Abstract
Current management strategy of the invasive fruit fly Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) exploits different tools but relies mainly on chemical control. In the invaded areas, the local natural enemy community mostly consists of generalist pupal parasitoids unable to control the pest efficiently. Conversely, in the pest native area, there are more specialized sympatric larval parasitoids attacking D. suzukii. Following foreign explorations and quarantine risk assessments, the larval endoparasitoid Ganaspis brasiliensis (Ihering) (Hymenoptera: Figitidae) was selected as the best candidate for classical biological control programs. In 2021, the first ever propagative biocontrol program using a Japanese G1 lineage of G. brasiliensis started in Italy. Here we report the results of the first year of releases in the province of Trento (Northeast Italy), wherein G. brasiliensis was released in 12 locations. Pre- and post-release samplings on fresh and fallen fruits were performed around the release points to assess the recapture rate, the impact of the exotic parasitoid on D. suzukii and its potential interactions with local non-target species. After releases, G. brasiliensis was recovered at 50% of the locations. The exotic parasitoid only emerged from D. suzukii, mostly from fresh fruit still on the plant. Post-overwintering monitoring revealed the presence of a four G. brasiliensis individuals at two release locations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Drosophila suzukii Matsumura (Diptera: Drosophilidae), native to southeast Asia, has gradually invaded North America and Europe (2008), South America (2012) and Africa (2017) becoming a global economic threat to soft-skinned fruit crops (Cini et al. 2012; Asplen et al. 2015; Anfora 2020; Hassani et al. 2020). The control of this highly polyphagous pest primarily relies on local area management, mostly on the use of insecticides (Tait et al. 2021). However, this approach is often not environmentally friendly and economically sustainable because of pest re-infestations occurring shortly after treatment from surrounding vegetation (Tait et al. 2020). On the other hand, an area-wide management approach would deal better with such mobile pest by reducing the total pest population within crop and non-crop areas (Haye et al. 2016).
In this sense, biological control strategies to suppress D. suzukii have been intensively studied, and include the use of predators, parasitoids, pathogens and competitors (Tait et al. 2021). In particular, it has been shown that parasitoids can regulate the population of Drosophilids, either by directly causing high mortality rates (Janssen et al. 1988; Driessen & Hemerik 1991) or by affecting competitive interactions between sibling host species (Boulétreau et al. 1991). In the invaded areas, two cosmopolitan pupal parasitoids have been identified to successfully parasitize D. suzukii: Trichopria drosophilae (Perkins) (Hymenoptera: Diapriidae) and Pachycrepoideus vindemiae (Rondani) (Hymenoptera: Pteromalidae) (Miller et al. 2015), with the first being released in Italy and Mexico and the latter in North America for augmentative biological control (Rossi Stacconi et al. 2019; Gonzalez-Cabrera et al. 2019; Hogg et al. 2022). However, natural parasitism by these generalist pupal parasitoids is below 10% (Lee et al. 2019) and the introduction specialist parasitoids from the native area of D. suzukii for classical biological control remains a preferable option (Lee et al. 2019).
Classical biological control can be an effective strategy to control invasive insect pests (Hajek et al. 2016) and can offer protection to natural ecosystem (van Driesche et al. 2010). Although its application and number of introductions gradually decreased since the 1970s, this method gained higher establishment and success rate in recent days, regaining momentum and trust (Cock et al. 2016). This positive trend is also the result of higher attention given from national and international regulatory bodies into providing appropriate tools for risk assessment, import and release of exotic biocontrol agents (BCA), all necessary steps to limit potential negative impacts on biodiversity in areas of introduction (Barratt et al. 2018).
In its native environment, D. suzukii is regulated by a multitude of natural enemies and, among these, at least 14 parasitoid species (Daane et al. 2016). However, field collections and laboratory studies have shown that two larval parasitoids of the Figitidae family are the most specialized towards D. suzukii: Leptopilina japonica Novković & Kimura and Ganaspis brasiliensis (Ihering) (Girod et al. 2018a, b; Giorgini et al. 2019; Daane et al. 2021). In particular, for G. brasiliensis, five clades (G1 to G5) where identified on a genetic basis (Nomano et al. 2017; Giorgini et al. 2019), with the G3 attacking few hosts in the Drosophila melanogaster Meigen species group and the G1 being considered a specialist on D. suzukii, thus rapidly gaining researchers’ interest as a candidate for classical biological control. Adventive populations of L. japonica have been recently identified in northeastern Italy (Puppato et al. 2020) and in the northwestern North America (Abram et al. 2020a), whereas the G1 clade of G. brasiliensis was only recorded in British Columbia (Canada) and Washington (USA) (Abram et al. 2020a; Beers et al. 2022).
