Abstract
Background
The spotted wing drosophila, Drosophila suzukii, is an invasive pest causing significant economic losses worldwide. Current pest control strategies mainly rely on insecticides, which negatively impact fruit marketability and the sustainability of integrated pest management (IPM) programs. In addition, pesticides can have dramatic consequences on non-target species when persisting in the environment at low concentrations after field applications. In this context, chemical control can strongly interfere with the releases of the G1 strain of the Asian larval parasitoid Ganaspis cf. brasiliensis, which is currently the adopted classical biological control agent to manage D. suzukii infestations worldwide.
Methods
Probit analysis was used to assess the baseline toxicity of acetamiprid, cyazypyr, lambda-cyhalothrin, phosmet, and spinosad on G1 G. cf. brasiliensis adults through residual contact exposure in the laboratory. Then, adult parasitoids were exposed to insecticide low Lethal Concentrations (LC5 and LC30) and their mortality was checked daily to assess the survival of treated wasps.
Results
Lambda-cyhalothrin showed the highest toxicity on the parasitoid with a LC50 of 1.38 × 10–3 g active ingredient (a.i.) /L, while cyazypyr seemed the safer active ingredient with an estimated LC50 of 0.20 g a.i./L without affecting parasitoids at sublethal doses. Spinosad and phosmet significantly reduced wasp survival at both LC30 and LC5, while lambda-cyhalothrin and acetamiprid affected parasitoid lifespan only at LC30. Spinosad, lambda-cyhalothrin and phosmet LC30 caused the major survival reductions, followed by acetamiprid LC30. The least significant reduction in parasitoid survival was 21.6% by spinosad LC5.
Conclusions
Overall, this study highlighted the importance of carefully selecting insecticides to minimize adverse effects on non-target organisms. In particular, cyazypyr was the most promising candidate to integrate inoculative biological control with chemical treatments. By contrast, the application of phosmet, spinosad and lambda-cyhalothrin should be avoided alongside parasitoid field releases. Although acetamiprid is less used against D. suzukii in the field than the other tested molecules, it should be used with caution due to its sublethal toxicity on the parasitoid. These results provide the first evidence of G. cf. brasiliensis susceptibility to insecticides in order to promote sustainable and efficient pest management strategies.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Classical biological control (CBC) is considered the most promising strategy to control the spotted wing drosophila, Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) (Lisi et al. 2022), which is an Asian pest of soft-skinned fruits causing severe economic losses worldwide (De Ros et al. 2020; Boughdad et al. 2021). The CBC approach consists of the importation of specialist natural enemies from the pest’s native range (Heimpel and Mills 2017). This is because they are more efficient in targeting the prey/host and providing a stable control of D. suzukii populations than natural enemies resident in the pest infested areas (Wang et al. 2020a, b). Indeed, some pupal parasitoid species showed promising effectiveness under laboratory conditions in invaded countries (Kaçar et al. 2017), but not in the field because too generalist (Lee et al. 2019), while larval parasitoid success is prevented by the lack of co-evolution with D. suzukii due to a strong immune response of the pest against the wasp eggs and larvae (Kacsoh and Schlenke 2012). In this context, shortage of effective natural enemies prompted the need for foreign explorations in South Korea, China, and Japan targeting D. suzukii co-evolved parasitoids (Daane et al. 2016; Girod et al. 2018a; Giorgini et al. 2019).
Field surveys and laboratory quarantine investigations identified the larval parasitoid Ganaspis cf. brasiliensis (Ihering) (Hymenoptera: Figitidae) as the most suitable candidate for the CBC program (Wang et al. 2020a, b; Biondi et al. 2021; Daane et al. 2021), although genetic and molecular studies raised uncertainty on the parasitoid’s taxonomic status. Indeed, four to five genetic groups (G1-G5), mainly differing in the host range, were identified in the G. cf. brasiliensis complex (Nomano et al. 2017; Giorgini et al. 2019; Seehausen et al. 2020). Among these, the G1 strain resulted as the most host-specific lineage mostly parasitizing D. suzukii larvae within fresh and ripe fruits (Girod et al. 2018a; Seehausen et al. 2022).
These features prompted various governments, such as the Italian and the American one, to grant approvals for an area-wide release program of the parasitoid G1 strain (Beers et al. 2022; Lisi et al. 2022), and first release efforts confirmed the parasitoid’s ability to disperse, overwinter and parasitize D. suzukii in the field (Fellin et al. 2023). Despite these encouraging results, assessing the parasitoid’s compatibility within the integrated pest management (IPM) is crucial for ensuring greater synergy (Tait et al. 2021; Kenis et al. 2023). In this context, the abundant presence of D. suzukii close to harvest season has induced growers to rely on chemical control, which is mainly based on conventional and broad-spectrum insecticides sprayed according to calendar schedules (Tait et al. 2021). Among these, pyrethroids, carbamates, organophosphates and diamides are the most common insecticides used by conventional farmers (Shawer et al. 2018; Shaw et al. 2019; Tait et al. 2021), while spinosad, azadirachtin and pyrethrins are among the best options for the organic management of D. suzukii (Gress and Zalom 2019; Noble et al. 2023). To date, most of the toxicological studies have focused on application methods, insecticide resistance and lethal toxicity towards D. suzukii (Van Timmeren et al. 2018; Mermer et al. 2021, 2023; Noble et al. 2023). Toxicity and sublethal effects of insecticides have been evaluated for several non-target biocontrol arthropods species (Desneux et al. 2007), including predators (Biondi et al. 2012; Ricupero et al. 2020) and parasitoids (Biondi et al. 2013; Teder and Knapp 2019), but the agrochemical impact on D. suzukii parasitoids is still neglected. In particular, only few studies showed that synthetic neurotoxicants and spinosad can affect viability of pupal parasitoids (Cossentine and Ayyanath 2017; Schlesener et al. 2019; Morais et al. 2022), and strongly compromise their effectiveness as biological control agents at sublethal concentrations (Lisi et al. 2023). However, no toxicological reports are available for G. cf. brasiliensis and other D. suzukii larval parasitoids.
