomics, and proteomics along with advanced sequencing tools, have expedited the discovery of PDEs. Additionally, modern techniques such as molecular dynamic simulations and machine learning have facilitated enzyme remodeling, enhancing the efficiency of plastic breakdown. In-silico studies contribute not only to enzyme mining but also to improving the efficiency of plastic degradation. Protein engineering approaches (directed evolution and rational design) have resulted in groundbreaking studies in the mitigation of other classes of plastics such as PEs, PAs, and PUs. Nevertheless, future research must focus on enhancing the scalability and efficacy of enzymatic and microbial degradation techniques for practical, real-world applications. The development of multifunctional enzymes, enzyme promiscuity, role of product inhibition, optimizing process parameters, understanding enzyme kinetics, and process dynamics can be used in synergy with identifying novel microbes, enzymes, and enzymatic pathways followed by its systematic regulation for filling research gaps and developing potential strategies to overcome the global plastic pollution catastrophe. However, the vast problem of plastic waste accumulation cannot be mitigated solely through biological techniques. Effective plastic waste management necessitates stringent recycling and waste sorting policies as well.
Availability of data and materials
No datasets were generated or analysed during the current study.
Abbreviations
- PDE:
-
Plastic-degrading enzyme
- PET:
-
Polyethylene terephthalate
- LDPE:
-
Low-density polyethylene
- HDPE:
-
High-density polyethylene
- MPs:
-
Microplastics
- NPs:
-
Nano plastics
- PA:
-
Polyamide
- PS:
-
Polystyrene
- PP:
-
Polypropylene
- PEG:
-
Polyethylene glycol
- PVC:
-
Polyvinyl chloride
- PCL:
-
Polycaprolactone
- PBAT:
-
Polybutylene adipate terephthalate
- CBMs:
-
Carbohydrate-binding modules
- PBMs:
-
Polyhydroxyalkanoate binding modules
- SEM:
-
Scanning electron microscopy
- ATR-FTIR:
-
Attenuated total reflectance Fourier transform infrared spectroscopy
- WCA:
-
Water contact angle
- GPC:
-
Gel permeation chromatography
- LC-MS:
-
Liquid chromatography-mass spectrometry
- BLAST:
-
Basic local alignment search tool
- NMR:
-
Nuclear magnetic resonance
- HPLC:
-
High-performance liquid chromatography
- Xc :
-
Degree of crystallinity
- MHET:
-
Mono-(2-hydroxyethyl) terephthalate
- BHET:
-
Bis-(2-hydroxyethyl) terephthalate
- TPA:
-
Terephthalic acid
- UV:
-
Ultra-violet
References
Charles D, Kimman L. Plastic Waste Makers Index. 2023. https://cdn.minderoo.org/content/uploads/2023/02/04205527/Plastic-Waste-Makers-Index-2023.pdf.
Plastics – the fast Facts 2023 • Plastics Europe. https://plasticseurope.org/knowledge-hub/plastics-the-fast-facts-2023/. Accessed 19 June 2024.
Plastic consumption in India from 1990 to 2021. 2023. https://www.statista.com/statistics/1154434/india-net-plastic-consumption/. Accessed 19 Dec 2023.
Borrelle SB, Ringma J, Lavender Law K, Monnahan CC, Lebreton L, McGivern A, et al. Predicted growth in plastic waste exceeds efforts to mitigate plastic pollution. Science. 1979;2020(369):1515–8.
Mor S, Ravindra K. Municipal solid waste landfills in lower- and middle-income countries: Environmental impacts, challenges and sustainable management practices. Process Safety and Environmental Protection. 2023;174:510–30.
Waste to Energy: Considerations for Informed Decision-making | International Environmental Technology Centre. https://www.unep.org/ietc/resources/publication/waste-energy-considerations-informed-decision-making. Accessed 19 June 2024.
Plastic and Climate: The Hidden Costs of a Plastic Planet - Center for International Environmental Law. https://www.ciel.org/plasticandclimate/. Accessed 21 Feb 2024.
Ali I, Tan X, Li J, Peng C, Wan P, Naz I, et al. Innovations in the development of promising adsorbents for the remediation of microplastics and nanoplastics – A critical review. Water Res. 2023;230:119526.
Pencik O, Durdakova M, Molnarova K, Kucsera A, Klofac D, Kolackova M, et al. Microplastics and nanoplastics toxicity assays: A revision towards environmental-relevance in water environment. J Hazard Mater. 2023;454:131476.
Moeck C, Davies G, Krause S, Schneidewind U. Microplastics and nanoplastics in agriculture—A potential source of soil and groundwater contamination? Grundwasser. 2022;28(1):23–35.
Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N Engl J Med. 2024;390:900–10.
Naz S, Chatha AMM, Khan NA, Ullah Q, Zaman F, Qadeer A, et al. Unraveling the ecotoxicological effects of micro and nano-plastics on aquatic organisms and human health. Front Environ Sci. 2024;12:1390510.
Zhao J, Lan R, Wang Z, Su W, Song D, Xue R, et al. Microplastic fragmentation by rotifers in aquatic ecosystems contributes to global nanoplastic pollution. Nat Nanotechnol. 2023;19(3):406–14.
Liu S, Wang Z, Xiang Q, Wu B, Lv W, Xu S. A comparative study in healthy and diabetic mice followed the exposure of polystyrene microplastics: Differential lipid metabolism and inflammation reaction. Ecotoxicol Environ Saf. 2022;244:114031.
Ragaert K, Delva L, Van Geem K. Mechanical and chemical recycling of solid plastic waste. Waste Management. 2017;69:24–58.
Vollmer I, Jenks MJF, Mayorga González R, Meirer F, Weckhuysen BM. Plastic Waste Conversion over a Refinery Waste Catalyst. Angew Chem Int Ed. 2021;60:16101–8.
Yoshida S, Hiraga K, Takehana T, Taniguchi I, Yamaji H, Maeda Y, et al. A bacterium that degrades and assimilates poly(ethylene terephthalate). Science. 1979;2016(351):1196–9.