In 2021, after the evaluation of a comprehensive risk assessment submitted by Italian scientific institutions and phytosanitary services, the Italian Ministry of Ecological Transition authorized the release of G. brasiliensis G1 in seven regions and two autonomous provinces within the frame of a national biological control program (Rossi Stacconi pers. comm.). Here we report the results of the monitoring program following the first-year releases of G. brasiliensis in the province of Trento (Northeastern Italy), where the exotic parasitoid was released at 12 locations.
Materials and methods
Parasitoid rearing and releases
Parasitoids were reared according to the mass rearing protocol specified in Rossi Stacconi et al. (2022). For host, we used laboratory-reared D. suzukii that originated from multiple field collections of live adults at different locations of Trento Province (TN) Italy, during 2020 and 2021. The starting colony of G. brasiliensis derived from wild individuals collected during surveys in Naganuma park (Hachioji, Tokyo, Japan) from 2015 to 2017 (Girod et al. 2018a) and were provided by CABI’s Swiss centre (Delémont, Switzerland). Parasitoid releases were performed at 12 locations in the Trento province, Italy (Table 1). Locations were selected according to presence of suitable unmanaged D. suzukii hosts, known presence of the pest and absence of ecological vulnerability constraints (protected areas). The sites were distributed along an altitudinal range (Table 1) and categorized as valley bottom sites (0–249 m a.s.l.), hillside sites (250–699 m a.s.l.) and mountain sites (700 + m a.s.l.). All sites were located at the margins of wooded areas, providing natural corridors for parasitoid movements. Four sites (#1, #2, #10 and #12) were in proximity (< 50 m) of crops considered non-host for D. suzukii (apple orchards and vineyards), four sites (#4, #5, #8 and #11) were in proximity of cherry orchards and four sites (#3, #6, #7 and #9) were in proximity of multiple small fruit orchards (blueberry, blackberry, raspberry and strawberry). At each site, a single release point was selected and parasitoid releases were performed once a week during three consecutive weeks. The dates of the releases were set according with local host plants’ phenology (Table 1). Each release consisted of 200 three-day-old adults of G. brasiliensis (sex ratio 50:50 M:F).
Field surveys
The authorization to release G. brasiliensis was received on August 17th 2021 and parasitoid releases started within one week. Field surveys were performed before (1–2 pre-release samplings) and after (six post-release samplings) the first release event (Table 1). At each site, at least one pre-release sampling and five post-release samplings were conducted. Due to the lack of experimental evidence comparing the outcomes of different sampling methods (Abram et al. 2022a), the twelve release locations were divided into two subgroups, establishment sites (n = 3) and specificity sites (n = 9), each adopting a different sampling methodology. The goal of monitoring establishment sites (ESs) was to verify the ability of G. brasiliensis to disperse and reproduce in the new environments after releasing; to this aim, large quantities of fruits were collected from a wide area surrounding the release sites (see below). On the other hand, the goal of monitoring in the specificity sites (SSs) was to assess the host specificity of G. brasiliensis towards D. suzukii and other non-target species, and its interactions with other parasitoids. To this aim, at each site a fruit was collected from few selected host patches and all fly puparia deriving from it were sorted prior to eclosion. In 2020 and 2021, monitoring activity was also conducted in multiple control sites (Supplementary Table S1) where no release of G. brasiliensis was performed. Monitoring in control sites was carried out according to the sampling protocol described for ESs, with the aim to assess the presence of adventive populations of the parasitoid in the area of the study (Trento province, Italy) and supporting results from the pre-release monitoring in ESs and SSs. In the ESs, post-overwintering surveys were conducted on May 27th and June 21th 2022 to check the survival of G. brasiliensis after the cold season.
Fruit collections
In the ESs, multiple fruit collections were conducted on host plants located in an area within 200 m from the release point. To account for spatial variation in host densities and parasitism levels, all plant patches included in the monitoring area and carrying fruit susceptible to D. suzukii attack were sampled and kept individually. In the SSs, fruit collection was carried out from only four pre-selected host plant patches located within 100 m from the release point. For both sampling methodologies, all sampling points were georeferenced using SMASH Digital Field Mapping (HydroloGIS S.r.l, Bolzano, Italy) to calculate their distance from the release site. At each sampling point and fruit quantity permitting, sub-samplings were carried out both from the plants (fresh fruit), randomly picking over the whole vertical height of the canopy of each selected host plant patch, and from the underlying ground (overripe and rotten fruit). Each sub-sample aimed to fill a 280 ml plastic container (Unipak 5011–21, Berry Superfos, Bologna, Italy) corresponding to 50–80 g of fruit, depending on the size and the water content of individual fruit. All sub-samples were incubated for three weeks in separate containers under optimal condition ranges (21–24 °C; 65–75% RH; L:d 16:8 photoperiod). Incubation was carried out in modified plastic containers similar to those described by Abram et al. (2022a). Within each container, fruit was placed over blotting paper covering a layer of sand (1 cm). Such method allowed for a better control over humidity, as sand worked as moisture retainer and prevented formation of liquid pools.