In this scenario, we first aimed at evaluating the baseline toxicity of five insecticides on adults of G1 G. cf. brasiliensis exposed by residual contact to chemicals. Then, we assessed sublethal effects of low insecticide concentrations (Lethal Concentrations 5 and 30%, LC5 and LC30) on parasitoid survival. These results provide a first screening on insecticide toxicity on G. cf. brasiliensis and can support ongoing biological control efforts in Europe and the US.
Material and methods
Insect colonies
An isofemale colony of D. suzukii was established in September 2015 from a field sampling on infested wild blackberries (Rubus sp.) in the Catania province (Sicily, Italy). Adult flies were fed with a nutrient honey-water solution (1:1) and rearing conditions were kept constant with a photoperiod of 16: 8 (L:D) at 24 ± 2 °C and 60 ± 10% R.H. inside insect cages (BugDorm®, MegaView, Taiwan, 32.5 × 32.5 × 32.5 cm). A cornmeal artificial diet within Dutscher rearing tubes (Ø x h: 25 × 95 mm) was provided to adult females as oviposition substrate and food source to the larvae after the egg hatching. Artificial diet was prepared according to the following recipe. Briefly, 45 g Agar, 125 g cornmeal, 200 g sugar, 70 g yeast in granules were added in 4.8 L of boiling water, and 3.3 g of methyl paraben (Methyl-4-hydroxybenzoate) dissolved in 33.3 mL of 95% ethanol and 25 g of 1 M propionic acid were added to avoid the formation of mould and bacteria.
A colony of G1 G. cf. brasiliensis was initiated with specimens provided by the Sicilian Phytosanitary Service (Regione Siciliana), in the context of the classical biological control program (Lisi et al. 2022) in collaboration with the Edmund Mach Foundation (FEM) (San Michele all’Adige, Italy), in July 2021. Wasps were originally sampled, in 2017, in Japan during foreign explorations targeting D. suzukii co-evolved parasitoids (Girod et al. 2018b) and then reared in quarantine laboratories at CABI (Delemont, Switzerland) and later at FEM until government approval for field releases (Lisi et al. 2022; Fellin et al. 2023). Parasitoids were then reared at the Department of Agriculture, Food and Environment of the University of Catania (Catania, Italy), according to Rossi-Stacconi et al. (2022), on fresh blueberries previously infested by D. suzukii.
Insecticides
Commercial formulations of acetamiprid (Epik®, Sipcam Italia), cyazypyr (Benevia®2021, FMC Agro Italia), lambda-cyhalothrin (Karate Zeon CC®, Syngenta Italia S.p.a), phosmet (Spada® 200 EC, Gowan Italia) and spinosad (Laser®, Corteva AgriScience Italia S.r.l) were tested for their lethal and sublethal toxicity on adults of G. cf. brasiliensis.
Spinosad is a naturally derived insecticide compatible with conventional and organic pest management programs, while the other molecules are synthetic insecticides currently employed in D. suzukii agroecosystems (Table 1). Phosmet was recently banned for field application in European Union (EU pesticide database 2022). Label information of each pesticide is shown in Table 1.
Chemical residual exposure and insecticide baseline toxicity on Ganaspis cf. brasiliensis
The experimental arenas were composed by a PVC cylindric-shaped section (88 cm3) interposed between two glass plates (9 × 9 cm) held together by two elastic strands. To prevent fumigant effect of insecticide residues inside the arena, forced ventilation was triggered by a ventilator (100 L/h) (Air fizz 100®, Ferplast Spa) able to recycle air approximately once an hour in five experimental arenas, through PVC tubes connections. The airflow movement in and out was ensured by two specular holes (diameter 0.3 cm) covered with a fine mesh net integrated into the plastic section. A third hole in the PVC section allowed for the introduction of wasps with a mouth aspirator and the placement of a cotton dispenser filled with a honey-water solution (1:1) to feed adult wasps during the chemical exposure (Fig. 1).
Lateral (left) and ventral (right) view of the experimental arena used to expose Ganaspis cf. brasiliensis adults to dry insecticide residues. (1) ventilator triggering forced ventilation inside the arena to prevent fumigation (each ventilator was connected to five experimental units, despite the figure shows only one); (2) hole to introduce wasps in the arena and place cotton dispenser filled with a honey-water solution; (3) specular hole covered with a fine mesh net to ensure the air movement outside the arena
For each tested insecticide concentration, glass plates were sprayed with 1.0–1.2 mL of insecticide solution under a constant pressure of 34, 5 kPa using the Potter spray tower (Burkard Manufacturing Co. Ltd) and ensuring a standard deposit of 1.5–1.8 mg/cm2 of insecticide solution on each surface area (Suma et al. 2009). Control arenas were sprayed with distilled water. The treated glass plates were allowed to dry for 1 h inside a laminar flow hood and were then assembled to expose wasps to the inner and contaminated surfaces inside the arena. At the end of the laboratory trials, glass sections were cleaned with a 10% KOH solution before reuse.
Insecticide concentration-mortality response was assessed by exposing five G. cf. brasiliensis adult couples, three days old, in each arena for 48 h. For each insecticide, concentration-mortality regression lines were assessed by testing a range of six to eight decreasing concentrations starting from the label field rate (Table 1). Following preliminary observations, acetamiprid, cyazypyr and lambda-cyhalothrin label field doses were decreased with a geometrical ratio. While spinosad and phosmet were tested by decreasing the label rate with a logarithmic ratio. Each concentration was replicated three to six times and consisted of five G. cf. brasiliensis adult couples tested. Insecticide dilutions were prepared individually in a laminar flow hood using distilled water, starting from the recommended label dose for each chemical compound (Table 1). Mortality of treated wasps was assessed after 48 h of exposure by stimulating wasps with a fine paintbrush and considering them dead in absence of parasitoid movements after being touched.
