Abstract
Background
Sarcopenia is a syndrome characterized by a progressive decline in muscle mass and strength, with subsequent deterioration of functional performance and increased morbidity and mortality. Its emergence may be associated with disorders that are not limited to the elderly. The multifactorial nature of sarcopenia is a major barrier to diagnosis. Several risk factors contribute to the development of sarcopenia, including age, gender, and amount of physical activity. Additionally, the pathophysiology of sarcopenia involves inflammatory conditions, endocrinal dysfunction, and metabolic alterations. Several studies have proposed numerous molecules that may be linked to the pathogenesis of sarcopenia and could be useful in the future; however, there is an unmet need to discover a sensitive, reliable, and cost-effective biomarker of muscle aging.
Main text
The objective of this research is to highlight different biomarkers of sarcopenia that reflect its multifactorial pathophysiology. A narrative review was carried out through a series of literature searches in the database MEDLINE/PubMed focusing on sarcopenia biomarkers. The following search terms were used: “sarcopenia,” “osteosarcopenia,” “muscle ageing,” “muscle failure,” “sarcopenic obesity,” “weakness,” “biomarkers,” “frailty,” “comorbidity,” “functional disability,” and “inflamm-aging.” The studies were observational and peer-reviewed. They were all carried out at a referral center, hospital, or in the community. The articles chosen all contained information about sarcopenia. Case reports and articles that did not assess people's muscle aging and sarcopenia were not considered.
Conclusion
Despite the availability of numerous functional, imaging, and biological sarcopenia markers, the inherent limitations of the assessment tools make it difficult to objectively measure the various sarcopenia domains. A valid and reliable biomarker of sarcopenia has yet to be identified. The identification of “gold standard” evaluation techniques that should be systematically used is also impacted by the variability of the populations to be assessed. In this context, the establishment of an international consensus adopting a multi-biomarker approach may be of utmost importance to tackle the different aspects of this multifactorial health-related problem.
Similar content being viewed by others
Background
Sarcopenia is a disorder characterized by a generalized decline in muscle mass and strength. It is directly linked to physical impairment, poor quality of life, and high mortality. Although it is primarily a disease of the elderly, other disorders, such as inflammatory conditions, inactivity, and malnutrition, may also contribute to its development [1].
According to reports, sarcopenia affects 5 to 13% of people between the ages of 60 and 70, but it affects 11 to 50% of people over the age of 80. Since the age of 40, people begin to lose 1% to 2% of their muscle annually. At age 70, skeletal muscle mass declines by 25–30%, and muscle strength declines even more noticeably by up to 40% [2].
In 1989, Rosenberg used the term “sarcopenia” (Greek: “sarx” or flesh + “penia” or loss) to describe this age-related loss of muscle mass [3]. In 2008, the term dynapenia was proposed to describe age-related loss of muscle strength in the absence of neurological or muscular disorder. It was linked to the functional impairment of the neuromuscular apparatus [4]. Over the upcoming decades, although many committees published their consensus diagnostic definition of sarcopenia, the most widely recognized one was that proposed by the European Working Group on Sarcopenia in Older People (EWGCOP) [5]. The first definition of sarcopenia that was published by EWGSOP in 2010 aided in the identification and care of people at risk of or suffering from sarcopenia. This operational definition by EWGSOP states that low muscle mass and poor physical performance are necessary for the diagnosis of sarcopenia [6]. Eight years later, an update (EWGSOP2) was released as many aspects of sarcopenia and muscle role in health and diseases were explored. Muscle strength was placed at the top of the diagnostic algorithm, and several measures of muscle strength, muscle mass, and/or physical performance were included with gender-specific cut-off points for some of these measurements for diagnosing sarcopenia in daily clinical practice. Furthermore, EWGSOP2 advised using the sarcopenia questionnaire (SARC-F questionnaire) as a formal method of collecting self-reports from patients with symptoms suggestive of sarcopenia [7].
The multifactorial nature of sarcopenia presents the most difficult diagnostic challenge. There are many risk factors for sarcopenia including age, gender, and level of physical activity [1, 8]. Sarcopenia could be either age-related (primary sarcopenia) or disease-related (secondary sarcopenia). The age-related loss of skeletal muscle mass is caused by a decrease in the number of myofibers and the atrophy of individual myofibers. It affects mainly the fast-twitch muscles in a slow, progressive course, and subsequently, these changes are irreversible [9]. While secondary sarcopenia is mainly due to a decrease in the cross-sectional area of myofibers, it tends to affect the slow-twitch muscles in an acute and severe manner; however, these changes are usually reversible [10]. Moreover, it has been reported that aging is associated with a progressive reduction in the number of motor units and morphological changes in neuromuscular synapses [11]. Additionally, protein synthesis in muscle decreases with aging, and protein anabolism is suppressed in the muscles of the elderly even when the same amounts of amino acids are present in the blood, which is known as anabolic resistance [12]. All the aforementioned changes result in the functional decline of skeletal muscles and muscle atrophy.
