Abstract
Background
Obstructive sleep apnea syndrome (OSAS) is a common disease that has a prevalence of 6 to 13% of the adult population. It is characterized by recurrent obstruction partial or total upper airway and subsequent paroxysmal nocturnal hypoxia, leading to intermittent arousals from sleep and excessive daytime sleepiness.
This work aimed to evaluate the relationship between the hematological parameters in CBC with differential as a new biomarker showing systemic inflammation and as an indicator of OSAS severity.
Patient and methods
This retrospective cross-sectional analysis included 100 subjects with OSA from those attending Chest departments in Benha University Hospital from 2021 to 2022 and 2022 to 2023 period. All patients were subjected to full history taking and clinical examination, electrocardiogram, chest X-ray posteroanterior view, full night of polysomnography, and complete blood count with differential.
Results
There was a statistically significant difference between mild; moderate and severe OSA patients regarding platelets to lymphocyte ratio. A statistically significant difference between mild and severe OSA regarding neutrophil to lymphocyte ratio was found. There was a statistically significant positive correlation between OSA severity and platelet level, N/L, and P/L ratio.
Conclusion
The hematological indices including neutrophil to lymphocyte ratio and platelet to lymphocyte ratio could be alternatives to expensive time-consuming biochemical markers to evaluate the inflammation and severity in the OSAS population.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSAS) is a popular disease with moderate to severe sleep apnea affecting 6 to 13% of adult persons [1]. It is recognized by recurrent partial or complete upper airway closure and subsequent paroxysmal nocturnal hypoxia, resulting in episodic sleep arousals and precipitous daytime sleepiness [2].
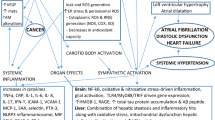
OSA could result in complications such as cardiovascular disorders (CVD) and diabetes [3]. And CVD includes a large proportion of complications [4,5,6]. It is recognized that CVD complications of OSAS patients may be accompanied by endothelial dysfunction, exaggerated oxidative stress, systemic inflammation, and stimulation of the sympathetic system.
The chronic systematic inflammation of OSAS represents a crucial part of the evolution of CVD [12]. Recent research implies that both WBC and NLR are good indices of inflammation [13,14,15,16,17]. Neutrophils chiefly mediate innate immune response by secreting mediators while lymphocytes mediate adaptive immune response by regulating inflammation [18]. Also, some research showed that platelet was activated and aggregated in cases with OSAS, which was also important in inflammation [19, 20].
Recently, studies introduced PLR as a novel inflammatory marker to predict the adverse outcomes of CVD [14,15,16,17, 21]. It is found that HCT was increased in OSAS patients which might be called secondary erythrocytosis and this was suggested in view of the hypoxemic state [22]. Red cell distribution width (RDW), which assessed the variability of erythrocytes, was also reported to be increased in relation to inflammation in OSAS [23].
Patients and methods
This retrospective cross-sectional analysis included 100 patients with OSA from those attending Chest departments in Benha University Hospital from 1 January 2021 to 1 January 2023 period. Inclusion criteria included patients with diagnosed OSA by polysomnography and age equal to or more than 18 years. Exclusion criteria: patients known to have cardiovascular, renal, or hepatic diseases were excluded by history and laboratory (LFTs, KFTs). Patients with hematological disorders and malignancies were excluded by history. Patients diagnosed with obesity hypoventilation were excluded by ABG, complex sleep apnoea, central sleep apnea Cheyne-Stokes sleeping disorder, or REM-induced OSAS were excluded from the PSG results. Written consent was taken from all patients, and all of them were subjected to full history taking and clinical examination, Electrocardiogram. Chest X-ray posteroanterior view, CBC with differential leucocytic count, and a full night of polysomnography with a diagnosis of OSA severity by AHI using Somnoscreen plus PSG, Somnomdics, Germany. All those data were taken from the recordings in the sleep lab. All data were collected, tabulated, and statistically analyzed.
Statistical analysis
The statistical analysis was conducted using the Software, Statistical Package for Social Science, (SPSS Inc. Released 2009-PASW Statistics for Windows Version 26.0. Chicago: SPSS Inc.). All variables were tested for normality of distribution using the Shapiro–Wilk test. All P values were two-sided. Statistical significance was accepted at p value < 0.05. A p value > 0.05 was considered non-significant.
Results
The study population consisted of 100 patients divided into three groups according to disease severity (OSA classification) (mild: 5 ≤ apnea–hypopnea index (AHI) < 15; moderate: 15 ≤ AHI < 30; and severe: 30 ≤ AHI). This article divided patients into three groups according to body mass index (BMI (normal weight: BMI < 25; overweight: 25 ≤ BMI ≤ 30; and obesity: 30 < BMI) (Table 1).