Effects of insecticide sublethal concentrations on parasitoid survival
The survival of 17–20 male and female parasitoids following sublethal chemical exposure was tested for each insecticide and concentration. Ganaspis cf. brasiliensis adults, 3–4 days old, were exposed to the estimated LC30 or LC5 of each insecticide, as described in the baseline toxicity trials, and control arenas were treated with distilled water. After chemical exposure, survived wasps were individually moved into Dutscher rearing tubes (Ø x h: 28.5 × 95 mm). A moistened cottony ball was placed on the bottom of the tube to ensure proper humidity and honey drops were applied once a week on the inner part of the tube lid as food source. Parasitoid mortality was monitored daily to calculate survival of treated male and female wasps following the sublethal chemical exposure.
Data analysis
The baseline toxicity of tested insecticides on G. cf. brasiliensis adults, exposed via residual contact, was assessed using a probit regression model through a logarithmic transformation of the data (Finney, 1971). The dose-mortality relationships were considered valid when there was no significant deviation between the observed and expected data (P > 0.05).
Survival raw data were analysed for normality and homogeneity of variance using the Kolmogorov–Smirnov and Levene tests. A general linear model (GLM) analysis was performed to assess the effects of the “insecticide” (five insecticides and untreated control), “concentration” (LC30 and LC5), and “sex” (male and female wasps), as well as their interactions, on the survival of exposed parasitoids. Additional one-way ANOVA followed by Tukey post-hoc test (P < 0.05) was used for multiple mean comparisons among insecticides within each tested concentration. The unpaired student t test (P < 0.05) was employed to reveal significant differences between the two concentrations of each tested insecticide. All statistical analyses were performed at 95% level of significance using IBM SPSS Statistics for Windows version 20.00 (IBM Corp. 2011. Armonk, NY: IBM Corp.), and Excel®(Microsoft) was used to generate means, standard errors (SE) and graphs.
Results
Insecticide baseline toxicity on Ganaspis cf. brasiliensis
The probit model was fitted to the observed data for all the treatments (i.e., there was no significant differences between the observed data and the expected data withall treatments being at P > 0.05) and the estimation of lethal concentrations was considered valid (Table 2). Parasitoids treated only with distilled water survived throughout the period assessment.
Following 48 h of exposure to insecticide residues, the highest toxicity towards G. cf. brasiliensis was estimated for lambda-cyhalothrin, as it exhibited the lowest LC50 values, followed by spinosad, phosmet, and acetamiprid, respectively. Cyazypyr was the least toxic molecule to G. cf. brasiliensis showing the highest concentration required to kill 50% of treated wasps. The same toxicological trend was observed for the two sublethal concentrations, although spinosad LC5 was more toxic than lambda-cyhalothrin LC5 with values of 1.20 × 10–5 and 1.4 × 10–4 g a.i./L, respectively (Table 2).
Except for cyazypyr, all chemical molecules had lower estimated LCs values than their label field dose. The ratio between the recommended field doses and LC30 ranged from 0.53 to 274.7 for cyazypyr and fosmet, respectively, and from 2.81 to 10169 for LC5 of cyazypyr and spinosad.
Effects of insecticide sublethal concentrations on parasitoid survival
The survival of parasitoids exposed to chemical residues on glass plates was significantly affected by the factor “insecticide” (F5 = 17.412; P < 0.001), “concentration” (F1 = 29.808; P < 0.001), and their interaction “insecticide × concentration” (F5 = 2.884; P = 0.014). The independent variable “sex” did not significantly affect the parasitoid survival alone (F1 = 0.120; P = 0.729) and in its interactions with the other factors (“insecticide × sex” F5 = 0.418; P = 0.836; “concentration × sex” F1 = 0.262; P = 0.609; “insecticide × concentration × sex” F5 = 0.383; P = 0.860), therefore differences among treatments were studied combining male and female survival.
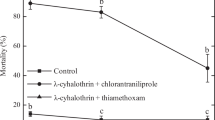
Wasps belonging to the control treatment survived 34.8 ± 1.2 days (Fig. 2). Both LC30 (F5209 = 11.689; P < 0.001) and LC5 (F5216 = 7.576; P < 0.001) affected parasitoid survival. Cyazypyr was the only insecticide that did not affect parasitoid survival at both sublethal doses and no significant differences were found between the two tested concentrations (t = − 0.978, df = 70, P = 0.216). On the contrary, the LC30 and LC5 of acetamiprid (t = 6.403, df = 70, P = 0.014) and lambda-cyhalothrin (t = − 3.407, df = 70, P < 0.001) differently affected parasitoid survival. Indeed, both insecticides decreased insect survival only at LC30 by 33.5 and 42.3%, respectively, while their LC5 did not reduced wasp survival (Fig. 2). On the contrary, phosmet and spinosad were the most toxic compounds towards G. cf. brasiliensis at sublethal doses because both insecticide concentrations significantly interfered with parasitoid survival. In particular, phosmet decreased wasp lifespan by 41.6 and 31.1% at LC30 and LC5, respectively, and no statistical differences in parasitoid survival were observed between the two sublethal concentrations (t = − 1.597, df = 68, P = 0.057) (Fig. 2). Spinosad reduced G. cf. brasiliensis survival by 43.8 and 21.6% at LC30 and LC5, and no significant differences in wasp lifespan were found between the two concentrations (t = − 2.862, df = 71, P = 0.120) (Fig. 2).