Hu Q, Jayasinghe-Arachchige VM, Prabhakar R. Degradation of a main plastic pollutant polyethylene terephthalate by two distinct proteases (Neprilysin and cutinase-like enzyme). J Chem Inf Model. 2021;61:764–76.
Gricajeva A, Nadda AK, Gudiukaite R. Insights into polyester plastic biodegradation by carboxyl ester hydrolases. J Chem Technol Biotechnol. 2022;97:359–80.
Zampolli J, Mangiagalli M, Vezzini D, Lasagni M, Ami D, Natalello A, et al. Oxidative degradation of polyethylene by two novel laccase-like multicopper oxidases from Rhodococcus opacus R7. Environ Technol Innov. 2023;32:103273.
Dimarogona M, Nikolaivits E, Kanelli M, Christakopoulos P, Sandgren M, Topakas E. Structural and functional studies of a Fusarium oxysporum cutinase with polyethylene terephthalate modification potential. Biochimic Biophys Acta. 2015;1850:2308–17.
Liu M, Zhang T, Long L, Zhang R, Ding S. Efficient enzymatic degradation of poly (ɛ-caprolactone) by an engineered bifunctional lipase-cutinase. Polym Degrad Stab. 2019;160:120–5.
Yang Y, Yang J, Wu WM, Zhao J, Song Y, Gao L, et al. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 1. Chemical and physical characterization and isotopic tests. Environ Sci Technol. 2015;49:12080–6.
Yang Y, Yang J, Wu WM, Zhao J, Song Y, Gao L, et al. Biodegradation and mineralization of polystyrene by plastic-eating mealworms: Part 2. Role of gut microorganisms. Environ Sci Technol. 2015;49:12087–93.
Caruso G. Plastic Degrading microorganisms as a tool for bioremediation of plastic contamination in aquatic environments. J Pollut Effects Control. 2015;3:3. https://doi.org/10.4172/2375-4397.1000e112.
Bornscheuer UT. Feeding on plastic. Science. 1979;2016(351):1154–5.
Parida D, Sangtani R, Bala K. Microplastics: The stemming environmental challenge and the quest for the missing mitigation strategies. Int Biodeterior Biodegradation. 2023;179: 105581.
Ferronato N, Maalouf A, Mertenat A, Saini A, Khanal A, Copertaro B, et al. A review of plastic waste circular actions in seven developing countries to achieve sustainable development goals. Waste Management and Research. 2023. https://doi.org/10.1177/0734242X231188664/ASSET/IMAGES/LARGE/10.1177_0734242X231188664-FIG5.JPEG.
Tournier V, Duquesne S, Guillamot F, Cramail H, Taton D, Marty A, et al. Enzymes’ power for plastics degradation. Chem Rev. 2023;123:5701.
Kaushal J, Khatri M, Arya SK. Recent insight into enzymatic degradation of plastics prevalent in the environment: A mini - review. Cleaner Engineering and Technology. 2021;2:100083.
Singh N, Hui D, Singh R, Ahuja IPS, Feo L, Fraternali F. Recycling of plastic solid waste: A state of art review and future applications. Compos B Eng. 2017;115:409–22.
Alsabri A, Tahir F, Al-Ghamdi SG. Environmental impacts of polypropylene (PP) production and prospects of its recycling in the GCC region. Mater Today Proc. 2022;56:2245–51.
PET market volume worldwide 2015-2030 | Statista. https://www.statista.com/statistics/1245264/polyethylene-terephthalate-market-volume-worldwide/. Accessed 2 Dec 2023.
Ru J, Huo Y, Yang Y. Microbial degradation and valorization of plastic wastes. Front Microbiol. 2020;11:507487.
Urase T, Okumura H, Panyosaranya S, Inamura A. Emission of volatile organic compounds from solid waste disposal sites and importance of heat management. Waste Management & Research. 2008;26:534–8.
Tsuchida D, Kajihara Y, Shimidzu N, Hamamura K, Nagase M. Hydrogen sulfide production by sulfate-reducing bacteria utilizing additives eluted from plastic resins. Waste Management & Research. 2010;29:594–601.
Sinha V, Patel MR, Patel JV. Pet waste management by chemical recycling: A review. Journal of Polymers and the Environment. 2010;18:8–25.
Zhang J, Wang X, Gong J, Gu Z. A study on the biodegradability of polyethylene terephthalate fiber and diethylene glycol terephthalate. J Appl Polym Sci. 2004;93:1089–96.
Webb HK, Arnott J, Crawford RJ, Ivanova EP. Plastic degradation and its environmental implications with special reference to poly(ethylene terephthalate). Polymers (Basel). 2013;5:1–18.
Vlasopoulos A, Malinauskaite J, Żabnieńska-Góra A, Jouhara H. Life cycle assessment of plastic waste and energy recovery. Energy. 2023;277: 127576.
Kumar S, Panda AK, Singh RK. A review on tertiary recycling of high-density polyethylene to fuel. Resour Conserv Recycl. 2011;55:893–910.
Vollmer I, Jenks MJF, Roelands CP, Obin R, White J, Van Harmelen T, et al. Plastic recycling beyond mechanical recycling: Giving new life to plastic waste. 2020:9:15402–23. https://doi.org/10.1002/anie.201915651.
Rispoli O, Ajibade OO. Comparative life cycle assessment of a novel sustainable road pavement system adopting recycled plastic from PET bottles and carbonated aggregate. Heliyon. 2024;10: e24354.
Javid F, Ali G, Rehman A, Naeem R, Ali I, Naz I. Assessment of plastic degradation by indigenous bacteria from waste disposal sites. Emerg Contam. 2024;10: 100323.
Lin Z, Jin T, Xu X, Yin X, Zhang D, Geng M, et al. Screening and degradation characteristics of plastic-degrading microorganisms in film-mulched vegetable soil. Int Biodeterior Biodegradation. 2024;186:105686.