Laboratory handling of collected fruit
Two methodologies were adopted to assess emergence of the different fly and parasitoid species depending on the collection site (ESs or SSs). For fruit collected in the ESs, emerged individuals (flies and parasitoids) from each sub-sample were regularly removed from the container three times per week and stored in 70% ethanol until taxonomic identification. For samples collected in the SSs, Drosophila puparia were collected from containers as they formed. Based on size, color and the unique morphology of the spiracles of D. suzukii puparium (EPPO 2013; Abram et al. 2022a), puparia were divided in D. suzukii and non-D. suzukii and kept in separate containers over moist filter paper until emergence of flies or parasitoids. This method allowed for an accurate estimation of parasitization rate on target and non-target by G. brasiliensis and by other larval and pupal parasitoid species. For both methodologies, at the end of the incubation period, containers were carefully inspected and all remaining uneclosed puparia were collected, re-constituted in water for 24 h and dissected to check for presence of dead hosts or parasitoids.
Data analysis
The number of G. brasiliensis and other parasitoids as well as that of hosts emerged from the samples was recorded. Identification was conducted on a morphological basis using taxonomic keys (Markow and O’Grady 2005; Lue et al 2016; Buffington and Forshage 2016; Forshage & Nordlander 2008). After identification, samples of the released and recovered G. brasiliensis and samples of non-target drosophilids were deposited in the permanent collection of the Trento’s Science Museum (Trento, Italy) and made available for further verifications and analysis. Descriptive analyses were performed on the occurrence of G. brasiliensis with other species. For data collected in the SSs, the total percentage parasitism and the percentage of aborted parasitism were assessed based on the protocol described in Abram et al (2022a).
Results
Occurrence of G. brasiliensis
No G. brasiliensis emerged from fruit collected in the control sites (Supplementary Table S1) or at the release sites during pre-release sampling (Tables. 2, 3). During post-release sampling, a total of 87 G. brasiliensis individuals was obtained from fruit collected at six release locations (#1, #4, #8, #9, #10 and #12). (Fig. 1). Among these, 42 emerged from six fruit samples collected in the ESs and 45 from 11 fruit samples collected in the SSs (Tables 2, 3). In the ESs, all G. brasiliensis individuals derived from plant-sampled fruit, whereas in the SSs 31.1% G. brasiliensis eclosed from ground-sampled fruit (Tables 2, 3). Overall, eclosions spanned from samples collected during week 34 (August 23rd–29th) to week 39 (September 27th–October 3rd), with all sites but #12 scoring occurrence of G. brasiliensis from multiple weeks. In the SSs, eclosions of G. brasiliensis occurred at all the altitudinal ranges, with individuals being recorded from one valley bottom, one hillside and two mountain sites. Most of the fruit samples from which G. brasiliensis was recovered were wild blackberry (Rubus ulmifolius Schott—nine samples), the remaining samples consisted in elderberry (Sambucus nigra (L.)—three samples), cropped blackberry (Rubus fruticosus (L.)—two samples), alder buckthorn (Frangula alnus Mill.—two samples) and cropped raspberry (Rubus idaeus (L.)—one sample) (Fig. 2, 3, 4). In the SSs, all fruit samples containing G. brasiliensis were collected in the proximity of the release point (0–10 m), representing 18% of all collected fruit samples. In the ESs, 33.3% of G. brasiliensis individuals were recorded at 0–10 m (26.6% of the fruit samples), 47.6% at 20–50 m (27.5% of the fruit samples) and 19.1% at 100 m far from the release point on a single fruit sample. During the post-overwintering surveys, four G. brasiliensis individuals were recorded at two locations: a single female eclosed from St. Lucie cherry (Prunus mahaleb L.) sampled from plant on May 27th 2022 at the site #10, while two males and one female eclosed from two common mulberry (Morus alba L.) samples collected from ground and from plant on June 21st 2022 at site #12.