Mean (± SE) Ganaspis cf. brasiliensis adult survival following LC30 and LC5 chemical exposure by residual contact. Different capital letters indicate significant differences among insecticides within the LC30 exposure, while different lower-case letters indicate significant differences in the LC5 treatments (Tukey post hoc tests, P ≤ 0.05). Asterisks show significant differences between the LC30 and LC5 for each tested insecticide according to the unpaired student t test (P ≤ 0.05)
Discussion
Understanding parasitoid susceptibility to insecticides is a key aspect to integrate biological control agents into pest management programs (Desneux et al. 2007; Guedes et al. 2017). Insecticides tested in this study are among the molecules mostly used by conventional farmers to manage D. suzukii infestations, with spinosad being one of the most effective options also for the organic production (Tait et al. 2021). Chemical applications are generally performed during the crop ripening season when fruit become susceptible to the fly (Tait et al. 2021) and G. cf. brasiliensis is released to parasitize larvae within the fruits (Lisi et al. 2022). However, it is well known that pesticides can persist in the environment (e.g., fruits, leaves, flowers, soil, etc.) at sublethal concentrations after their application compromising the behaviour and physiology of arthropods survived to the residual insecticide exposure (Desneux et al. 2007). In this context, investigating the toxicity of insecticide sublethal concentrations on G. cf. brasiliensis by residual contact bioassays would allow to understand the long-term consequences of synthetic insecticide applications on this natural enemy. To this aim, the median lethal concentration (LC50) has been the most used parameter to assess the toxicity of pesticides in (eco) toxicological studies (Stark and Banks 2003). Here, we reported the results of the lethal and sublethal toxicity of five insecticides on G. cf. brasiliensis. The results of concentration-mortality responses revealed significant variability among the tested agrochemicals. For instance, cyazypyr is widely considered a low-risk insecticide with minor or no harm to invertebrates and non-target organisms (Tiwari and Stelinski 2013).
In the present study, cyazypyr resulted as the least toxic insecticide toward G. cf. brasiliensis, showing an estimated LC50 equal to 0.20 g a.i./L, which is 0.375-fold higher than the recommended field dose against D. suzukii (Table 2). These results align with those of other studies testing cyazypyr toxicity towards biological control agents of insect pests. For example, Zhang et al. (2021) estimated a cyazypyr LC50 of 0.22 g a.i./L on the parasitoid Encarsia formosa (Gahan) (Hymenoptera: Aphelinidae), following insecticide residual exposure. Interestingly, Ahumada and Chorbadjian (2019) reported a cyazypyr LC50 on Chrysoperla defreitasi (Brooks) (Neuroptera: Chrysopidae) three times higher than that estimated for G. cf. brasiliensis in this study, thus elucidating potential differences in cyazypyr susceptibility between parasitoids and predators. On the other hand, our results showed lambda-cyhalothrin as the most toxic compound toward the parasitoid, with a LC50 of 1.38 × 10–3 g a.i./L (Table 2), followed by spinosad, phosmet and acetamiprid. Similar findings were also reported on the larval parasitoid Cotesia flavipes (Cameron) (Hymenoptera: Braconidae) exposed to the dry residues of 12 insecticides commonly used against its host Chilo partellus (Swinhoe) (Lepidoptera: Pyralidae) (Akhtar et al. 2021). In particular, pyrethroids, organophosphates and carbamates exhibited high lethal toxicity towards the parasitic wasp, and lambda-cyhalothrin showed a LC50 equal to 1.85 × 10–3 g a.i./L, which is comparable to that obtained on G. cf. brasiliensis. At the same time, acetamiprid and other neonicotinoids were considered safer to C. flavipes than the aforementioned pesticides (Akhtar et al. 2021).
Spinosad is gaining increased relevance in IPM strategies against D. suzukii because it stands out as one of the most effective options in both organic and conventional farming (Gress and Zalom 2019; Tait et al. 2021). The LC50 estimated for spinosad against G. cf. brasiliensis was equal to 3.94 × 10–3 g a.i./L (Table 2), which is very close to the 4.94 × 10–3 g a.i./L estimated on the larval wasp Oomyzus sokolowskii (Kurdjumov) (Hymenoptera: Eulophidae), following exposure to spinosad residues (Cordero et al. 2007). Moreover, Cordero et al. (2007) showed that spinosad and acetamiprid exhibited higher toxicity on Diadegma insulare (Cresson) (Hymenoptera: Ichneumonidae) than O. sokolowskii, with LC50 values of 3.4 × 10–4 and 23.9 × 10–3 g a.i./L for spinosad and 4.94 × 10–3 and 35.2 × 10–3 g a.i./L for acetamiprid, respectively (Cordero et al. 2007). Despite several reports confirming the toxicity results obtained here on G. cf. brasiliensis, it is worth noting that insecticide impact on parasitoids could depend on multiple biotic and abiotic factors, even within the same experimental conditions (Prashar and Shah 2016), as shown by Cordero et al. (2007).
Unsurprisingly, lambda-cyhalothrin LC30 and both concentrations of spinosad and phosmet were the most impactful insecticides on G. cf. brasiliensis, along with a moderate toxicity of acetamiprid LC30 in reducing survival of treated parasitoids. Several studies reported reduction of wasp lifespan following residual exposures to pyrethroids at non-lethal concentrations (Bayram et al. 2010; Garcia 2011; Desneux et al. 2006). Lambda-cyhalothrin significantly reduced Aphidius colemani Viereck (Hymenoptera: Braconidae) survival at sublethal concentrations estimated for its host (Alfaro-Tapia et al. 2021) and for the parasitoid itself (D’Ávila et al. 2018), following wasp exposure on pesticide dry residues. In the latter case, D’Ávila et al. (2018) also showed that A. colemani was about 20-fold more susceptible to spinosad than lambda-cyhalothrin and imidacloprid sublethal doses, with very low concentrations of spinosad residues (i.e., 200 ng a.i./cm2) reducing wasp survival by half.
Similarly, spinosad significantly reduced the survival of fall armyworm parasitoids (Penagos et al., 2005), and decreased Trichogramma brassicae (Parsaeyan et al. 2020) and T. chilonis Ishii (Hymenoptera: Trichogrammatidae) (Wang et al. 2012) survival more than organophosphates and diamides at LC30. In addition, Schlesener et al. (2019) classified spinosad and phosmet as moderately harmful on the D. suzukii pupal parasitoid Pachycrepoideus vindemmiae (Rondani) (Hymenoptera: Pteromalidae), while acetamiprid was categorized slightly harmful.