Gao R, Liu R, Sun C. A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J Hazard Mater. 2022;431:128617.
Kim HR, Lee HM, Yu HC, Jeon E, Lee S, Li J, et al. Biodegradation of polystyrene by Pseudomonas sp isolated from the gut of superworms (Larvae of Zophobas atratus). Environ Sci Technol. 2020;54:6987–96.
Ganesh Kumar A, Hinduja M, Sujitha K, Nivedha Rajan N, Dharani G. Biodegradation of polystyrene by deep-sea Bacillus paralicheniformis G1 and genome analysis. Sci Total Environ. 2021;774:145002.
Skariyachan S, Patil AA, Shankar A, Manjunath M, Bachappanavar N, Kiran S. Enhanced polymer degradation of polyethylene and polypropylene by novel thermophilic consortia of Brevibacillus sps. and Aneurinibacillus sp. screened from waste management landfills and sewage treatment plants. Polym Degrad Stab. 2018;149:52–68.
Wang P, Zhao J, Ruan Y, Cai X, Li J, Zhang L, et al. Degradation of Polypropylene by the Pseudomonas aeruginosa Strains LICME WZH-4 and WGH-6. J Polym Environ. 2022;30:3949–58.
Wilkes RA, Aristilde L. Degradation and metabolism of synthetic plastics and associated products by Pseudomonas sp.: capabilities and challenges. J Appl Microbiol. 2017;123:582–93.
Cai Z, Li M, Zhu Z, Wang X, Huang Y, Li T, et al. Biological degradation of plastics and microplastics: A recent perspective on associated mechanisms and influencing factors. Microorganisms. 2023;11:1661.
Tao X, Ouyang H, Zhou A, Wang D, Matlock H, Morgan JS, et al. Polyethylene degradation by a Rhodococcous Strain isolated from naturally weathered plastic waste enrichment. Environ Sci Technol. 2023;57:13901–11.
Thakur B, Singh J, Singh J, Angmo D, Vig AP. Biodegradation of different types of microplastics: Molecular mechanism and degradation efficiency. Sci Total Environ. 2023;877:162912.
Yao Z, Seong HJ, Jang YS. Environmental toxicity and decomposition of polyethylene. Ecotoxicol Environ Saf. 2022;242:113933.
Khandare SD, Chaudhary DR, Jha B. Marine bacterial biodegradation of low-density polyethylene (LDPE) plastic. Biodegradation. 2021;32:127–43.
Skariyachan S, Megha M, Kini MN, Mukund KM, Rizvi A, Vasist K. Selection and screening of microbial consortia for efficient and ecofriendly degradation of plastic garbage collected from urban and rural areas of Bangalore. India Environ Monit Assess. 2015;187:1–14.
Tiong E, Koo YS, Bi J, Koduru L, Koh W, Lim YH, et al. Expression and engineering of PET-degrading enzymes from Microbispora, Nonomuraea, and Micromonospora. Appl Environ Microbiol. 2023;89(11):1-12.
Mohanan N, Wong MCH, Budisa N, Levin DB. Polymer-degrading enzymes of Pseudomonas chloroaphis PA23 display broad substrate preferences. Int J Mol Sci. 2023;24:4501.
Gao R, Liu R, Sun C. A marine fungus Alternaria alternata FB1 efficiently degrades polyethylene. J Hazard Mater. 2022;431:128617.
Sowmya H V, Ramalingappa, Krishnappa M, Thippeswamy B. Degradation of polyethylene by Trichoderma harzianum—SEM, FTIR, and NMR analyses. Environ Monit Assess. 2014;186:6577–86.
Jeon HJ, Kim MN. Functional analysis of alkane hydroxylase system derived from Pseudomonas aeruginosa E7 for low molecular weight polyethylene biodegradation. Int Biodeterior Biodegradation. 2015;103:141–6.
Parthasarathy A, Miranda RR, Eddingsaas NC, Chu J, Freezman IM, Tyler AC, et al. Polystyrene degradation by Exiguobacterium sp. RIT 594: Preliminary evidence for a pathway containing an atypical Oxygenase. Microorganisms. 2022;10:1619.
Adıgüzel AO, Şen F, Könen-Adıgüzel S, Kıdeyş AE, Karahan A, Doruk T, et al. Identification of cutinolytic esterase from microplastic-Associated microbiota using functional metagenomics and its plastic degrading potential. Mol Biotechnol. 2023;1–18. https://doi.org/10.1007/s12033-023-00916-7.
Nikolaivits E, Taxeidis G, Gkountela C, Vouyiouka S, Maslak V, Nikodinovic-Runic J, et al. A polyesterase from the Antarctic bacterium Moraxella sp. degrades highly crystalline synthetic polymers. J Hazard Mater. 2022;434:128900.
Zhang Y, Lin Y, Gou H, Feng X, Zhang X, Yang L. Screening of polyethylene-degrading bacteria from Rhyzopertha dominica and evaluation of its key enzymes degrading polyethylene. Polymers (Basel). 2022;14:5127.
Parida D, Katare K, Ganguly A, Chakraborty D, Konar O, Nogueira R, et al. Molecular docking and metagenomics assisted mitigation of microplastic pollution. Chemosphere. 2024;351:141271.
Di Pippo F, Venezia C, Sighicelli M, Pietrelli L, Di Vito S, Nuglio S, et al. Microplastic-associated biofilms in lentic Italian ecosystems. Water Res. 2020;187:116429.
Rozman U, Filker S, Kalčíková G. Monitoring of biofilm development and physico-chemical changes of floating microplastics at the air-water interface. Environ Pollut. 2023;322:121157.
Uekert T, Singh A, DesVeaux JS, Ghosh T, Bhatt A, Yadav G, et al. Technical, economic, and environmental comparison of closed-loop recycling technologies for common plastics. ACS Sustain Chem Eng. 2023;11:965–78.