Moreover, the pathophysiology of sarcopenia includes inflammatory conditions, obesity and endocrinal dysfunction. Furthermore, muscle-related myokines and cytokines have been linked to autocrine regulation of muscle metabolism, as well as paracrine and endocrine effects on other tissues like bones and fats, a phenomenon known as bone, muscle, and fat cross-talk. A number of chronic diseases and sedentary lifestyle factors (such as malnutrition, obesity, and lack of physical activity) may also contribute to the development of sarcopenia [13,14,15,16].
Despite recent advances in the assessment of muscle mass and strength, the numerous mechanisms underlying the development and prediction of sarcopenia are not fully understood; however, a series of biomarkers that may potentially help characterize the different mechanisms of sarcopenia allows for the identification of those with early sarcopenia and the implementation of a personalized, effective management strategy for the optimal prevention, and treatment of those patients [16,17,18].
Main text
Method: search strategy
A narrative review was carried out by conducting a series of literature searches in the database MEDLINE/PubMed for English language articles focusing on sarcopenia biomarkers. A combination of medical subject headings and keywords was used in the search strategy. The following search terms were used: “sarcopenia,” “osteosarcopenia,” “muscle ageing,” “muscle failure,” “sarcopenic obesity,” “weakness,” “biomarkers,” “frailty,” “comorbidity,” “functional disability,” “inflamm-aging,” and “apoptosis.” Sources published within the last 7 years were given preference. The researchers extracted data using a standardized data collection form, which was then discussed among the authors. The studies were observational and peer-reviewed. The studies were all carried out at a referral center, hospital, or in the community. The articles chosen all contained information about sarcopenia. Case reports and articles that did not assess people's muscle health and sarcopenia were not considered.
Biomarkers of sarcopenia
Actually, the skeletal muscle is no longer considered a simple contractile tissue but an interface of more complex connections. In addition to muscle loss and contractile dysfunction, sarcopenia also includes metabolic and endocrinal alterations as well as low-grade age-related systemic inflammation (also known as “inflamm-aging”). The process of muscle loss involves a significant decline in protein regeneration coupled with an accelerated protein lysis and apoptosis [19,20,21].
A biomarker was defined by the National Institutes of Health Biomarkers Definitions Working Group as “a characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” [22]. According to this definition, the term biomarker refers to a broad sub-category of medical signs that can be measured accurately and with reproducibility. A biomarker could be a simple clinical tool, a specific molecule in the biofluid, or an imaging biomarker where a specific biological feature could be detected by imaging. Specific sarcopenia biomarkers that might be linked to clinical evaluation allow for the detection of subjects suffering from or at risk of developing sarcopenia as well as the monitoring of the efficacy of preventative and therapeutic measures. The ideal biomarker of sarcopenia needs to be accurate, specific, reliable, cost-effective, and available [16, 18]. In the next section, we will discuss some of the biomarkers of sarcopenia based on different pathophysiologic mechanisms.
Muscle mass biomarkers
Muscle mass is the amount of skeletal muscles in the body, while lean body mass refers to the non-adipose tissue mass (total body weight − body fat weight). Several tools, such as anthropometric parameters (e.g., calf circumference, mid-arm muscle circumference), bioelectric impedance analysis (BIA), imaging techniques, and biochemical markers, are used to objectively assess muscle mass [18, 23, 24] (Table 1).
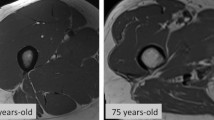
Imaging biomarkers such as dual-energy X-ray absorptiometry (DXA), ultrasonography (US), computed tomography (CT), or magnetic resonance imaging (MRI) are commonly used to qualify muscle or lean body mass. These imaging techniques can provide measures of muscle mass, muscle cross-sectional area (CSA), and muscle density. Skeletal muscle index (SMI) is an important parameter in the assessment of muscle mass. It is calculated by dividing the total appendicular skeletal mass (ASM) (kg) by standing height (m2) [27, 28].
However, each imaging technique has significant shortcomings. Actually, body thickness, hydration level, and extracellular fluid buildup have a significant negative impact on the DXA results. Additionally, DXA cannot quantify intramuscular adipose tissue. On the other hand, high costs, and complicated technology, prevent the widespread use of CT and MRI. The test subject receives substantial doses of ionizing radiation from CT as well. [18] Using anthropometry, BIA, or ultrasonography, although cost-effective and commonly applicable, is either inaccurate or not adequately standardized to be used as a diagnostic tool. This paved the way for the creation and validation of new muscle mass biomarkers that can be measured in biofluid samples and used economically to detect and monitor the condition. Such biochemical markers would also make healthcare professionals more knowledgeable about sarcopenia, eventually encouraging its inclusion in best practices [31].