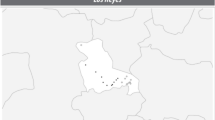
Distribution map of the release sites within the Province of Trento (Italy). Squares and circles indicate locations with and without G. brasiliensis occurrence during the monitoring, respectively. Location numbers refer to those listed in Table 1
Relative eclosion percentage of flies and parasitoids in fruit collected from different host plants during post-release monitoring in the establishment sites. For each host plant species, the number of samples collected followed by the number of fly and parasitoid individuals eclosed are indicated in brackets
Relative eclosion percentage of flies and parasitoids from the puparia of D. suzukii (a) and of other host flies (b) collected from plant- and ground-sampled fruit during post-release monitoring in the specificity sites. For each host plant species, the number of samples collected followed by the number of fly and parasitoid individuals eclosed are indicated in brackets
Interactions of G. brasiliensis with other species
Drosophila suzukii was the main host eclosing from fruit samples representing 98.5% and 43.9% of all the flies obtained from plant-sampled and ground-sampled fruit, respectively (Tables 2, 3). Other host species consisted in D. melanogaster (0.98% and 36.8% from plant-sampled and ground-sampled fruit, respectively), Drosophila simulans Sturtevant (0.37% and 11.6%), Drosophila immigrans Sturtevant (0.15% and 4.1%), Drosophila subobscura Collin (1.5% from ground-sampled fruit), Drosophila hydei Sturtevant (1.1% from ground-sampled fruit), Drosophila busckii Coquillett (0.9% from ground-sampled fruit) and Drosophila funebris (Fabricius) (0.1% from ground-sampled fruit). In the SSs, the occurrence of G. brasiliensis was recorded only from D. suzukii pupae collected in post-release samplings (Table 3). Likewise, in the ESs all G. brasiliensis individuals eclosed from fruit samples in which the recorded host eclosion was 100% D. suzukii. Ganaspis brasiliensis individuals accounted for 4.44% and 3.74% of all the parasitoids recorded in the ESs and SSs, respectively. Several other parasitoid species were recorded from both ESs and SSs during post-release monitoring. The most abundant was the exotic larval parasitoid L. japonica representing 71.22% and 94.18% of all parasitoid individuals eclosed in ESs and SSs, respectively. Among the local species, pupal parasitoids were P. vindemiae (9.50% in ESs and 1.49% in SSs), T. drosophilae (3.69% in ESs and 0.58% in SSs) and Vrestovia fidenas (Walker) (0.04% in ESs), while larval parasitoids were Leptopilina heterotoma (Thomson) (6.04% in ESs), Leptopilina boulardi (Barbotin) (4.89% in ESs) and Asobara tabida (Nees) (0.17% in ESs). In berry samples from the SSs in which G. brasiliensis was present, apparent parasitism was on average 2.3% (0.2–13.1), accounting for 17.9% (span: 1.8–67.8%) of the total apparent parasitism recorded in those samples (G. brasiliensis + other parasitoids). When G. brasiliensis parasitism occurred, L. japonica was always present and more abundant than G. brasiliensis. Local larval parasitoid species never eclosed from plant-sampled fruit and were absent in the two ground-sampled fruit samples attacked by G. brasiliensis (Tables 2, 3). When dissecting D. suzukii uneclosed puparia (< 0.7% of the total puparia), no sign of aborted parasitism was recorded.
Discussion
Implementing a propagative (classical) biocontrol program involves efforts that are equally distributed between the pre- and post-release phases. Pre-release work seeks to draft a comprehensive risk assessment for the candidate exotic BCA, considering all positive and negative consequences of its potential introduction in a new environment (Barratt et al. 2010). Post-release work mainly aims to verify (1) the initial survival and potential establishment of the released BCA and (2) the interactions of the BCA with the target species and with other local organisms (non-targets) (van Driesche et al. 2010). In our post-release study, the results of the first-year monitoring showed that G. brasiliensis can reproduce and overwinter in the release area. In particular, the sites at which the parasitoid was recovered during the post-overwintering surveys are both located at the valley bottom and characterized by a temperate climate with hot summer and no dry season (Beck et al. 2018). This is consistent with the CLIMEX model prediction from Wang et al. (2020), indicating the suitability of most of the temperate European areas for the establishment of the exotic parasitoid. Despite the low number of parasitoids released per site (300 females and 300 males), G. brasiliensis was recovered in 50% of the sites during the six weeks following the first release event (weeks 34–39) and regardless the altitudinal range. Moreover, post-overwintering monitoring showed that, at least at two locations, G. brasiliensis was able to survive the cold season and to start new generations in spring. These findings strongly suggest a climatic suitability of the Province of Trento for the parasitoid. However, further data need to be collected in the next years, particularly in relation to the ability of the exotic BCA to overwinter in release area (Hokkanen and Sailer 1985). In fact, winter 2021–2022 has been relatively mild compared with the local climatic historical series and this unusual temperature trend may have favored the survival of G. brasiliensis. Preliminary laboratory trials assessing survival of different G. brasiliensis stages at low temperature suggest that the parasitoid overwinters as pharate adult within the host puparium (Rossi Stacconi pers. comm.), but further experiments are needed for confirmation. The few records of G. brasiliensis adults scored during the post-overwintering monitoring activity may appear as a rather disappointing result. However, recent examples of successful classical biological control shed a positive light on this data. For example, the parasitoid Torymus sinensis Kamijo (Hymenoptera: Torymidae) was released for the first time in 2005 and 2006 at three sites located in Piedmont (Italy) to control the invasive Asian chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae). In 2007, out of 36.000 galls sampled at the same release sites, a total of only 30 adults of T. sinensis were collected (Quacchia et al. 2008). By 2009, the parasitoid emerged from 4.08% of the collected galls and after seven years from its first release (in 2012) T. sinensis parasitization rate was steadily above 80% (Ferraccini et al. 2019).