Traxler I, Laske S, Fischer J. Closed-loop recycling of polypropylene: A case study on mechanical recycling of separately collected yogurt cups in Austria. Resour Conserv Recycl. 2024;205:107537.
Yu S, Li Q, Zhang Y, Zhou H. New possibility for PET plastic recycling by a tailored hydrolytic enzyme. Green Energy & Environment. 2024;9:163–5.
Li A, Wu L, Cui H, Song Y, Zhang X, Li X. Unlocking a Sustainable Future for Plastics: A Chemical-Enzymatic Pathway for Efficient Conversion of Mixed Waste to MHET and Energy-Saving PET Recycling. ChemSusChem. 2024;17:e202301612.
Oda K, Wlodawer A. Development of enzyme-based approaches for recycling PET on an industrial scale. Biochemistry. 2024. https://doi.org/10.1021/ACS.BIOCHEM.3C00554/ASSET/IMAGES/MEDIUM/BI3C00554_0031.GIF.
Navone L, Moffitt K, Hansen KA, Blinco J, Payne A, Speight R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Management. 2020;102:149–60.
Aguiar MIS, Sousa AF, Teixeira G, Tavares APM, Ferreira AM, Coutinho JAP. Enhancing plastic waste recycling: Evaluating the impact of additives on the enzymatic polymer degradation. Catal Today. 2024;429:114492.
Orlando M, Molla G, Castellani P, Pirillo V, Torretta V, Ferronato N. Microbial enzyme biotechnology to reach plastic waste circularity: Current status, problems and perspectives. 2023. https://doi.org/10.3390/ijms24043877.
Fotopoulou K, Fotopoulou KN, Karapanagioti HK, Fotopoulou KN, Karapanagioti HK, Takada H. Degradation of various plastics in the environment. 2017. https://doi.org/10.1007/698_2017_11.
Mohanan N, Montazer Z, Sharma PK, Levin DB. Microbial and Enzymatic Degradation of Synthetic Plastics. Front Microbiol. 2020;11: 580709.
Gupta D, Modi G. Breaking down the plastics paradox: polymer degrading microorganisms. Bulgarian Chemical Communications. 2023;55:2023.
Islam S, Apitius L, Jakob F, Schwaneberg U. Targeting microplastic particles in the void of diluted suspensions. Environ Int. 2019;123:428–35.
Shi L, Zhu L. Recent advances and challenges in enzymatic depolymerization and recycling of PET wastes. ChemBioChem. 2024;25:e202300578.
Schubert S, Schaller K, Bååth JA, Hunt C, Borch K, Jensen K, et al. Reaction pathways for the enzymatic degradation of poly(ethylene terephthalate): What characterizes an efficient PET-hydrolase? ChemBioChem. 2023;24:e202200516.
Zhang K, Hamidian AH, Tubić A, Zhang Y, Fang JKH, Wu C, et al. Understanding plastic degradation and microplastic formation in the environment: A review. Environ Pollut. 2021;274:116554.
Chen CC, Dai L, Ma L, Guo RT. Enzymatic degradation of plant biomass and synthetic polymers. Nature Reviews Chemistry. 2020;4:114–26.
Zhu X, Rochman CM, Hardesty BD, Wilcox C. Plastics in the deep sea – A global estimate of the ocean floor reservoir. Deep Sea Research Part I: Oceanographic Research Papers. 2024;206:104266.
Rynek R, Tekman MB, Rummel C, Bergmann M, Wagner S, Jahnke A, et al. Hotspots of floating plastic particles across the North Pacific Ocean. Environ Sci Technol. 2024;58:4302–13.
De-la-Torre GE, Santillán L, Dioses-Salinas DC, Yenney E, Toapanta T, Okoffo ED, et al. Assessing the current state of plastic pollution research in Antarctica: Knowledge gaps and recommendations. Chemosphere. 2024;355:141870.
Vaksmaa A, Hernando-Morales V, Zeghal E, Niemann H. Microbial Degradation of Marine Plastics: Current State and Future Prospects. In: Joshi SJ, Deshmukh A, Sarma H, editors. Biotechnology for Sustainable Environment. Singapore: Springer; 2021. p. 111–154.
Burrows S, Colwell J, Costanzo S, Kaserzon S, Okoffo E, Ribeiro F, et al. UV sources and plastic composition influence microplastic surface degradation: Implications for plastic weathering studies. J Hazardous Mater Adv. 2024;14:100428.
Wu X, Zhao X, Chen R, Liu P, Liang W, Wang J, et al. Size-dependent long-term weathering converting floating polypropylene macro- and microplastics into nanoplastics in coastal seawater environments. Water Res. 2023;242:120165.
Ciuffi B, Fratini E, Rosi L. Plastic pretreatment: The key for efficient enzymatic and biodegradation processes. Polym Degrad Stab. 2024;222:110698.
Kong Y, Hay JN. The enthalpy of fusion and degree of crystallinity of polymers as measured by DSC. Eur Polym J. 2003;39:1721–7.
Chukhchin DG, Malkov AV, Tyshkunova IV, Mayer LV, Novozhilov EV. Diffractometric method for determining the degree of crystallinity of materials. Crystallography Reports. 2016;61:371–5.
Uzun İ. Methods of determining the degree of crystallinity of polymers with X-ray diffraction: a review. J Polymer Res. 2023;30(10):1–45.
Thomsen TB, Almdal K, Meyer AS. Significance of poly(ethylene terephthalate) (PET) substrate crystallinity on enzymatic degradation. N Biotechnol. 2023;78:162–72.
Kawai F. The current state of research on PET hydrolyzing enzymes available for biorecycling. Catalysts. 2021;11:1–10.
Urbanek AK, Rymowicz W, Mirończuk AM. Degradation of plastics and plastic-degrading bacteria in cold marine habitats. Appl Microbiol Biotechnol. 2018;102:7669–78.
Ciuffi B, Fratini E, Rosi L. Plastic pretreatment: The key for efficient enzymatic and biodegradation processes. Polym Degrad Stab. 2024;222:110698.