Interestingly, this neonicotinoid was reported to reduce Peristenus spretus (Chen et van Achterberg) and P. relictus (Ruthe) (Hymenoptera: Braconidae) survival in a range of concentrations (Yang and Lu 2023) similar to the LCs estimated in this study for G. cf. brasiliensis. However, few studies are available for cyazypyr sublethal toxicity on wasp survival. Current literature showed that residual exposures of Trichogramma atopovirilia (Oatman & Platner) (Hymenoptera: Trichogrammatidae) to cyazypyr increased, even not significantly, survival of treated wasps (Cantori et al. 2023), while Tamarixia triozae (Burks) (Hymenoptera: Eulophidae) adults fed on sucrose solution contaminated with cyazypyr at 0.12 g a.i./L lived half than untreated wasps (Liu et al. 2012). In this context, it is well known that pesticide sublethal impact in insects is influenced by a plethora of biotic and abiotic variables and interacting factors. In particular, the pesticide penetration route into the insect body, particularly at sublethal concentrations, could play a key role in determining the amount of insecticide molecules affecting the organism, and therefore the toxicity degree. For example, ingestion is considered the primary route of penetration in insect body both for acetamiprid and cyazypyr (Sparks et al. 2001; Balabanidou et al. 2018), therefore higher oral than contact penetration rate could explain their low and absent toxicity on G. cf. brasiliensis at sublethal doses, as also supported by previous reports (Cantori et al. 2023; Liu et al. 2012). On the other hand, spinosad is highly toxic when ingested and by residual contact, causing quick death of several organisms under both routes of exposure (Biondi et al. 2012; Bacci et al., 2016; Eger & Lindenberg, 1998).
Indeed, in our study spinosad reduced parasitoid survival even when tested at a concentration 10169 times lower than the label rate (Fig. 2). Phosmet and lambda-cyhalothrin act as contact insecticides, penetrating insect cuticle and crossing biological membranes and tissues due to their biochemical structure (He et al. 2008). Therefore, their strong impairment on G. cf. brasiliensis lifespan could be related to a higher penetration rate into the parasitoid body than the other tested molecules. However, many other features would influence the non-target effects of these insecticides at very low concentrations (Pazini et al. 2019). Special emphasis should be placed on their secondary mechanisms of action under a sublethal scenario, which can strongly interfere with the behaviour and physiology of surviving insects (Guedes et al. 2016). Indeed, several studies revealed that pesticide sublethal doses may interfere with the feeding and mating behaviour, as well as many other physiological processes influencing parasitoid longevity. This suggests that alterations of a specific key life-history trait of the parasitoid could indirectly affect other behavioural and/or physiological features and directly impact on the entire biocontrol service provided by natural enemies (Desneux et al. 2007).
According to these results, the significance of evaluating insecticide sublethal effects on parasitoid survival is closely related to the general assumption that longevity is a main component of individual fitness and population dynamic in hymenopteran parasitoids (Jervis 2007). Indeed, the longer parasitoids can survive, the longer they can mate and lay eggs, ultimately leading to increased parasitism and host mortality (Jervis 2007). In this context, decreased wasp survival by pesticides can result in cascading effects at the population level, which mainly depend on the parasitoid biological features (Desneux et al. 2007). Ganaspis cf. brasiliensis is a weakly pro-ovigenic species (Wang et al. 2018), which means that females are characterized by a short oviposition period, short lifespan and by decreased fecundity with increased female age (Jervis et al. 2001). From a practical perspective, pesticides persisting at sublethal concentrations in the agroecosystem decrease parasitoid survival, therefore they would cause an additional decrease of the available oviposition timeframe and the effectiveness of parasitoids. Beside our hypothesis, it is worth noting that life table analysis should be used to better understand the implications of reduced survival at the population level (Desneux et al. 2007). To the best of our knowledge, there are no reports of successful programs integrating parasitoid release with chemical control for the integrayed management of D. suzukii, although different reports against other pests showed the feasibility of combining biological and chemical control (Mansour and Biondi 2021; Wright and Verkerk 1995). As classical biological control programs continue to be developed against this invasive pest worldwide, these results could be used as a proxy for future ecotoxicology research focusing on other key traits of G. cf. brasiliensis, such as fertility, fecundity and parasitism behaviour. Moreover, further routes by which the parasitoid could be exposed to insecticides (e.g., topical exposure of adult parasitoid, juvenile parasitoid intoxication while developing on treated hosts) need to be investigated, as well as semi-field and field evaluations are required to achieve a comprehensive understanding of parasitoid susceptibility to insecticides, therefore on its potential compatibility with chemical control within the current D. suzukii IPM programs.
Conclusions
This study sheds light on the susceptibility of G1 G. cf. brasiliensis to five insecticides commonly used for managing D. suzukii infestations. Cyazypyr would be the most recommended insecticide to integrate parasitoid releases with the current IPM strategies, as a result of its low toxicity and lack of sublethal effects on the parasitoid lifespan. Acetamiprid was considered as a moderate risk molecule because it was the least impactful insecticides among the toxic ones. Moreover, it is not frequently employed in the agroecosystem affected by D. suzukii. The application of lambda-cyhalothrin, phosmet and spinosad would not be recommended due to their strong non-target effects at sublethal and low lethal concentrations. Further research on other key traits of G. cf. brasiliensis and field trials would allow to better evaluate the toxicological profile of these insecticides and integrate this biological control agent within the current IPM strategies.
Availability of data and materials
The datasets during and/or analysed during the current study available from the corresponding author on reasonable request.