Two different monitoring approaches were adopted for establishment and specificity sites, allowing for different evaluations on the impact of G. brasiliensis in the release area. Monitoring in the ESs aimed to sample large quantity of fruit from a wide sampling area in order to maximize the probability to recover G. brasiliensis, to assess its spread capacity from the release point and to provide an overview of the host plants exploited by the parasitoid during host search. On the other hand, most of the sites were monitored as SSs sampling a reduced amount of fruit samples in order to evaluate in detail parasitism parameters and host specificity of G. brasiliensis, As expected, the occurrence rate of G. brasiliensis was higher in ESs (66.6% of the sites) than in SSs (44.4% of the sites), although in the SSs were found more fruit samples attacked by the BCA (n. 11/184) than in the ESs (n. 6/229). However, looking at the distribution of the attacked fruit samples, the ESs monitoring approach seems to provide a more accurate representation of the parasitoid movements within the sampled area compared to the SSs approach, showing some parasitoid activity away from the release point. Despite the practical differences, data from the two monitoring approaches were consistent with the fact that G. brasiliensis G1 behaves as a specialist on D. suzukii, never eclosing from puparia of other species (SSs) or from fruit samples infested with other flies than D. suzukii (ESs). This aspect of the biology of G. brasiliensis G1 was already observed in its native range (Daane et al. 2016; Girod et al. 2018a), in quarantine facility studies (Girod et al. 2018b; Daane et al. 2021) and in field cage releases (Seehausen et al. 2022), but this is the first study reporting the parasitoid’s strict association with D. suzukii in natural conditions outside its native range. A second aspect that has been further confirmed by our study, is that G. brasiliensis tends to avoid competition by attacking its hosts mostly within fresh fruit still on the plant (Giorgini et al. 2019), whereas local parasitoids of drosophilids parasitize their hosts on decaying fruit. Although chemical ecology studies have not yet been conducted on olfactory preference of G. brasiliensis G1 towards infested and uninfested fruit, such avoidance behavior was already suggested in a recent study considering the competitive parasitization outcomes of three solitary larval parasitoids of drosophilids:; G. brasiliensis G3, L. japonica and Asobara japonica Belokobylskij (Hymenoptera: Braconidae) (Wang et al. 2019). The study showed that G. brasiliensis G3 is outcompeted by both the other parasitoids and, in response to that, it discriminates against parasitized hosts. Also, we observed that the ovipositor of the released strain of G. brasiliensis is significantly shorter compared to that of other larval parasitoids recovered from the monitoring (Supplementary Figure S1). Such morphological feature would allow G. brasiliensis to only parasitize host larvae located just below the fruit skin of fresh fruits (i.e., 1st and 2nd instars) and making it extremely difficult for the exotic parasitoid to attack hosts buried in a rotten substrate. In terms of impact on biodiversity, these functional traits (preference towards fresh fruit and ovipositor morphology) exhibited by G. brasiliensis creates a specific trophic niche for the BCA (Cohen 1977) and reduces the risk of negative impacts on non-target organisms, such as local larval parasitoids.
Overall, the result of our monitoring showed that L. japonica was the dominant parasitoid species in all locations where G. brasiliensis was released. This adventive exotic species was reported in Italy (Province of Trento) for the first time in 2019 at one location (Puppato et al. 2020) and, since then, increasing catches have been reported. In its native range, L. japonica is frequently found on D. suzukii, which is considered a preferred host (Girod et al. 2018b; Giorgini et al. 2019), and recent studies reported that this parasitoid reproduces on a relatively narrow host range, although not narrow enough to be considered as a candidate for classical biocontrol programs (Girod et al. 2018a; Daane et al. 2021). Likely, the presence of D. suzukii as one of the most abundant species feeding on cultivated and wild fruit have been a key factor contributing to the rapid dispersal and population growth of L. japonica in the Alpine region and a similar trend can be expected for G. brasiliensis. However, population changes induced by biological control programs often require years to reach stable endpoints and the outcomes are function of many dependent and independent variables, including the intrinsic characteristics of the pest, the BCA and the target ecosystem (Hokkanen and Sailer 1985). Abram et al. (2022b) recently reported that in Canada (British Columbia) the two adventive parasitoids, G. brasiliensis and L. japonica, have re-built the same close association with D. suzukii observed in the pest’s native range, with the latter being the dominant species. Extensive fruit samples carried out in 2020 showed D. suzukii parasitization by the two parasitoids to be time-structured and widespread across a variety of habitats and host plants. Such observations have been made a short time after the first reports of G. brasiliensis and L. japonica in the area (one and four years, respectively), and most likely the parasitoids’ dispersal is still ongoing (Abram et al. 2022b). In the area of the present study, D. suzukii is considered a major pest of soft fruit and, more than a decade after the introduction, its impact still accounts for 9% of the potential revenues, mostly due to costly control measures (De Ros et al. 2020). Realistic expectations for impact of classical biological control in Italy might be that G. brasiliensis will contribute in restoring part of the top-down pest suppression existing in the D. suzukii native area. However, such contribution will likely be difficult to quantify in terms of success or failure, as it is expected to be regionally variable and to add to that provided by other local and adventive biocontrol organisms (Abram et al. 2020b). In this sense, the establishment of stable populations of G. brasiliensis and their relative contribution to D. suzukii larval parasitism would be a first step towards success of the classical biological control program. Once this is accomplished, an evaluation should be done in the long run to assess whether the increased parasitism has resulted in a reduction of management costs.