Zettler ER, Mincer TJ, Amaral-Zettler LA. Life in the “plastisphere”: Microbial communities on plastic marine debris. Environ Sci Technol. 2013;47:7137–46.
Ogonowski M, Motiei A, Ininbergs K, Hell E, Gerdes Z, Udekwu KI, et al. Evidence for selective bacterial community structuring on microplastics. Environ Microbiol. 2018;20:2796–808.
Liu P, Dai J, Bie C, Li H, Zhang Z, Guo X, et al. Bioaccessibility of microplastic-associated antibiotics in freshwater organisms: Highlighting the impacts of biofilm colonization via an in vitro protocol. Environ Sci Technol. 2022;56:12267–77.
Sheng Y, Ye X, Zhou Y, Li R. Microplastics (MPs) act as sources and vector of pollutants-Impact hazards and preventive measures. Bull Environ Contam Toxicol. 2021;107(4):722–9.
Du Y, Liu X, Dong X, Yin Z. A review on marine plastisphere: biodiversity, formation, and role in degradation. Comput Struct Biotechnol J. 2022;20:975–88.
Frey B, Aiesi M, Rast BM, Rüthi J, Julmi J, Stierli B, et al. Searching for new plastic-degrading enzymes from the plastisphere of alpine soils using a metagenomic mining approach. PLoS One. 2024;19:e0300503.
Wright RJ, Bosch R, Langille MGI, Gibson MI, Christie-Oleza JA. A multi-OMIC characterisation of biodegradation and microbial community succession within the PET plastisphere. Microbiome. 2021;9:1–22.
Ray AS, Rajasekaran M, Uddin M, Kandasamy R. Laccase driven biocatalytic oxidation to reduce polymeric surface hydrophobicity: An effective pre-treatment strategy to enhance biofilm mediated degradation of polyethylene and polycarbonate plastics. Science of The Total Environment. 2023;904:166721.
Giraldo-Narcizo S, Guenani N, Sánchez-Pérez AM, Guerrero A. Accelerated polyethylene terephthalate (PET) enzymatic degradation by room temperature alkali pre-treatment for reduced polymer crystallinity. ChemBioChem. 2023;24:e202200503.
Patel A, Chang AC, Perry S, Soong YHV, Ayafor C, Wong HW, et al. Melt processing pretreatment effects on enzymatic depolymerization of poly(ethylene terephthalate). ACS Sustain Chem Eng. 2022;10:13619–28.
Gallorini R, Ciuffi B, Real Fernández F, Carozzini C, Ravera E, Papini AM, et al. Subcritical hydrothermal liquefaction as a pretreatment for enzymatic degradation of polyurethane. ACS Omega. 2022;7:37757–63.
Skariyachan S, Manjunath M, Shankar A, Bachappanavar N, Patil AA. Application of Novel Microbial Consortia for Environmental Site Remediation and Hazardous Waste Management Toward Low- and High-Density Polyethylene and Prioritizing the Cost-Effective, Eco-friendly, and Sustainable Biotechnological Intervention. In: Hussain C, editor. Handbook of Environmental Materials Management. Cham: Springer; 2019. p. 431–478.
Salinas J, Carpena V, Martínez-Gallardo MR, Segado M, Estrella-González MJ, Toribio AJ, et al. Development of plastic-degrading microbial consortia by induced selection in microcosms. Front Microbiol. 2023;14:1143769.
Quartinello F, Kremser K, Schoen H, Tesei D, Ploszczanski L, Nagler M, et al. Together is better: The rumen microbial community as biological toolbox for degradation of synthetic polyesters. Front Bioeng Biotechnol. 2021;9:684459.
Cao Z, Yan W, Ding M, Yuan Y. Construction of microbial consortia for microbial degradation of complex compounds. Front Bioeng Biotechnol. 2022;10:1051233.
Lu H, Diaz DJ, Czarnecki NJ, Zhu C, Kim W, Shroff R, et al. Machine learning-aided engineering of hydrolases for PET depolymerization. Nature. 2022;604(7907):662–7.
Jin J, Arciszewski J, Auclair K, Jia Z. Enzymatic polyethylene biorecycling: Confronting challenges and shaping the future. J Hazard Mater. 2023;460: 132449.
Ziemert N, Alanjary M, Weber T. The evolution of genome mining in microbes – a review. Nat Prod Rep. 2016;33:988–1005.
Kim DW, Ahn JH, Cha CJ. Biodegradation of plastics: mining of plastic-degrading microorganisms and enzymes using metagenomics approaches. Journal of Microbiology. 2022;60:969–76.
Howard SA, McCarthy RR. Modulating biofilm can potentiate activity of novel plastic-degrading enzymes. NPJ Biofilms Microbiomes. 2023;9(1):1–10.
Pérez-García P, Danso D, Zhang H, Chow J, Streit WR. Exploring the global metagenome for plastic-degrading enzymes. Methods Enzymol. 2021;648:137–57.
Richter PK, Blázquez-Sánchez P, Zhao Z, Engelberger F, Wiebeler C, Künze G, et al. Structure and function of the metagenomic plastic-degrading polyester hydrolase PHL7 bound to its product. https://doi.org/10.1038/s41467-023-37415-x.
Zampolli J, Orro A, Manconi A, Ami D, Natalello A, Di Gennaro P. Transcriptomic analysis of Rhodococcus opacus R7 grown on polyethylene by RNA-seq. Sci Rep. 2021;11(1):1–14.
Wright RJ, Bosch R, Gibson MI, Christie-Oleza JA. Plasticizer degradation by marine bacterial isolates: A proteogenomic and metabolomic characterization. Environ Sci Technol. 2020;54:2244–56.
Buchholz PCF, Feuerriegel G, Zhang H, Perez-Garcia P, Nover LL, Chow J, et al. Plastics degradation by hydrolytic enzymes: The plastics-active enzymes database—PAZy. Proteins. 2022;90:1443–56.