Muscle-specific biomarkers such as Procollagen type III N-terminal peptide (P3NP), peptides derived from collagen type VI turnover, and skeletal muscle-specific isoform of troponin T (sTnT) were investigated as potential biomarkers of loss of muscle mass, however, their lack of specificity limits their use as reliable makers of sarcopenia [32,33,34].
A recently developed method for precisely measuring total body muscle mass is the D3-creatine dilution method. The technique uses creatine's irreversible conversion to creatinine and the latter’s excretion in urine to estimate the size of the total body creatine pool as an analog for the total mass of skeletal muscles. This method’s estimates of the total body muscle mass exhibit remarkable agreement with whole-body MRI scans [35]. Unlike the DXA, the D3-creatine dilution method's measurement of muscle mass is strongly positively correlated with physical performance and predicts incidents of falls and functional decline [36,37,38,39]. However, urinary creatine excretion is also altered in other organ dysfunctions, such as testicular damage [40].
Members of the transforming growth factor (TGF) superfamily, myostatin, and growth differentiation factor-15 (GDF-15), are antagonists of skeletal muscle myogenesis and growth inhibitors. Myostatin is a myokine secreted from muscle cells and adipose tissues and is involved in the pathogenesis of sarcopenia [41]. The use of antibodies against myostatin was found to improve muscle mass and grip strength [42]. A myostatin inhibitor called folistatin (FST) appears to be an intriguing tool for assessing the amount of muscle damage [43].
Irisin (IR) is a peptide secreted by the skeletal muscles, particularly after exercise. It is produced by the cleavage of a fibronectin type III domain-containing protein 5 (FNDC5). It may be classified as an adipomyokine, as it is secreted by the adipose tissues. Studies demonstrated a significant correlation between the circulating levels of IR, lean mass, and hand grip strength [44]. Interestingly, both obese patients and healthy individuals showed a direct correlation between IR and FST. Additionally, it was found that the expression of IR mRNA positively correlated with FST mRNA expression in muscular biopsies from both groups [45]. These results are very intriguing because they highlight the potential existence of a quiet, mutual relationship between FST and IR in skeletal muscles.
Cathepsin D is an aspartic endopeptidase, a type of lysosomal proteolytic enzyme found in all animal cells. The level of cathepsin D has been found to be significantly higher in the serum of sarcopenic patients relative to normal people [46]. Moreover, a negative relationship was reported between cathepsin D levels and gait speed [46]. In a recent study, when a predictive model with cathepsin D, age, and BMI was created (AUC = 0.908) to enhance its diagnostic performance, the sarcopenia group had levels of cathepsin D that were 2.2 times higher than the control group [47].
Muscle strength biomarkers
Muscle strength is defined as the amount of force generated by a dynamic muscle contraction [48]. The most popular, simple, and widely used tests for assessment of muscle strength of the upper and lower limbs are the Isometric handgrip strength (IHG) by hand dynamometer and the five-time sit-to-stand test (5STS) [49]. Other tests such as isokinetic knee extension and flexion strength have been evaluated in clinical research as predictors of sarcopenia (Table 2).
Low IHG has been associated with poor physical activity, and mobility impairment in cross-sectional [51,52,53] and prospective studies [54, 55]. Moreover, in a study including a well-functioning elderly population, low muscle mass did not explain the strong relationship between strength and mortality, indicating that when estimating the risk of events, muscle strength could be more significant than muscle mass [56]. Additionally, the strong correlations between IHG and lower extremity muscle power, knee extension torque, and calf cross-sectional muscle area highlight the fact that sarcopenia is a generalized rather than a localized disorder [57].
Isokinetic dynamometry is used mainly to assess muscle strength in athletes. Particularly in elderly people with sarcopenia, it can give valuable data about muscle strength [58]. It focuses on lower extremity musculature such as knee extensors and flexors. Isokinetic knee extension strength was measured as a parameter of muscle strength in several studies with participants in a sitting position; the most commonly used angular velocity was 60°/s, and peak torque (Nm) was the most commonly recorded measure [50, 53]. However, cut-off values for knee extension strength are lacking.
Functional biomarkers
Around the third decade of life, physical function starts to decline, with a more severe decrease occurring after the age of 50, which raises the possibility that the initial decline in physical performance may be an early indicator of sarcopenia [59]. However, the term “physical performance” refers to a broad concept that encompasses a number of elements, not just muscle power, strength, and mobility.