References
Abram PK, McPherson AE, Kula R, Hueppelsheuser T, Thiessen J, Perlman SJ, Curtis CI, Fraser JL, Tam J, Carrillo J, Gates M, Scheffer S, Lewis M, Buffington M (2020a) New records of Leptopilina, Ganaspis, and Asobara species associated with Drosophila suzukii in North America, including detections of L. japonica and G. brasiliensis. J Hymenoptera Res 78:1–17
Abram PK, Mills NJ, Beers EH (2020b) Classical biological control of invasive stink bugs with egg parasitoids–what does success look like? Pest Manag Sci 76(6):1980–1992
Abram PK, Wang X, Hueppelsheuser T, Franklin MT, Daane KM, Lee JC, Lue CH, Girod P, Carrillo J, Wong WHL, Kula RR, Gates MW, Hogg BN, Moffat CE, Hoelmer KA, Sial AA, Buffington ML (2022a) A coordinated sampling and identification methodology for larval parasitoids of spotted-wing drosophila. J Econ Entomol 115(4):922–942
Abram PK, Franklin MT, Hueppelsheuser T, Carrillo J, Grove E, Eraso P, Acheampong S, Keery L, Girod P, Tsuruda M, Clausen M, Buffington ML, Moffat CE (2022b) Adventive larval parasitoids reconstruct their close association with spotted-wing drosophila in the invaded North American range. Environ Entomol 51(4):670–678
Anfora G (2020) Drosophila suzukii. EPPO datasheets on pests recommended for regulation. Available online. https://gd.eppo.int
Asplen MK, Anfora G, Biondi A, Choi DS, Chu D, Daane KM, Gibert P, Gutierrez PG, Hoelmer KA, Hutchinson WD, Isaacs R, Zhi-Lin J, Kárpáti Z, Kimura MT, Pascual M, Philips CR, Plantamp C, Ponti L, Vétek G, Vogt H, Walton VM, Yu Y, Zappalà L, Desneux N (2015) Invasion biology of spotted wing Drosophila (Drosophila suzukii): a global perspective and future priorities. J Pest Sci 88:469–494
Barratt BIP, Howarth FG, Withers TM, Kean JM, Ridley GS (2010) Progress in risk assessment for classical biological control. Biol Contr 52(3):245–254
Barratt BIP, Moran VC, Bigler F, van Lenteren JC (2018) The status of biological control and recommendations for improving uptake for the future. BioControl 63:155–167
Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF (2018) Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci Data 5:180214
Beers EH, Beal D, Smytheman P, Abran PK, Schmidt-Jeffris R, Moretti E, Daane KM, Looney C, Lue CH, Buffington M (2022) First records of adventive populations of the parasitoids Ganaspis brasiliensis and Leptopilina japonica in the United States. J Hymen Res 91:11–25
Boulétreau M, Fouillet P, Allemand R (1991) Parasitoids affect competitive interactions between the sibling species Drosophila melanogaster and D simulans. Redia 74:171–177
Buffington ML, Forshage M (2016) Redescription of Ganaspis brasiliensis (Ihering, 1905), new combination, (Hymenoptera: Figitidae) a natural enemy of the invasive Drosophila suzukii (Matsumura, 1931) (Diptera: Drosophilidae). Proc Ent Soc Washington 118(1):1–13
Cini A, Ioriatti C, Anfora G (2012) A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bull Insectol 65(1):149–160
Cock MJ, Murphy ST, Kairo MT, Thompson E, Murphy RJ, Francis AW (2016) Trends in the classical biological control of insect pests by insects: an update of the BIOCAT database. BioControl 61:349–363
Cohen JE (1977) Food webs and the dimensionality of trophic niche space. Proc Natl Acad Sci 74(10):4533–4536
Daane KM, Wang XG, Biondi A, Miller B, Riedl H, Shearer PW, Guerrieri E, Giorgini M, Buffington M, van Achterberg K, Song Y, Kang T, Yi H, Jung C, Lee DW, Chung BK, Hoelmer KA, Walton VM (2016) First exploration of parasitoids of Drosophila suzukii in South Korea as potential classical biological agents. J Pest Sci 89:823–835
Daane KM, Wang XG, Hogg BN, Biondi A (2021) Potential host ranges of three Asian larval parasitoids of Drosophila suzukii. J Pest Sci 94:1171–1182
De Ros G, Grassi A, Pantezzi T (2020) Recent trends in the economic impact of Drosophila suzukii. In: Garcia FRM (ed) Drosophila suzukii Management. Springer, Cham, pp 11–27
Driessen G, Hemerik L (1991) Aggregative responses of parasitoids and parasitism in populations of Drosophila breeding in fungi. Oikos 61:96–107