The median age (IQR) was 43 years [39, 51]. About 85% of the study cases were males and 15% were females. Most patients (97%) were under 65 years of age. Median BMI (IQR) was 35 (31.3, 39)}. Seventy-nine patients (79%) were obese (BMI > 30) [35] compared with 16% overweight and 5% normal weight. Median AHI (IQR) was 56.45 (35.35, 77.98). Eighty patients (80%) reported a severe grade of AHI compared with 15% moderate and 5% mild. Median DI (IQR) was 56.9 (29.85, 79.05) (Table 1). We found that the median neutrophile count was 3.94 with IQR of (2.73, 5.33). The median lymphocyte count was 2.7 with IQR of (2.19, 3.25). Median platelet count was 247 with an IQR of (205, 291.25). Median HCT% was 46.85 with IQR of (44.2, 49.6). Median RDW-CV% was 12.03 with IQR of (11.3, 13.08). The median N/L ratio was 1.6 with an IQR of (1.1, 2.2). Median P/L was 94 with IQR of (80, 116.5) (Table 2).
Comparison between mild, moderate, and severe groups of OSA revealed that there were significant differences between the three groups regarding BMI, AHI, DI, and P/L ratio (p = 0.018, 0.001, 0.001, 0.024) respectively, where the severe OSA group recorded the greatest BMI (35.6 compared with 29 and 34), AHI (62.1 compared with 7.5 and 23.5), DI (65.7 compared with 13.8 and 22) and P/L ratio (95 compared with 63 and 95). There was a statistically significant difference between the mild and severe groups regarding the median N/L ratio (p = 0.041) (Table 3).
Spearman’s correlation analysis showed that there was a statistically significant positive correlation between OSA classification and BMI (rho = 0.248, p = 0.013), platelet level (rho = 0.233, p = 0.02), N/L ratio (rho = 0.224, p = 0.025) and P/L ratio (rho = 0.218, p = 0.03). No statistically significant correlation was detected between AHI and other parameters (p > 0.05) (Table 4).
Univariate analysis by ordinal logistic regression revealed that BMI, platelets count, and P/L ratio were significant positive predictors for OSA classification (p = 0.029, 0.048, 0.017) respectively (Table 5).
Discussion
In the current work, there was a statistically significant difference between the three groups of OSA severity regarding platelet to lymphocyte ratio, and there was a statistically significant difference between mild and severe OSA regarding median neutrophil to lymphocyte ratio. There was a statistically significant positive correlation between OSA severity and platelet level, neutrophil to lymphocyte, and platelet to lymphocyte ratios, while the relation between OSA and other parameters (HT, RDW, − CV%, and neutrophils) was statistically nonsignificant. In the current study, univariate analysis by ordinal logistic regression revealed that platelets count, and P/L ratio were the most significant positive predictors for OSA severity (0.048, 0.017) respectively.
The chronic systematic inflammation of OSAS may play an important role in the progression of CVD [12]. Recent studies suggest that both WBC and NLR are good indicators of inflammation [13,14,15,16,17]. Neutrophils mainly mediate innate immune response by secreting mediators while lymphocytes mediate adaptive immune response by regulating inflammation [18]. Besides, some studies reported platelet was activated and aggregated in patients with OSAS, which was also relevant in inflammation [19, 20].
Many, studies introduced PLR as a novel inflammatory marker to predict the adverse outcomes of CVD [14,15,16,17, 21]. In view of hypoxemic states, Choi et al. 2006 found that HCT was elevated in OSAS patients which might be called secondary erythrocytosis [22]. Ozsu, et al. 2012 reported that red cell distribution width (which assessed the variability of erythrocytes) was increased in relation to inflammation in OAS, this disagrees with our results, which show a nonsignificant correlation between OSA severity and both HCT and RWD [23].
Mindan et al. (2018) results showed that there was a positive correlation between the levels of hematological indices including (WBC, LYM, NLR, MPV, PDW, PLR, RDW, and HCT) and the severity of obstructive sleep apnea [23]. These results agreed with our work; which showed a positive correlation between OSA severity and platelets; platelet to lymphocyte and neutrophil to lymphocyte ratios. Mindan et al. (2018) proposed that these hematological indices could be alternatives to markers (like IL6 and CRP) to evaluate the inflammation in OSAS patients, which was useful for assessing the severity of OSAS [24].