References
Ahumada MI, Chorbadjian RA. Laboratory assays of the insecticidal activity of cyantraniliprole and imidacloprid on Brevicoryne brassicae, Myzus persicae (Hemiptera: Aphididae) and Trialeurodes vaporariorum (Hemiptera: Aleyrodidae) pests species and a biological control agent Chrysoperla defreitasi (Neuroptera: Chrysopidae). Chil J Agric Res. 2019;79:658–63.
Akhtar ZR, Tariq K, Handler AM, Ali A, Ullah F, Ali F, et al. Toxicological risk assessment of some commonly used insecticides on Cotesia flavipes, a larval parasitoid of the spotted stem borer Chilo partellus. Ecotoxicol. 2021;30:448–58.
Alfaro-Tapia A, Alvarez-Baca JK, Figueroa CC, Fuentes-Contreras E. Sub-Lethal Effects of λ-cyhalothrin on behavior and development of the parasitoid Aphidius colemani (Hymenoptera: Braconidae) on kdr-resistant and susceptible green peach aphid, Myzus persicae (Hemiptera: Aphididae). J Econ Entomol. 2021;114:2032–42.
Bacci L, Lupi D, Savoldelli S, Rossaro B. A review of Spinosyns, a derivative of biological acting substances as a class of insecticides with a broad range of action against many insect pests. J. Ent. Acar. Res. 2016;48:40-52.
Balabanidou V, Grigoraki L, Vontas J. Insect cuticle: a critical determinant of insecticide resistance. Curr Opin Insect Sci. 2018;27:68–74.
Bayram A, Salerno G, Onofri A, Conti E. Sub-lethal effects of two pyrethroids on biological parameters and behavioral responses to host cues in the egg parasitoid Telenomus busseolae. Biol Control. 2010;53:153–60.
Beers EH, Beal D, Smytheman P, Abram PK, Schmidt-Jeffris R, Moretti E, et al. First records of adventive populations of the parasitoids Ganaspis brasiliensis and Leptopilina japonica in the United States. J Hymenopt Res. 2022;91:11–25.
Biondi A, Desneux N, Siscaro G, Zappalà L. Using organic-certified rather than synthetic pesticides may not be safer for biological control agents: selectivity and side effects of 14 pesticides on the predator Orius laevigatus. Chemosphere. 2012;87:803–12.
Biondi A, Zappalà L, Stark JD, Desneux N. Do biopesticides affect the demographic traits of a parasitoid wasp and its biocontrol services through sublethal effects? PLoS ONE. 2013;8:e76548.
Biondi A, Wang X, Daane KM. Host preference of three Asian larval parasitoids to closely related Drosophila species: implications for biological control of Drosophila suzukii. J Pest Sci. 2021;94:273–83.
Boughdad A, Haddi K, El Bouazzati A, Nassiri A, Tahiri A, El Anbri C, Biondi A. First record of the invasive spotted wing Drosophila infesting berry crops in Africa. J Pest Sci. 2021;94:261–71.
Cantori LV, Iost Filho FH, Pazini JDB, Diniz AJF, Yamamoto PT, Parra JRP. Is integrated management of Gymnandrosoma aurantianum possible with Trichogramma atopovirilia and novel products used in citrus orchards in Brazil? InSects. 2023;14:419.
Cordero RJ, Bloomquist JR, Kuhar TP. Susceptibility of two diamondback moth parasitoids, Diadegma insulare (Cresson) (Hymenoptera; Ichneumonidae) and Oomyzus sokolowskii (Kurdjumov) (Hymenoptera; Eulophidae), to selected commercial insecticides. Biol Control. 2007;42:48–54.
Cossentine JE, Ayyanath MM. Limited protection of the parasitoid Pachycrepoideus vindemiae from Drosophila suzukii host-directed spinosad suppression. Entomol Exp Appl. 2017;164:78–86.
D’Ávila VA, Barbosa WF, Guedes RN, Cutler GC. Effects of spinosad, imidacloprid, and lambda-cyhalothrin on survival, parasitism, and reproduction of the aphid parasitoid Aphidius colemani. J Econ Entomol. 2018;111:1096–103.
Daane KM, Wang XG, Biondi A, Miller B, Miller JC, Riedl H, et al. First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J Pest Sci. 2016;89:823–35.
Daane KM, Wang X, Hogg BN, Biondi A. Potential host ranges of three Asian larval parasitoids of Drosophila suzukii. J Pest Sci. 2021;94:1171–82.
De Ros G, Grassi A, Pantezzi T. Recent trends in the economic impact of Drosophila suzukii. In: Garcia FRM, editor. Drosophila suzukii management. Switzerland: Springer; 2020. p. 11–27.
Desneux N, Ramirez-Romero R, Kaiser L. Multistep bioassay to predict recolonization potential of emerging parasitoids after a pesticide treatment. Environ Toxicol Chem. 2006;25:2675–82.
Desneux N, Decourtye A, Delpuech JM. The sublethal effects of pesticides on beneficial arthropods. Annu Rev Entomol. 2007;52:81–106.
Eger Jr JE, Lindenberg LB. Utility of spinosad for insect control in Florida vegetables. Proc. Fla. State Hort. Soc. 1998;111:55-7
European Union pesticides database, 2022. Accessed on 03/02/2024. https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances
Fellin L, Grassi A, Puppato S, Saddi A, Anfora G, Ioriatti C, Rossi-Stacconi MV. First report on classical biological control releases of the larval parasitoid Ganaspis brasiliensis against Drosophila suzukii in northern Italy. Biocontrol. 2023;68:1–12.
Finney DJ. Probit analysis. Cambridge: Cambridge University Press. 1971.
Garcia P. Sublethal effects of pyrethroids on insect parasitoids: what we need to further know. In: Stoytcheva M, editor. Pesticides: formulations, effects, fate. Rijeka: BoD–Books on Demand; 2011. p. 477–494.
Giorgini M, Wang XG, Wang Y, Chen FS, Hougardy E, Zhang HM, et al. Exploration for native parasitoids of Drosophila suzukii in China reveals a diversity of parasitoid species and narrow host range of the dominant parasitoid. J Pest Sci. 2019;92:509–22.