Physical performance tests are used in conjunction with muscle strength and mass measurements. They include the gait speed, the Short Physical Performance Battery (SPPB), and the stair climb power test (Table 3). A strong correlation was found between physical performance measures, body composition, and skeletal muscle parameters [60, 61]. In addition, they have the ability to predict health-related outcomes, such as mortality, morbidity, and disability [62,63,64,65]. SPPB in particular had proven to be a reliable and sensitive tool (sensitivity 82%) in diagnosing severe sarcopenia when using the cut-point of ≤ 8 [66]. Unfortunately, each has its own characteristics and only captures specific aspects of muscle functioning, resulting in different sets of possibilities in sarcopenia measurement.
Non-specific sarcopenia biomarkers
Inflammatory biomarkers
Aging is characterized by a state of low-grade systemic inflammation called “inflamm-aging" phenomenon [70]. In this situation, pro-inflammatory cytokines are unregulated, with subsequent decreased anti-inflammatory cytokine levels. Inflammatory aging contributes to accelerated muscle loss and prevents muscle regeneration, which promotes sarcopenia [71]. Actually, decreased muscle mass, strength, and physical function are closely linked to elevated levels of C-reactive protein, tumor necrosis factor, interleukin-8, interleukin-6, granulocyte-monocyte colony-stimulating factor, interferon, and high-temperature requirement serine protease A1 in older adults [72, 73].
Hormonal biomarkers
There is a great deal of evidence that changes in sex hormone levels in the blood may be linked to defects in muscle protein homeostasis. A significant decline in hormonal levels may contribute to a reduction in the ability to synthesize proteins and repair muscle damage with subsequent muscle mass loss, which implies a gradual shifting towards the catabolic state. Studies have shown that the onset of sarcopenia is influenced by sex hormones, particularly testosterone and dehydroepiandrosterone sulfate (DHEAS), whose levels decline with age [74]. Testosterone has anti-catabolic, anti-inflammatory, and anabolic effects on muscle [75]. DHEAS may have an impact on how well muscles function, and its age-related decline is a significant contributor to the loss of muscle mass and strength in older people [74]. Insulin-like growth factor 1 (IGF-1), and growth hormone (GH) levels are also reduced in sarcopenia. IGF-1 is an anabolic hormone that promotes muscle regeneration and mediates the effects of GH. IGF-1 administration has been shown to speed up the functional recovery of injured skeletal muscle [76].
Neuromuscular junction (NMJ) dysfunction biomarkers
Dysfunction of the NMJ is one of the symptoms of sarcopenia [77]. Studies showed that people who are sarcopenic have significantly more circulating C-terminal agrin fragments (CAF) than people who are not sarcopenic [78, 79]. Agrin binds to acetylcholine receptors at the postsynaptic terminal, where it aggregates them as a crucial part of the neuromuscular junction. Agrin is cleaved into CAF22 by proteolytic cleavage in sarcopenia and other catabolic disorders, which results in dysfunction of the NMJ. In patients with pulmonary diseases, sarcopenia has been linked to an increase in CAF22 levels [80]. Furthermore, serum CAF22 levels were consistently higher in accelerated sarcopenic patients than in healthy subjects. However, serum CAF22 levels did not correlate with either the SPPB or the SARC-F questionnaire [17].
Metabolic biomarkers
Several metabolic biomarkers known as metabolomics were strongly linked with muscle mass and quality in the elderly. In particular, lower plasma concentrations of the branched-chain amino acids leucine and isoleucine were found in sarcopenic older individuals [81]. Additionally, it was reported that the circulating levels of essential amino acids were lower in frail older people compared to their non-frail peers [82].
The gut microbiota appears to play a role in regulating several muscle metabolic pathways [83]. However, the causal correlations between age-related changes in muscles and gut microbiota had not been clearly investigated. The age-related disruption of the barrier function of the gut mucosa and subsequent gut dysbiosis may trigger inflammation and contribute to immune system dysregulation [84]. Animal studies demonstrated that a lack of gut microbiota was associated with a reduction in muscle mass [85, 86]. Similarly, studies on antibiotics that alter the microbiota, such as metronidazole, found a significant decrease in muscle mass in the hind limb and muscle fiber volume in the tibialis anterior muscle of mice. In vitro studies have also revealed that gut microbial products like indoxyl sulfate and p-cresol sulfate can have a direct effect on muscle mass [87].