The relationship between OSAS and accompanying changes in hematological parameters is complicated and can be explained by the following: acute and chronic hypoxia may be associated with MPV, PDW, and HCT changes. It was found that MPV and PDW were negatively related to average SpO2 and minimum SpO2 and implied that hypoxia could activate platelet function [19]. Rahangdale et al. (2011) demonstrated that a high level of oxygen desaturation was linked with higher platelet surface adhesion molecules, activated glycoprotein receptor expression, platelet-monocyte aggregation, and platelet-neutrophil aggregation. A hypoxemic state is interrelated with high hematocrit levels, as oxyhemoglobin desaturation can stimulate erythropoiesis, leading to increased hematocrit [25]. Svatikova et al. reported that ANP (atrial natriuretic peptide) was increased overnight in those untreated OSAS patients, and ANP levels decreased with CPAP treatment. It indicated that hemoconcentration might lead to increased hematocrit [26]. Another mechanism appears to be sympathetic overactivity. It results in many pathophysiological changes such as recurrent arousals and increased inspiratory effort. OSAS patients exhibited high levels of sympathetic nerve activity even when they were fully awake, which contributed to platelet activation and CVD [27].
Larsson et al. (1989) suggested that platelet aggregability was increased by high levels of circulating catecholamine in vivo. Therefore, hematological indices associated with platelet activation (e.g., PLR) might change in OSAS patients caused by catecholamine discharge [28].
Some researchers declared that nuclear factor kappa B (NF-κB), a master transcription factor that regulated the downstream inflammatory gene expression, was found to be selectively activated by hypoxia and reoxygenation [29]. NF-κB activity also resulted in an increased number of circulating neutrophils and monocytes. And the apoptosis of neutrophils was dysregulated in the process of OSAS [30]. Both lead to elevated levels of neutrophils in the peripheral blood of OSAS patients. As for lymphocytes, OSAS patients combined with CVDs were found to have a lower lymphocyte level compared to those without CVDs, which could be due to the uncontrolled inflammatory pathway [31].
Moreover, some researchers demonstrated that lower lymphocyte counts were related to activation of the hypothalamus-hypophysis-adrenal (THA) axis, increased production of systemic cortisol levels, and altered sleeping habits [32]. The NLR, a novel marker of systemic inflammation, was associated with many chronic diseases and could be an indicator used to predict CVDs in OSAS patients [33]. On the other hand, many pro-inflammatory cytokines, such as IL6, could significantly promote the production and activation of platelets, which contributed to the changes in those hematological parameters including PLR, MPV, and PDW [34].
Conclusion
The hematological indices including neutrophil to lymphocyte ratio and platelet to lymphocyte ratio could be alternatives to expensive time-consuming biochemical markers to evaluate the inflammation and severity in the OSAS population because they were comparatively cheap, readily measurable, easy, and practical laboratory markers.
Limitations
Small study size, not all hematological markers like MPV and PDW were included.
Availability of data and materials
All data generated or analyzed during this study are included in this published article (and its Supplementary Information files).
Change history
29 February 2024
A Correction to this paper has been published: https://doi.org/10.1186/s43168-024-00258-1
Abbreviations
- OSA:
-
Obstructive sleep apnea
- ANP:
-
Atrail natriuretic peptid
- RDW:
-
Red cell distribution width
- THA:
-
Hypothalamus-hypophysis-adrenal
- HCT:
-
Hematocrit
- BMI:
-
Body mass index
- CVD:
-
Cardiovascular disease
- OSAS:
-
Obstructive sleep apnea syndrome
- N/L:
-
Neutrophil lymphocyte ratio
- P/L:
-
Platelet lymphocyte ratio
References
Peppard PE, Young T, Barnet JH et al (2013) Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol 177:1006–1014
American Academy of Sleep Medicine. International Classification of Sleep Disorders. 3rd ed. IL (2014): American Academy of Sleep Medicine.