Girod P, Lierhmann O, Urvois T, Turlings TC, Kenis M, Haye T. Host specificity of Asian parasitoids for potential classical biological control of Drosophila suzukii. J Pest Sci. 2018;91:1241–50.
Girod P, Borowiec N, Buffington M, Chen G, Fang Y, Kimura MT, et al. The parasitoid complex of D. suzukii and other fruit feeding Drosophila species in Asia. Sci Rep. 2018b;8:11839.
Gress BE, Zalom FG. Identification and risk assessment of spinosad resistance in a California population of Drosophila suzukii. Pest Manag Sci. 2019;75:1270–6.
Guedes RNC, Smagghe G, Stark JD, Desneux N. Pesticide-induced stress in arthropod pests for optimized integrated pest management programs. Ann Rev Entomol. 2016;61:43–62.
Guedes RNC, Walse SS, Throne JE. Sublethal exposure, insecticide resistance, and community stress. Curr Opin Insect Sci. 2017;21:47–53.
He LM, Troiano J, Wang A, Goh K. Environmental chemistry, ecotoxicity, and fate of lambda-cyhalothrin. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. New York: Springer; 2008. p. 71–91.
Heimpel GE, Mills NJ. Biological control. Cambridge: Cambridge University Press. 2017.
Jervis MA. Insects as natural enemies: a practical perspective. Dordrecht: Springer Science & Business Media. 2007.
Jervis MA, Heimpel GE, Ferns PN, Harvey JA, Kidd NAC. Life-history strategies in parasitoid wasps: a comparative analysis of “ovigeny.” J Anim Ecol. 2001;70:442–58.
Kaçar G, Wang XG, Biondi A, Daane KM. Linear functional response by two pupal Drosophila parasitoids foraging within single or multiple patch environments. PLoS ONE. 2017;12:e0183525.
Kacsoh BZ, Schlenke TA. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS ONE. 2012;7:e34721.
Kenis M, Benelli G, Biondi A, Calatayud PA, Day R, Desneux N, Wu K. Invasiveness, biology, ecology, and management of the fall armyworm, Spodoptera frugiperda. Entomol Gen. 2023;43:187–241.
Lee JC, Wang X, Daane KM, Hoelmer KA, Isaacs R, Sial AA, Walton VM. Biological control of spotted-wing drosophila (Diptera: Drosophilidae)-current and pending tactics. J Integr Pest Manag. 2019;10:13.
Lisi F, Biondi A, Cavallaro C, Zappalà L, Campo G, Roversi PF, et al. Current status of Drosophila suzukii classical biological control in Italy. Acta Hortic. 2022;1354:193–200.
Lisi F, Mansour R, Cavallaro C, Alınç T, Porcu E, Ricupero M, et al. Sublethal effects of nine insecticides on Drosophila suzukii and its major pupal parasitoid Trichopria drosophilae. Pest Manag Sci. 2023;79:5003–14.
Liu TX, Zhang YM, Peng LN, Rojas P, Trumble JT. Risk assessment of selected insecticides on Tamarixia triozae (Hymenoptera: Eulophidae), a parasitoid of Bactericera cockerelli (Hemiptera: Trizoidae). J Econ Entomol. 2012;105:490–6.
Mansour R, Biondi A. Releasing natural enemies and applying microbial and botanical pesticides for managing Tuta absoluta in the MENA region. Phytoparasitica. 2021;49:179–94.
Mermer S, Pfab F, Tait G, Isaacs R, Fanning PD, Timmeren V, et al. Timing and order of different insecticide classes drive control of Drosophila suzukii, a modeling approach. J Pest Sci. 2021;94:743–55.
Mermer S, Rossi-Stacconi MV, Tait G, Pfab F, Sial AA, Disi JO, et al. Comparing the effectiveness of different insecticide application orders for suppressing Drosophila suzukii Matsumura (Diptera: Drosophilidae) infestation: experimental and modeling approaches. J Econ Entomol. 2023;116:899–908.
Morais MC, Rakes M, Pasini RA, Grützmacher AD, Nava DE, Bernardi D. Toxicity and transgenerational effects of insecticides on Trichopria anastrephae (Hymenoptera: Diapriidae). Neotrop Entomol. 2022;51:143–50.
Noble R, Shaw B, Walker A, Whitfield EC, Deakin G, Harris A, Fountain MT. Control of spotted wing drosophila (Drosophila suzukii) in sweet cherry and raspberry using bait sprays. J Pest Sci. 2023;96:623–33.
Nomano FY, Kasuya N, Matsuura A, Suwito A, Mitsui H, Buffington ML, Kimura MT. Genetic differentiation of Ganaspis brasiliensis (Hymenoptera: figitidae) from East and Southeast Asia. Appl Entomol Zool. 2017;52:429–37.
Parsaeyan E, Saber M, Safavi SA, Poorjavad N, Biondi A. Side effects of chlorantraniliprole, phosalone and spinosad on the egg parasitoid, Trichogramma Brassicae. Ecotoxicol. 2020;29:1052–61.
Pazini JDB, Padilha AC, Cagliari D, Bueno FA, Rakes M, Zotti MJ, Grützmacher AD. Differential impacts of pesticides on Euschistus heros (Hem.: Pentatomidae) and its parasitoid Telenomus podisi (Hym.: Platygastridae). Sci Rep. 2019;9:6544.
Penagos DI, Cisneros J, Hernández O, Williams T. Lethal and sublethal effects of the naturally derived insecticide spinosad on parasitoids of Spodoptera frugiperda (Lepidoptera: Noctuidae). Biocontrol Sci Technol. 2005;15:81–95.
Prashar P, Shah S. Impact of fertilizers and pesticides on soil microflora in agriculture. Agric Res. 2016;19:331–61.