Naveed M, Naveed R, Aziz T, Azeem A, Afzal M, Waseem M, et al. Biodegradation of PVCs through in-vitro identification of Bacillus albus and computational pathway analysis of ABH enzyme. Biodegradation. 2024;35:451–68.
Amjad K, Shah T, Khan Z, Haider G, Sheikh Z, Adnan F, et al. Genome-wide identification and characterization of lipases from ascomycetes, and molecular docking analysis with various plastics. 2024. Preprint. https://doi.org/10.21203/RS.3.RS-3951591/V1.
Watts T, Khoba K, Purty RS. In silico approach for evaluating the degradation efficiency of plastic degrading enzyme mono(2-hydroxyethyl) terephthalic acid hydrolase (MHETase) of selected bacteria. Bioremediat J. 2023. https://doi.org/10.1080/10889868.2023.2279201.
Pawano O, Jenpuntarat N, Streit WR, Pérez-García P, Pongtharangkul T, Phinyocheep P, et al. Exploring untapped bacterial communities and potential polypropylene-degrading enzymes from mangrove sediment through metagenomics analysis. Front Microbiol. 2024;15:1347119.
Salam LB. Metagenomic investigations into the microbial consortia, degradation pathways, and enzyme systems involved in the biodegradation of plastics in a tropical lentic pond sediment. World J Microbiol Biotechnol. 2024;40(6):1–37.
Kassab A, Al Nabhani D, Mohanty P, Pannier C, Ayoub GY. Advancing plastic recycling: Challenges and opportunities in the integration of 3D printing and distributed recycling for a circular economy. Polymers. 2023;15(19):3881.
Ellis LD, Rorrer NA, Sullivan KP, Otto M, McGeehan JE, Román-Leshkov Y, et al. Chemical and biological catalysis for plastics recycling and upcycling. Nat Catal. 2021;4:539–56.
Lee S, Lee YR, Kim SJ, Lee JS, Min K. Recent advances and challenges in the biotechnological upcycling of plastic wastes for constructing a circular bioeconomy. Chem Eng J. 2023;454:140470.
Zhu B, Wang D, Wei N. Enzyme discovery and engineering for sustainable plastic recycling. Trends in Biotechnology. 2022;40:22–37.
Wang Y, Xue P, Cao M, Yu T, Lane ST, Zhao H. Directed evolution: Methodologies and applications. Chemical Reviews. 2021;121:12384–444.
Bell EL, Smithson R, Kilbride S, Foster J, Hardy FJ, Ramachandran S, et al. Directed evolution of an efficient and thermostable PET depolymerase. Nat Catal. 2022;5:673–81.
Tan L-T, Hiraishi T, Sudesh K, Maeda M. Directed evolution of poly[(R)-3-hydroxybutyrate] depolymerase using cell surface display system: functional importance of asparagine at position 285. Appl Microbiol Biotechnol. 2013;97:4859–71.
Apitius L, Rübsam K, Jakesch C, Jakob F, Schwaneberg U. Ultrahigh-throughput screening system for directed polymer binding peptide evolution. Biotechnol Bioeng. 2019;116:1856–67.
Zhou X, Zhou X, Xu Z, Zhang M, Zhu H. Characterization and engineering of plastic-degrading polyesterases jmPE13 and jmPE14 from Pseudomonas bacterium. Front Bioeng Biotechnol. 2024;12:1349010.
Then J, Wei R, Oeser T, Gerdts A, Schmidt J, Barth M, et al. A disulfide bridge in the calcium binding site of a polyester hydrolase increases its thermal stability and activity against polyethylene terephthalate. FEBS Open Bio. 2016;6:425–32.
Son HF, Joo S, Seo H, Sagong HY, Lee SH, Hong H, et al. Structural bioinformatics-based protein engineering of thermo-stable PETase from Ideonella sakaiensis. Enzyme Microb Technol. 2020;141:109656.
Oda M, Yamagami Y, Inaba S, Oida T, Yamamoto M, Kitajima S, et al. Enzymatic hydrolysis of PET: functional roles of three Ca2+ ions bound to a cutinase-like enzyme, Cut190*, and its engineering for improved activity. Appl Microbiol Biotechnol. 2018;102:10067–77.
Son HF, Cho IJ, Joo S, Seo H, Sagong HY, Choi SY, et al. Rational protein engineering of thermo-stable PETase from Ideonella sakaiensis for highly efficient PET degradation. ACS Catal. 2019;9:3519–26.
Cui Y, Chen Y, Liu X, Dong S, Tian Y, Qiao Y, et al. Computational redesign of a PETase for plastic biodegradation under ambient condition by the GRAPE strategy. ACS Catal. 2021;11:1340–50.
Shirke AN, Basore D, Butterfoss GL, Bonneau R, Bystroff C, Gross RA. Toward rational thermostabilization of Aspergillus oryzae cutinase: Insights into catalytic and structural stability. Proteins. 2016;84:60–72.
Thumarat U, Nakamura R, Kawabata T, Suzuki H, Kawai F. Biochemical and genetic analysis of a cutinase-type polyesterase from a thermophilic Thermobifida alba AHK119. Appl Microbiol Biotechnol. 2012;95:419–30.
Thumarat U, Kawabata T, Nakajima M, Nakajima H, Sugiyama A, Yazaki K, et al. Comparison of genetic structures and biochemical properties of tandem cutinase-type polyesterases from Thermobifida alba AHK119. J Biosci Bioeng. 2015;120:491–7.
Shirke AN, White C, Englaender JA, Zwarycz A, Butterfoss GL, Linhardt RJ, et al. Stabilizing leaf and branch compost cutinase (LCC) with glycosylation: Mechanism and effect on PET hydrolysis. Biochemistry. 2018;57:1190–200.
Gamerith C, Vastano M, Ghorbanpour SM, Zitzenbacher S, Ribitsch D, Zumstein MT, et al. Enzymatic degradation of aromatic and aliphatic polyesters by P. pastoris expressed cutinase 1 from Thermobifida cellulosilytica. Front Microbiol. 2017;8:938.