Dewan NA, Nieto FJ, Somers VK (2015) Intermittent hypoxemia and OSA: implications for comorbidities. Chest 147:266–274
Marin JM, Carrizo SJ, Vicente E et al (2005) Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet 365:1046–1053
Peker Y, Hedner J, Norum J et al (2002) Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med 166:159–165
Dempsey JA, Veasey SC, Morgan BJ et al (2010) Pathophysiology of sleep apnea. Physiol Rev 90:47–112
El Solh AA, Akinnusi ME, Baddoura FH et al (2007) Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med 175:1186–1191
Badran M, Ayas N, Laher I (2014) Cardiovascular complications of sleep apnea: role of oxidative stress. Oxid Med Cell Longev 2014:985258
Jelic S, Padeletti M, Kawut SM et al (2008) Inflammation, oxidative stress, and repair capacity of the vascular endothelium in obstructive sleep apnea. Circulation 117:2270–2278
Garvey JF, Taylor CT, McNicholas WT (2009) Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J 33:1195–1205
Tamisier R, Pepin JL, Remy J et al (2011) 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37:119–128
Nadeem R, Molnar J, Madbouly EM et al (2013) Serum inflammatory markers in obstructive sleep apnea: a meta-analysis. J Clin Sleep Med 9:1003–1012
Turak O, Ozcan F, Isleyen A et al (2013) The usefulness of neutrophil-to-lymphocyte ratio to predict in-hospital outcomes in infective endocarditis. Can J Cardiol 29:1672–1678
Lattanzi S, Cagnetti C, Provinciali L et al (2017) Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget 8:57489–57494
Lattanzi S, Cagnetti C, Rinaldi C et al (2018) Neutrophil-to-lymphocyte ratio improves outcome prediction of acute intracerebral hemorrhage. J Neurol Sci 387:98–102
Lattanzi S, Cagnetti C, Provinciali L et al (2016) Neutrophil-to-lymphocyte ratio predicts the outcome of acute intracerebral hemorrhage. Stroke 47:1654–1657
Yu S, Arima H, Bertmar C et al (2018) Neutrophil to lymphocyte ratio and early clinical outcomes in patients with acute ischemic stroke. J Neurol Sci 387:115–118
de Jager CP, van Wijk PT, Mathoera RB et al (2010) Lymphocytopenia and neutrophil-lymphocyte count ratio predict bacteremia better than conventional infection markers in an emergency care unit. Crit Care 14:R192
Nena E, Papanas N, Steiropoulos P et al (2012) Mean Platelet Volume and Platelet Distribution Width in non-diabetic subjects with obstructive sleep apnoea syndrome: new indices of severity? Platelets 23:447–454
Varol E, Ozturk O, Gonca T et al (2010) Mean platelet volume is increased in patients with severe obstructive sleep apnea. Scand J Clin Lab Invest 70:497–502
Dotsenko O, Chaturvedi N, Thom SA et al (2007) Platelet and leukocyte activation, atherosclerosis, and inflammation in European and South Asian men. J Thromb Haemost 5:2036–2042
Choi JB, Loredo JS, Norman D et al (2006) Does obstructive sleep apnea increase hematocrit? Sleep Breath 10:155–160
Ozsu S, Abul Y, Gulsoy A et al (2012) Red cell distribution width in patients with obstructive sleep apnea syndrome. Lung 190:319–326
Moher D, Liberati A, Tetzlaff J et al (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Rahangdale S, Yeh SY, Novack V et al (2011) The influence of intermittent hypoxemia on platelet activation in obese patients with obstructive sleep apnea. J Clin Sleep Med 7:172–178
Svatikova A, Shamsuzzaman AS, Wolk R et al (2004) Plasma brain natriuretic peptide in obstructive sleep apnea. Am J Cardiol 94:529–532
Abboud F, Kumar R (2014) Obstructive sleep apnea and insight into mechanisms of sympathetic overactivity. J Clin Invest 124:1454–1457
Larsson PT, Hjemdahl P, Olsson G et al (1989) Altered platelet function during mental stress and adrenaline infusion in humans: evidence for an increased aggregability in vivo as measured by filtragometry. Clin Sci (Lond) 76:369–376
Ryan S, Taylor CT, McNicholas WT (2005) Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation 112:2660–2667
Dyugovskaya L, Polyakov A, Lavie P et al (2008) Delayed neutrophil apoptosis in patients with sleep apnea. Am J Respir Crit Care Med 177:544–554
Altunayoglu Cakmak V, Ozsu S, Gulsoy A et al (2016) The significance of the relative lymphocyte count as an independent predictor of cardiovascular disease in patients with obstructive sleep apnea syndrome. Med Princ Pract 25:455–460
Acanfora D, Gheorghiade M, Trojano L et al (2001) Relative lymphocyte count: a prognostic indicator of mortality in elderly patients with congestive heart failure. Am Heart J 142:167–173
Uygur F, Tanriverdi H, Aktop Z et al (2016) The neutrophil-to-lymphocyte ratio in patients with obstructive sleep apnoea syndrome and its relationship with cardiovascular disease. Heart Lung 45:121–125
Norol F, Vitrat N, Cramer E et al (1998) Effects of cytokines on platelet production from blood and marrow CD34+ cells. Blood 91:830–843
Centers for Disease Control and Prevention. About Child and Teen BMI. 2018. Accessed at www.cdc.gov/healthyweight/assessing/bmi/childrens_BMI/about_childrens_BMI.html on June 3, 2020
Acknowledgements
The authors were grateful to all subjects who participated in this study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Rasha writed the discussion. Basma did the statistical analysis. Salwa collected data of the patients.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The Research Ethics Committee of the Faculty of Medicine, Benha University, approved the protocol. Written consent was obtained from the patient’s guardians. The reference number is RC 16–5-2023.
Consent for publication
Nothing to declare.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article has been updated to correct an author name.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hendy, R.M., Hani, B.M. & Mohammed, S.H. Hematological parameters as predictors of OSA severity. Egypt J Bronchol 18, 3 (2024). https://doi.org/10.1186/s43168-023-00252-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43168-023-00252-z