Ricupero M, Abbes K, Haddi K, Kurtulus A, Desneux N, Russo A, Zappalà L. Combined thermal and insecticidal stresses on the generalist predator Macrolophus pygmaeus. Sci Total Environ. 2020;729:138922.
Rossi-Stacconi MV, Wang XG, Stout A, Fellin L, Daane KM, Biondi A, et al. Methods for rearing the parasitoid Ganaspis brasiliensis, a promising biological control agent for the invasive Drosophila suzukii. JoVE. 2022;184:e63898.
Schlesener DCH, Wollmann J, Pazini JDB, Padilha AC, Grützmacher AD, Garcia FRM. Insecticide toxicity to Drosophila suzukii (Diptera: Drosophilidae) parasitoids: Trichopria anastrephae (Hymenoptera: Diapriidae) and Pachycrepoideus vindemmiae (Hymenoptera: Pteromalidae). J Econ Entomol. 2019;112:1197–206.
Seehausen ML, Ris N, Driss L, Racca A, Girod P, Warot S, Kenis M. Evidence for a cryptic parasitoid species reveals its suitability as a biological control agent. Sci Rep. 2020;10:19096.
Seehausen ML, Valenti R, Fontes J, Meier M, Marazzi C, Mazzi D, Kenis M. Large-arena field cage releases of a candidate classical biological control agent for spotted wing drosophila suggest low risk to non-target species. J Pest Sci. 2022;95:1057–65.
Shaw B, Hemer S, Cannon MF, Rogai F, Fountain MT. Insecticide control of Drosophila suzukii in commercial sweet cherry crops under cladding. InSects. 2019;10:196.
Shawer R, Tonina L, Tirello P, Duso C, Mori N. Laboratory and field trials to identify effective chemical control strategies for integrated management of Drosophila suzukii in European cherry orchards. Crop Prot. 2018;103:73–80.
Sparks TC, Crouse GD, Durst G. Natural products as insecticides: the biology, biochemistry and quantitative structure–activity relationships of spinosyns and spinosoids. Pest Manag Sci. 2001;57:896–905.
Stark JD, Banks JE. Population-level effects of pesticides and other toxicants on arthropods. Ann Rev Entomol. 2003;48:505–19.
Suma P, Zappala L, Mazzeo G, Siscaro G. Lethal and sub-lethal effects of insecticides on natural enemies of citrus scale pests. Biocontrol. 2009;54:651–61.
Tait G, Mermer S, Stockton D, Lee J, Avosani S, Abrieux A, Walton VM. Drosophila suzukii (Diptera: Drosophilidae): a decade of research towards a sustainable integrated pest management program. J Econ Entomol. 2021;114:1950–74.
Teder T, Knapp M. Sublethal effects enhance detrimental impact of insecticides on non-target organisms: a quantitative synthesis in parasitoids. Chemosphere. 2019;214:371–8.
Tiwari S, Stelinski LL. Effects of cyantraniliprole, a novel anthranilic diamide insecticide, against Asian citrus psyllid under laboratory and field conditions. Pest Manag Sci. 2013;69:1066–72.
Van Timmeren S, Mota-Sanchez D, Wise JC, Isaacs R. Baseline susceptibility of spotted wing Drosophila (Drosophila suzukii) to four key insecticide classes. Pest Manag Sci. 2018;74:78–87.
Wang DS, He YR, Guo XL, Luo YL. Acute toxicities and sublethal effects of some conventional insecticides on Trichogramma chilonis (Hymenoptera: Trichogrammatidae). J Econ Entomol. 2012;105:1157–63.
Wang XG, Nance AH, Jones JM, Hoelmer KA, Daane M. Aspects of the biology and reproductive strategy of two Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii. Biol Control. 2018;121:58–65.
Wang X, Biondi A, Daane KM. Functional responses of three candidate Asian larval parasitoids evaluated for classical biological control of Drosophila suzukii (Diptera: Drosophilidae). J Econ Entomol. 2020a;113:73–80.
Wang X, Lee JC, Daane KM, Buffington ML, Hoelmer KA. Biological control of Drosophila suzukii. CABI Rev. 2020b;15:1–19.
Wright DJ, Verkerk RH. Integration of chemical and biological control systems for arthropods: evaluation in a multitrophic context. Pestic Sci. 1995;44:207–18.
Yang Q, Lu Y. Impact of two neonicotinoid insecticides on the parasitoids of Apolygus lucorum: Peristenus spretus and Peristenus relictus. Crop Prot. 2023;167:106208.
Zhang Z, Li K, Xu W, Liang N, Chu D, Guo L. Characterization of the ryanodine receptor gene in Encarsia formosa (Gahan) and its expression profile in response to diamide insecticides. Pestic Biochem Phys. 2021;178:104921.
Acknowledgements
The authors would like to acknowledge the support of the Sicilian Phytosanitary Service (Regione Siciliana) for providing Ganaspis cf. brasiliensis individuals in the context of a classical biological control program of Drosophila suzukii in Italy.
Funding
The study was funded by the University of Catania, PhD grant to F.L. and by the European Union (Next Generation EU) and the Italian Ministry of Research for the PRIN 2022 project InStress (CUP: E53D23007650006) granted to A.B.
Author information
Authors and Affiliations
Contributions
Conceptualization: F.L.; L.F.; A.G.; M.V.R.S.; A.B.; methodology- investigation: F.L.; C.C.; A.B; resources: A.B.; M.V.R.S.; writing-original draft preparation: F.L.; writing-review and editing: all authors; funding acquisition: A.B.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Authors have nothing to declare.
Consent for publication
All authors gave consent to publish the present manuscript.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Lisi, F., Cavallaro, C., Fellin, L. et al. Non-target effects of neurotoxic insecticides on Ganaspis cf. brasiliensis, a classical biological control agent of the spotted wing Drosophila. CABI Agric Biosci 5, 48 (2024). https://doi.org/10.1186/s43170-024-00251-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43170-024-00251-0