Shirke AN, Su A, Jones JA, Butterfoss GL, Koffas MAG, Kim JR, et al. Comparative thermal inactivation analysis of Aspergillus oryzae and Thiellavia terrestris cutinase: Role of glycosylation. Biotechnol Bioeng. 2017;114:63–73.
Shirke AN, Basore D, Holton S, Su A, Baugh E, Butterfoss GL, et al. Influence of surface charge, binding site residues and glycosylation on Thielavia terrestris cutinase biochemical characteristics. Appl Microbiol Biotechnol. 2016;100:4435–46.
Liu B, He L, Wang L, Li T, Li C, Liu H, et al. Protein crystallography and site-direct mutagenesis analysis of the poly(ethylene terephthalate) hydrolase PETase from Ideonella sakaiensis. ChemBioChem. 2018;19:1471–5.
Pan Q, Nguyen TB, Ascher DB, Pires DEV. Systematic evaluation of computational tools to predict the effects of mutations on protein stability in the absence of experimental structures. Brief Bioinform. 2022;23(2):bbac025.
Sagong HY, Seo H, Kim T, Son HF, Joo S, Lee SH, et al. Decomposition of the PET film by MHETase using Exo-PETase function. ACS Catal. 2020;10:4805–12.
Bollinger A, Thies S, Knieps-Grünhagen E, Gertzen C, Kobus S, Höppner A, et al. A Novel polyester hydrolase from the marine bacterium Pseudomonas aestusnigri – structural and functional insights. Front Microbiol. 2020;11:114.
Araújo R, Silva C, O’Neill A, Micaelo N, Guebitz G, Soares CM, et al. Tailoring cutinase activity towards polyethylene terephthalate and polyamide 6,6 fibers. J Biotechnol. 2007;128:849–57.
Kawabata T, Oda M, Kawai F. Mutational analysis of cutinase-like enzyme, Cut190, based on the 3D docking structure with model compounds of polyethylene terephthalate. J Biosci Bioeng. 2017;124:28–35.
Austin HP, Allen MD, Donohoe BS, Rorrer NA, Kearns FL, Silveira RL, et al. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proceedings of the National Academy of Sciences. 2018;115:E4350-7.
Sevilla ME, Garcia MD, Perez-Castillo Y, Armijos-Jaramillo V, Casado S, Vizuete K, et al. Degradation of PET bottles by an engineered Ideonella sakaiensis PETase. Polymers (Basel). 2023;15(7):1779.
Silva C, Da S, Silva N, Matamá T, Araújo R, Martins M, et al. Engineered Thermobifida fusca cutinase with increased activity on polyester substrates. Biotechnol J. 2011;6:1230–9.
Ma Y, Yao M, Li B, Ding M, He B, Chen S, et al. Enhanced poly(ethylene terephthalate) hydrolase activity by protein engineering. Engineering. 2018;4:888–93.
Wei R, Oeser T, Zimmermann W. Synthetic polyester-hydrolyzing enzymes from thermophilic actinomycetes. In: Advances in applied microbiology. Academic Press Inc. USA; 2014:89:267–305.
Kari J, Olsen JP, Jensen K, Badino SF, Krogh KBRM, Borch K, et al. Sabatier principle for interfacial (heterogeneous) enzyme catalysis. ACS Catal. 2018;8:11966–72.
Choi J, Kim H, Ahn YR, Kim M, Yu S, Kim N, et al. Recent advances in microbial and enzymatic engineering for the biodegradation of micro- and nanoplastics. RSC Adv. 2024;14(14):9943–66.
Berselli A, Ramos MJ, Menziani MC. Novel PET-degrading enzymes: Structure-function from a computational perspective. ChemBioChem. 2021;22:2032–50.
Herrero Acero E, Ribitsch D, Dellacher A, Zitzenbacher S, Marold A, Steinkellner G, et al. Surface engineering of a cutinase from Thermobifida cellulosilytica for improved polyester hydrolysis. Biotechnol Bioeng. 2013;110:2581–90.
Ribitsch D, Hromic A, Zitzenbacher S, Zartl B, Gamerith C, Pellis A, et al. Small cause, large effect: Structural characterization of cutinases from Thermobifida cellulosilytica. Biotechnol Bioeng. 2017;114:2481–8.
Miyakawa T, Mizushima H, Ohtsuka J, Oda M, Kawai F, Tanokura M. Structural basis for the Ca2+-enhanced thermostability and activity of PET-degrading cutinase-like enzyme from Saccharomonospora viridis AHK190. Appl Microbiol Biotechnol. 2015;99:4297–307.
Biundo A, Steinkellner G, Gruber K, Spreitzhofer T, Ribitsch D, Guebitz GM. Engineering of the zinc-binding domain of an esterase from Clostridium botulinum towards increased activity on polyesters. Catal Sci Technol. 2017;7:1440–7.
Hiraishi T, Komiya N, Matsumoto N, Abe H, Fujita M, Maeda M. Degradation and adsorption characteristics of PHB depolymerase as revealed by kinetics of mutant enzymes with amino acid substitution in substrate-binding domain. Biomacromolecules. 2010;11:113–9.
Hiraishi T, Komiya N, Maeda M. Y443F mutation in the substrate-binding domain of extracellular PHB depolymerase enhances its PHB adsorption and disruption abilities. Polym Degrad Stab. 2010:95(8):1370–4.
Biundo A, Ribitsch D, Steinkellner G, Gruber K, Guebitz GM. Polyester hydrolysis is enhanced by a truncated esterase: Less is more. Biotechnol J. 2017;12:1-17
Sidar A, Albuquerque ED, Voshol GP, Ram AFJ, Vijgenboom E, Punt PJ. Carbohydrate Binding Modules: Diversity of domain architecture in amylases and cellulases from filamentous microorganisms. Front Bioeng Biotechnol. 2020;8:871.