EPPO (2013) PM 7/115 (1) Drosophila suzukii. EPPO Bull 43:417–424
Ferracini C, Ferrari E, Pontini M, Saladini MA, Alma A (2019) Effectiveness of Torymus sinensis: a successful long-term control of the Asian chestnut gall wasp in Italy. J Pest Sci 92:353–359
Forshage M, Nordlander G (2008) Identification key to european genera of eucoilinae (Hymenoptera, Cynipoidea, Figitidae). Insect Syst Evol 39(3):341–359
Giorgini M, Wang XG, Wang Y, Chen YW, Hougardy E, Zhang HM, Chen ZQ, Chen HY, Liu CX, Cascone P, Formisano G, Carvalho GA, Biondi A, Buffington M, Daane KM, Hoelmer KA, Guerrieri E (2019) Exploration for native parasitoids of Drosophila suzukii in China reveals a diversity of parasitoid species and narrow host range of the dominant parasitoid. J Pest Sci 92:509–522
Girod P, Lierhmann O, Urvois T, Turlings TCJ, Kenis M, Haye T (2018a) Host specificity of Asian parasitoids for potential classical biological control of Drosophila suzukii. J Pest Sci 91:1241–1250
Girod P, Borowiec N, Buffington M, Chen G, Fang Y, Kimura MT, Peris-Felipo FJ, Ris N, Wu H, Xiao C, Zhang J, Aebi A, Haye T, Kenis M (2018) The parasitoid complex of D suzukii and other fruit feeding Drosophila species in Asia. Sci Rep 8(1):1–8
Gonzalez-Cabrera J, Moreno-Carrillo G, Sanchez-Gonzalez JA, Mendoza-Ceballos MY, Arredondo-Bernal HC (2019) Single and combined release of Trichopria drosophilae (Hymenoptera: Diapriidae) to control Drosophila suzukii (Diptera: Drosophilidae). Neotrop Entomol 48:949–956
Hajek AE, Hurley BP, Kenis M (2016) Exotic biological control agents: a solution or contribution to arthropod invasions? Biol Invasions 18:953–969
Hassani IM, Behrman EL, Prigent SR, Gidaszewski N, Ravaomanarivo LHR, Suwalski A, Debat V, David JR, Yassin A (2020) First occurrence of the pest Drosophila suzukii (Diptera: Drosophilidae) in the Comoros Archipelago (Western Indian Ocean). Afr Entomol 28:78–83
Haye T, Girod P, Cuthbertson AGS, Wang XG, Daane KM, Hoelmer KA, Baroffio C, Zhang JP, Desneaux N (2016) Current SWD IPM tactics and their practical implementation in fruit crops across different regions around the world. J Pest Sci 89:643–651
Hogg BN, Lee JC, Rogers M, Worth L, Nieto DJ, Stahl JM, Daane KM (2022) Releases of the parasitoid Pachycrepoideus vindemiae for augmentative biological control spotted wing drosophila Drosophila Suzukii. Biol Contr 168:104865
Hokkanen HM, Sailer RI (1985) Success in classical biological control. Crit Rev Plant Sci 3(1):35–72
Janssen A, Driessen G, Haan MD, Roodbol N (1988) The impact of parasitoids on natural populations of temperate woodland Drosophila. Neth J Zool 38:61–73
Lee JC, Wang XG, Daane KM, Hoelmer KA, Isaacs R, Sial AA, Walton VM (2019) Biological control of spotted-wing drosophila (Diptera: Drosophilidae)-current and pending tactics. J Int Pest Manag 13:1–9
Lue CH, Driskell AC, Leips J, Buffington ML (2016) Review of the genus Leptopilina (Hymenoptera, Cynipoidea, Figitidae, Eucoilinae) from the Eastern United States, including three newly described species. J Hymen Res 53:135–176
Markow TA, O’Grady P (2005) Drosophila: a guide to species identification and use. Elsevier, Amsterdam
Miller B, Anfora G, Buffington M, Daane KM, Dalton DT, Hoelmer KM, Rossi Stacconi MV, Grassi A, Ioriatti C, Loni A, Miller JC, Ouantar M, Wang XG, Wiman NG, Walton VM (2015) Seasonal occurrence of resident parasitoids associated with Drosophila suzukii in two small fruit production regions of Italy and the USA. Bull Insectol 68(2):255–263
Nomano FY, Kasuya N, Matsuura A, Suwito A, Mitsui H, Buffington ML, Kimura MT (2017) Genetic differentiation of Ganaspis brasiliensis (Hymenoptera: figitidae) from East and Southeast Asia. Appl Entomol Zool 52(3):429–437
Puppato S, Grassi A, Pedrazzoli F, De Cristofaro A, Ioriatti C (2020) First report of Leptopilina japonica in Europe. Insects 11:611
Quacchia A, Moriya S, Bosio G, Scapin I, Alma A (2008) Rearing, release and settlement prospect in Italy of Torymus sinensis, the biological control agent of the chestnut gall wasp Dryocosmus kuriphilus. BioControl 53:829–839