Artificial intelligence (AI) as a biomarker of sarcopenia
In 2021, Chung et al. developed an AI diagnostic model of sarcopenia using transcriptome datasets that include a large number of different genes in muscle biopsies from sarcopenic patients and age-matched healthy subjects across three different ethnic groups. The model had the ability to successfully diagnose sarcopenia accurately (100% sensitivity, 94.12% specificity, and 95.83% accuracy) [88]. Moreover, AI may have a valuable role in the accurate measurement of imaging parameters of sarcopenia, such as abdominal musculature segmentation with deep learning, which provides a great chance to assess muscle mass and myosteatosis independently [29, 89]. It is worth noting that AI-assisted body composition measurement would improve the efficacy and accuracy of the sarcopenia assessment, decrease the inter-examiner variability, and aid in the establishment of normal reference cut-off values for different populations using a broader set of data via an AI-assisted technique, which could have a role in the development of standardized assessments [90,91,92,93].
Conclusion
The broad concept of a sarcopenia biomarker as an objective tool that can assess different sarcopenia domains with precision and reproducibility has allowed different clinical, laboratory, and imaging tools to emerge as potentially promising sarcopenia biomarkers. However, most of these tools lack a standardized quantitative cutoff value to define sarcopenia, which seems essential to predicting early sarcopenia and determining the treatment threshold.
Furthermore, it is challenging to accurately and consistently measure various aspects of sarcopenia due to the inherent limitations of the current assessment tools, such as their lack of specificity and variability based on different population characteristics. In this context, the establishment of an international consensus adopting a multi-biomarker approach may be of utmost importance to tackle the different aspects of this multifactorial health-related problem.
Availability of data and materials
The data sets used are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
Artificial intelligence
- ASM:
-
Appendicular skeletal mass
- AUC:
-
Area under the ROC curve
- BIA:
-
Bioelectric impedance analysis
- CAF:
-
C-terminal agrin fragments
- CC:
-
Calf circumference
- CSA:
-
Cross-sectional areas
- CT:
-
Computed tomography
- DHEAS:
-
Dehydroepiandrosterone sulfate
- DXA:
-
Dual-energy X-ray absorptiometry
- EWGCOP:
-
European Working Group on Sarcopenia in Older People
- FNDC5:
-
Fibronectin type III domain-containing protein 5
- FST:
-
Folistatin
- GDF-15:
-
Growth differentiation factor-15
- GH:
-
Growth hormone
- IGF-1:
-
Insulin-like growth factor 1
- IHG:
-
Isometric handgrip strength
- IR:
-
Irisin
- NMJ:
-
Neuromuscular junction
- MAMC:
-
Mid-arm circumference
- mRNA:
-
Messenger ribonucleic acid
- MRI:
-
Magnetic resonance imaging
- P3NP:
-
Procollagen type III N-terminal peptide
- SARC-F:
-
Sarcopenia questionnaire
- SMI:
-
Skeletal muscle index
- SPPB:
-
Short Physical Performance Battery
- STnT:
-
Skeletal muscle-specific isoform of troponin T
- TGF:
-
Transforming growth factor
- US:
-
Ultrasonography.
- 5STS:
-
Five-time sit-to-stand test
- °/s:
-
Degree per second
References
Marzetti E, Calvani R, Tosato M, Cesari M, di Bari M, Cherubini A, Collamati A, d’Angelo E, Pahor M, Bernabei R et al (2017) Sarcopenia: an overview. Aging Clin Exp Res 29:11–17
Papadopoulou SK (2020) Sarcopenia: a contemporary health problem among older adult populations. Nutrients 12(5):1293
Rosenberg IH (1997) Sarcopenia: origins and clinical relevance. J Nutr 127:990S-991S
Clark BC, Manini TM (2008) Sarcopenia ≠ dynapenia. J Gerontol A Biol Sci Med Sci 63(8):829–834
Dupuy C, Lauwers-Cances V, Guyonnet S, Gentil C, van Kan GA, Beauchet O, Schott AM, Vellas B, Rolland Y (2015) Searching for a relevant definition of sarcopenia: results from the cross-sectional EPIDOS study. J Cachexia Sarcopenia Muscle 6:144–154
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M (2010) Sarcopenia: European consensus on definition and diagnosis-report of the European working group on Sarcopenia in older people. Age Ageing 39:412–423
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyère O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Bahat G, Cruz-Jentoft A (2019) Putting sarcopenia at the forefront of clinical practice. Eur J Geriatr Gerontol 1:43–45