Mezzina MP, Pettinari MJ. Phasins, multifaceted polyhydroxyalkanoate granule-associated proteins. Appl Environ Microbiol. 2016;82:5060–7.
Burgin T, Pollard BC, Knott BC, Mayes HB, Crowley MF, McGeehan JE, et al. The reaction mechanism of the Ideonella sakaiensis PETase enzyme. Commun Chem. 2024;7(1):65.
Zhang B, Chen L, Chao J, Yang X, Wang Q. Research progress of microplastics in freshwater sediments in China. Environ Sci Pollut Res. 2020;27:31046–60.
Weber J, Petrović D, Strodel B, Smits SHJ, Kolkenbrock S, Leggewie C, et al. Interaction of carbohydrate-binding modules with poly(ethylene terephthalate). Appl Microbiol Biotechnol. 2019;103:4801–12.
Armenta S, Moreno-Mendieta S, Sánchez-Cuapio Z, Sánchez S, Rodríguez-Sanoja R. Advances in molecular engineering of carbohydrate-binding modules. Proteins. 2017;85:1602–17.
Dai L, Qu Y, Huang JW, Hu Y, Hu H, Li S, et al. Enhancing PET hydrolytic enzyme activity by fusion of the cellulose–binding domain of cellobiohydrolase I from Trichoderma reesei. J Biotechnol. 2021;334:47–50.
Ribitsch D, Yebra AO, Zitzenbacher S, Wu J, Nowitsch S, Steinkellner G, et al. Fusion of binding domains to Thermobifida cellulosilytica cutinase to tune sorption characteristics and enhancing PET hydrolysis. Biomacromolecules. 2013;14:1769–76.
Zhang Y, Chen S, Xu M, Cavoco-Paulo AP, Wu J, Chen J. Characterization of Thermobifida fusca cutinase-carbohydrate-binding module fusion proteins and their potential application in bioscouring∇. Appl Environ Microbiol. 2010;76:6870–6.
Zhang Y, Wang L, Chen J, Wu J. Enhanced activity toward PET by site-directed mutagenesis of Thermobifida fusca cutinase-CBM fusion protein. Carbohydr Polym. 2013;97:124–9.
Perz V, Zumstein MT, Sander M, Zitzenbacher S, Ribitsch D, Guebitz GM. Biomimetic approach to enhance enzymatic hydrolysis of the synthetic polyester poly(1,4-butylene adipate): Fusing binding modules to esterases. Biomacromolecules. 2015;16:3889–96.
Sánchez C. Fungal potential for the degradation of petroleum-based polymers: An overview of macro- and microplastics biodegradation. Biotechnol Adv. 2020;40:107501.
Ribitsch D, Acero EH, Przylucka A, Zitzenbacher S, Marold A, Gamerith C, et al. Enhanced cutinase-catalyzed hydrolysis of polyethylene terephthalate by covalent fusion to hydrophobins. Appl Environ Microbiol. 2015;81:3586–92.
Espino-Rammer L, Ribitsch D, Przylucka A, Marold A, Greimel KJ, Acero EH, et al. Two novel class ii hydrophobins from Trichoderma spp. Stimulate enzymatic hydrolysis of poly (ethylene terephthalate) when expressed as fusion proteins. Appl Environ Microbiol. 2013;79:4230–8.
Büscher N, Sayoga GV, Rübsam K, Jakob F, Schwaneberg U, Kara S, et al. Biocatalyst immobilization by anchor peptides on an additively manufacturable material. Org Process Res Dev. 2019;23:1852–9.
Rübsam K, Stomps B, Böker A, Jakob F, Schwaneberg U. Anchor peptides: A green and versatile method for polypropylene functionalization. Polymer (Guildf). 2017;116:124–32.
Barth M, Oeser T, Wei R, Then J, Schmidt J, Zimmermann W. Effect of hydrolysis products on the enzymatic degradation of polyethylene terephthalate nanoparticles by a polyester hydrolase from Thermobifida fusca. Biochem Eng J. 2015;93:222–8.
Gross C, Hamacher K, Schmitz K, Jager S. Cleavage product accumulation decreases the activity of cutinase during PET hydrolysis. J Chem Inf Model. 2017;57:243–55.
Wei R, Oeser T, Schmidt J, Meier R, Barth M, Then J, et al. Engineered bacterial polyester hydrolases efficiently degrade polyethylene terephthalate due to relieved product inhibition. Biotechnol Bioeng. 2016;113:1658–65.
Barth M, Honak A, Oeser T, Wei R, Belisário-Ferrari MR, Then J, et al. A dual enzyme system composed of a polyester hydrolase and a carboxylesterase enhances the biocatalytic degradation of polyethylene terephthalate films. Biotechnol J. 2016;11:1082–7.
Knott BC, Erickson E, Allen MD, Gado JE, Graham R, Kearns FL, et al. Characterization and engineering of a two-enzyme system for plastics depolymerization. PNAS. 2020;117:25476–85.
Biundo A, Subagia R, Maurer M, Ribitsch D, Syrén PO, Guebitz GM. Switched reaction specificity in polyesterases towards amide bond hydrolysis by enzyme engineering. RSC Adv. 2019;9:36217–26.
Erickson E, Shakespeare TJ, Bratti F, Buss BL, Graham R, Hawkins MA, et al. Comparative performance of PETase as a function of reaction conditions, substrate properties, and product accumulation. ChemSusChem. 2022;15:e202102517.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
Conceptualization: Bikash Kumar, Kiran Bala; Writing – literature survey, Formal analysis, Data curation, and original draft, revision: Swagata Lakshmi Dhali, Dinesh Parida, and Bikash Kumar, Writing - review & editing: Bikash Kumar, Kiran Bala.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Dhali, S.L., Parida, D., Kumar, B. et al. Recent trends in microbial and enzymatic plastic degradation: a solution for plastic pollution predicaments. Biotechnol Sustain Mater 1, 11 (2024). https://doi.org/10.1186/s44316-024-00011-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s44316-024-00011-0