Rossi Stacconi MV, Grassi A, Ioriatti C, Anfora G (2019) Augmentative releases of Trichopria drosophilae for the suppression of early season Drosophila suzukii populations. Biol Control 64:9–19
Rossi-Stacconi MV, Wang X, Stout A, Fellin L, Daane KM, Biondi A, Stahl JM, Buffington ML, Anfora G, Hoelmer KA (2022) Methods for rearing the parasitoid Ganaspis brasiliensis, a promising biological control agent for the invasive Drosophila suzukii. J vis Exp 184:e63898
Seehausen ML, Valenti R, Fontes J, Meier M, Marazzi C, Mazzi D, Kenis M (2022) Large-arena field cage releases of a candidate classical biological control agent for spotted wing drosophila suggest low risk to non-target species. J Pest Sci 95:1057–1065
Tait G, Cabianca A, Grassi A, Pfab F, Oppedisano T, Puppato S, Mazzoni V, Anfora G, Walton VM (2020) Drosophila suzukii daily dispersal between distinctly different habitats. Entomol Gen 40:25–37
Tait G, Mermer S, Stockton D, Lee JC, Avosani S, Abrieux A, Anfora G, Beers E, Biondi A, Burrack H, Cha D, Chiu JC, Choi MY, Cloonan K, Crava CM, Daane KM, Dalton DT, Diepenbrock L, Fanning P, Ganjisaffar F, Gómez MI, Gut L, Grassi A, Hamby K, Hoelmer KA, Ioriatti C, Isaacs R, Klick J, Kraft L, Loeb G, Rossi-Stacconi MV, Nieri R, Pfab F, Puppato S, Rendon D, Renkema J, Rodriguez-Saona C, Rogers M, Sassù F, Schöneberg T, Scott MJ, Seagraves M, Sial AA, Van Timmeren S, Wallingford A, Wang XG, Yeh DA, Zalom FG, Walton VM (2021) Drosophila suzukii (Diptera: Drosophilidae): a decade of research towards a sustainable integrated pest management program. J Econ Entomol 114(5):1950–1974
van Driesche RG, Carruthers RI, Center T, Hoddle MS, Hough-Goldstein J, Morin L, Smith L, Wagner DL, Blossey B, Brancatini V, Casagrande R, Causton CE, Coetzee JA, Cuda J, Ding J, Fowler SV, Frank JH, Fuester R, Goolsby J, Grodowitz M, Heard TA, Hill MP, Hoffmann JH, Huber J, Julien M, Kairo MTK, Kenis M, Mason P, Medal J, Messing R, Miller R, Moore A, Neuenschwander P, Newman R, Norambuena H, Palmer WA, Pemberton R, Perez Panduro A, Pratt PD, Rayamajhi M, Salom S, Sands D, Schooler S, Schwarzländer M, Sheppard A, Shaw R, Tipping PW, van Klinken RD (2010) Classical biological control for the protection of natural ecosystems. Biol Control 54:2–33
Wang XG, Hogg BN, Hougardy E, Nance AH, Daane KM (2019) Potential competitive outcomes among three solitary larval endoparasitoids as candidate agents for classical biological control of Drosophila suzukii. Biol Control 130:18–26
Wang X, Daane KM, Hoelmer KA, Lee JC (2020) Biological control of spotted-wing Drosophila: an update on promising agents. In: Garcia FRM (ed) Drosophila suzukii Management. Springer, Cham, pp 143–167
Acknowledgements
We thank Dr. Marc Kenis and Dr. Lukas Seehausen (CABI, Delémont, Switzerland) for providing the initial inoculum to start the rearing of G. brasiliensis G1 Tokio at Fondazione E. Mach.
Funding
Open access funding provided by Fondazione Edmund Mach - Istituto Agrario di San Michele all'Adige within the CRUI-CARE Agreement. The study was carried out within the frame of the Samurai Wasp Action Team (SWAT) project, funded by the Autonomous Province of Trento (Italy).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical approval
There are no ethical concerns regarding the organisms used in this research.
Additional information
Handling Editor: Josep Anton Jaques Miret
Insect releases
The release of the exotic parasitoid G. brasiliensis G1 were conducted under authorization of the Italian Ministry for Ecological Transition (D.M. 17 August 2021 n. 33)
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fellin, L., Grassi, A., Puppato, S. et al. First report on classical biological control releases of the larval parasitoid Ganaspis brasiliensis against Drosophila suzukii in northern Italy. BioControl 68, 1–12 (2023). https://doi.org/10.1007/s10526-022-10174-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10526-022-10174-2