Verdijk LB, Koopman R, Schaart G, Meijer K, Savelberg HH, van Loon LJ (2007) Satellite cell content is specifically reduced in type II skeletal muscle fibers in the elderly. Am J Physiol Endocrinol Metab 292:E151–E157
Wang Y, Pessin JE (2013) Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 16:243–250
Lepore E, Casola I, Dobrowolny G, Musarò A (2019) Neuromuscular junction as an entity of nerve-muscle communication. Cells 8:906
Burd NA, Gorissen SH, van Loon LJ (2013) Anabolic resistance of muscle protein synthesis with aging. Exerc Sport Sci Rev 41:169–173
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217
Picca A, Ponziani FR, Calvani R, Marini F, Biancolillo A, Coelho-Júnior HJ, Gervasoni J, Primiano A, Putignani L, Del Chierico F et al (2019) Gut microbial, inflammatory and metabolic signatures in older people with physical frailty and sarcopenia: results from the Biosphere study. Nutrients 12:65
Calvani R, Picca A, Marini F, Biancolillo A, Cesari M, Pesce V, Lezza AMS, Bossola M, Leeuwenburgh C, Bernabei R et al (2018) The “Biomarkers associated with Sarcopenia and Physical frailty in Elderly persons” (Biosphere) study: rationale, design and methods. Eur J Intern Med 56:19–25
Picca A, Calvani R, Cesari M, Landi F, Bernabei R, Coelho-Júnior HJ, Marzetti E (2020) Biomarkers of physical frailty and sarcopenia: coming up to the place? Int J Mol Sci 21(16):5635
Qaisar R, Karim A, Muhammad T et al (2021) Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep 11:8632
Tosato M, Marzetti E, Cesari M, Savera G, Miller RR, Bernabei R, Landi F, Calvani R (2017) Measurement of muscle mass in sarcopenia: from imaging to biochemical markers. Aging Clin Exp Res 29(1):19–27
Pascual-Fernández J, Fernández-Montero A, Córdova-Martínez A, Pastor D, Martínez-Rodríguez A, Roche E (2020) Sarcopenia: molecular pathways and potential targets for intervention. Int J Mol Sci 21(22):8844
Kalinkovich A, Livshits G (2017) Sarcopenic obesity or obese sarcopenia: a cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev 35:200–221
Tournadre A, Vial G, Capel F, Soubrier M, Boirie Y (2019) Sarcopenia. Joint Bone Spine 86(3):309–314
Biomarkers Definition Working Group Biomarkers and surrogate endpoints (2001) preferred definitions and conceptual framework. Clin Pharmacol Therapeutics 69:89–95
Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R et al (2016) Sarcopenia in daily practice: assessment and management. BMC Geriatr 16(1):170
González-Correa CH, Pineda-Zuluaga MC, Marulanda-Mejía F (2020) Skeletal muscle mass by bioelectrical impedance analysis and calf circumference for sarcopenia diagnosis. J Electr Bioimpedance 11(1):57–61
de Onis M, Habicht JP (1996) Anthropometric reference data for international use: recommendations from a World Health Organization Expert Committee. Am J Clin Nutr 64:650–658
Akın S, Mucuk S, Öztürk A et al (2015) Muscle function-dependent sarcopenia and cut-off values of possible predictors in community-dwelling Turkish elderly: calf circumference, midarm muscle circumference and walking speed. Eur J Clin Nutr 69(10):1087–1090
Sergi G, Trevisan C, Veronese N, Lucato P, Manzato E (2016) Imaging of sarcopenia. Eur J Radiol 85(8):1519–1524
Chianca V, Albano D, Messina C et al (2022) Sarcopenia: imaging assessment and clinical application. Abdom Radiol 47:3205–3216
Amini B, Boyle SP, Boutin RD, Lenchik L (2019) Approaches to assessment of muscle mass and myosteatosis on computed tomography: a systematic review. J Gerontol A Biol Sci Med Sci 74(10):1671–1678
Albano D, Messina C, Vitale J, Sconfienza LM (2020) Imaging of sarcopenia: old evidence and new insights. Eur Radiol 30(4):2199–2208
Ladang A, Beaudart C, Reginster JY et al (2023) Biochemical markers of musculoskeletal health and aging to be assessed in clinical trials of drugs aiming at the treatment of sarcopenia: consensus paper from an expert group meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d’Expérimentation en Santé (CARES SPRL), under the auspices of the world health organization collaborating center for the epidemiology of musculoskeletal conditions and aging. Calcif Tissue Int 112:197–217
Van der Voort EAM, Wakkee M, Veldt-Kok P, Darwish Murad S, Nijsten T (2017) Enhanced liver fibrosis test in patients with psoriasis, psoriatic arthritis and rheumatoid arthritis: a cross-sectional comparison with procollagen-3 N-terminal peptide (P3NP). Br J Dermatol 176:1599–1606
Nedergaard A, Sun S, Karsdal MA, Henriksen K, Kjaer M, Lou Y et al (2013) Type VI collagen turnover-related peptides-novel serological biomarkers of muscle mass and anabolic response to loading in young men. J Cachexia Sarcopenia Muscle 4:267–275. https://doi.org/10.1007/s13539-013-0114-x
Willumsen N, Bager C, Karsdal MA (2019) Matrix metalloprotease generated fragments of type VI collagen have serum biomarker potential in cancer—a proof of concept study. Transl Oncol 12:693–698
Clark RV, Walker AC, Miller RR, Semmes RLO, Ravussin E, Cefalu WT (2018) Creatine (methyl-d3) dilution in urine for estimation of total body skeletal muscle mass: accuracy and variability vs. MRI and DXA J Appl Physiol 124:1–9
Cawthon PM, Orwoll ES, Peters KE, Ensrud KE, Cauley JA, Kado DM, Stefanick ML, Shikany JM, Strotmeyer ES, Glynn NW, Caserotti P, Shankaran M, Hellerstein M, Cummings SR, Evans WJ, Osteoporotic Fractures in Men (MrOS) Study Research Group (2019) Strong relation between muscle mass determined by D3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol A Biol Sci Med Sci 74(6):844–852
Cawthon PM, Blackwell T, Cummings SR, Orwoll ES, Duchowny KA, Kado DM, Stone KL, Ensrud KE, Cauley JA, Evans WJ (2021) Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J Gerontol A Biol Sci Med Sci 76(1):123–130
Zanker J, Patel S, Blackwell T, Duchowny K, Brennan-Olsen S, Cummings SR, Evans WJ, Orwoll ES, Scott D, Vogrin S, Cauley JA, Duque G, Cawthon PM, Osteoporotic Fractures in Men (MrOS) Study Group (2020) Walking speed and muscle mass estimated by the D3-creatine dilution method are important components of sarcopenia associated with incident mobility disability in older men: a classification and regression tree analysis. J Am Med Dir Assoc 21(12):1997-2002.e1
Orwoll ES, Peters KE, Hellerstein M, Cummings SR, Evans WJ, Cawthon P (2020) The Importance of muscle versus fat mass in sarcopenic obesity: a re-evaluation using D3-Creatine muscle mass versus DXA lean mass measurements. J Gerontol Ser A Boil Sci Med Sci 75:1362–1368
Nahas K, le Net JL, Provost JP, Tomaszewski KE (1993) An investigation of urinary creatine excretion as a potential marker for testicular damage. Hum Exp Toxicol 12:173–176
Baczek J, Silkiewicz M, Wojszel ZB (2020) Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 12(8):2401
Choi SJ, Lee MS, Kang DH, Ko GJ, Lim HS, Yu BC, Park MY, Kim JK, Kim CH, Hwang SD, Kim JC, Won CW, An WS (2021) Myostatin/appendicular skeletal muscle mass (ASM) ratio, not myostatin, is associated with low handgrip strength in community-dwelling older women. Int J Environ Res Public Health 18(14):7344
Skrzypczak D, Skrzypczak-Zielińska M, Ratajczak AE, Szymczak-Tomczak A, Eder P, Słomski R, Dobrowolska A, Krela-Kaźmierczak I (2021) Myostatin and follistatin-new kids on the block in the diagnosis of sarcopenia in IBD and possible therapeutic implications. Biomedicines 9(10):1301
Chang JS, Kim TH, Nguyen TT, Park KS, Kim N, Kong ID (2017) Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int 17(11):2266–2273
Vamvini MT, Aronis KN, Panagiotou G, Huh JY, Chamberland JP, Brinkoetter MT, Petrou M, Christophi CA, Kales SN, Christiani DC, Mantzoros CS (2013) Irisin mRNA and circulating levels in relation to other myokines in healthy and morbidly obese humans Eur. J Endocrinol 169:829–834
Nagano K (2015) Alteration of cathepsin-D expression in atrophied muscles and apoptotic myofibers by hindlimb unloading in a low-temperature environment. J Phys Ther Sci 27(11):3585–3591
L’hôte C, Cordier B, Labasse A, Boileau C, Costes B, Henrotin Y (2021) Identification of new biomarkers for sarcopenia and characterization of cathepsin D biomarker. JCSM Rapid Commun 4:122–132
Bergquist R, Weber M, Schwenk M, Ulseth S, Helbostad JL, Vereijken B, Taraldsen K (2019) Performance-based clinical tests of balance and muscle strength used in young seniors: a systematic literature review. BMC Geriatr 19:9
Beaudart C, Rolland Y, Cruz-Jentoft AJ et al (2019) Assessment of muscle function and physical performance in daily clinical practice. Calcif Tissue Int 105:1–14
Binder EF, Schechtman KB, Ehsani AA et al (2002) Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc 50(12):1921–2198
Wiśniowska-Szurlej A, Ćwirlej-Sozańska A, Wołoszyn N, Sozański B, Wilmowska-Pietruszyńska A (2019) Association between handgrip strength, mobility, leg strength, flexibility, and postural balance in older adults under long-term care facilities. Biomed Res Int 23(2019):1042834
Yu H, Chen X, Dong R, Zhang W, Han P, Kang L, Ma Y, Jia L, Fu L, Hou L et al (2019) Clinical relevance of different handgrip strength indexes and cardiovascular disease risk factors: a cross-sectional study in suburb-dwelling elderly Chinese. J Formos Med Assoc 118:1062–1072
Martien S, Delecluse C, Boen F, Seghers J, Pelssers J, Van Hoecke A-S, Van Roie E (2015) Is knee extension strength a better predictor of functional performance than handgrip strength among older adults in three different settings? Arch Gerontol Geriatr 60:252–258
McLean RR, Shardell MD, Alley DE, Cawthon PM, Fragala MS, Harris TB, Kenny AM, Peters KW, Ferrucci L, Guralnik JM et al (2014) Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: The foundation for the National Institutes of Health (FNIH) sarcopenia project. J Gerontol Ser A Boil Sci Med Sci 69:576–583
Rantanen T, Avlund K, Suominen H, Schroll M, Frändin K, Pertti E (2002) Muscle strength as a predictor of onset of ADL dependence in people aged 75 years. Aging Clin Exp Res 14:10–15
Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, Tylavsky FA, Rubin SM, Harris TB (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61(1):72–77
Lauretani F, Russo CR, Bandinelli S, Bartali B, Cavazzini C, Di Iorio A, Corsi AM, Rantanen T, Guralnik JM, Ferrucci L (2003) Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985) 95(5):1851–1860
Van Roie E, Verschueren SM, Boonen S et al (2011) Force-velocity characteristics of the knee extensors: an indication of the risk for physical frailty in elderly women. Arch Phys Med Rehabil 92(11):1827–1832
Ferrucci L, Cooper R, Shardell M, Simonsick EM, Schrack JA, Kuh D (2016) Age-Related Change in mobility: perspectives from life course epidemiology and geroscience. J Gerontol A Biol Sci Med Sci 71(9):1184–1194
Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB (2002) Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: the health, aging and body composition study. J Am Geriatr Soc 50(5):897–904
Delmonico MJ, Harris TB, Lee JS, Visser M, Nevitt M, Kritchevsky SB, Tylavsky FA, Newman AB (2007) Alternative definitions of sarcopenia, lower extremity performance, and functional impairment with aging in older men and women. J Am Geriatr Soc 55(5):769–774
Newman AB, Simonsick EM, Naydeck EM, Kritchevsky SB, Nevitt M, Pahor M, Satterfield S, Brach JS, Studenski SA, Harris TB (2006) Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA 295(17):2018–2026
Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332(9):556–561
Cesari M, Kritchevsky SB, Penninx BWJH, Nicklas BJ, Simonsick EM, Newman AB, Tylavsky F, Brach JS, Satterfield S, Bauer DC, Visser M, Rubin S, Harris TB, Pahor M (2005) Prognostic value of usual gait speed in well-functioning older people-results from the health, aging and body composition study. J Am Geriatr Soc 53:1675–1680
Rolland Y, Lauwers-Cances V, Cesari M, Vellas B, Pahor M, Grandjean H (2006) Physical performance measures as predictors of mortality in a cohort of community-dwelling older French women. Eur J Epidemiol 21(2):113–122
Phu S, Kirk B, Bani Hassan E et al (2020) The diagnostic value of the Short Physical Performance Battery for sarcopenia. BMC Geriatr 20:242
Wall JC, Bell C, Campbell S, Davis J (2000) The Timed Get-up-and-Go test revisited: measurement of the component tasks. J Rehabil Res Dev 37(1):109–113
Treacy D, Hassett L (2018) The short physical performance battery. J Physiother 64(1):61
Ni M, Brown LG, Lawler D, Bean JF (2017) Reliability, validity, and minimal detectable change of four-step stair climb power test in community-dwelling older adults. Phys Ther 97(7):767–773
Liang Z, Zhang T, Liu H, Li Z, Peng L, Wang C, Wang T (2022) Inflammaging: the ground for sarcopenia? Exp Gerontol 15(168):111931
Pan L, Xie W, Fu X, Lu W, Jin H, Lai J, Zhang A, Yu Y, Li Y, Xiao W (2021) Inflammation and sarcopenia: a focus on circulating inflammatory cytokines. Exp Gerontol 15(154):111544
Rong YD, Bian AL, Hu HY, Ma Y, Zhou XZ (2018) Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr 18(1):308
Wu J, Lin S, Chen W et al (2023) TNF-α contributes to sarcopenia through caspase-8/caspase-3/GSDME-mediated pyroptosis. Cell Death Discov 9:76
Shin MJ, Jeon YK, Kim IJ (2018) Testosterone and sarcopenia. World J Mens Health 36(3):192–198
Wagers AJ, Conboy IM (2005) Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell 122:659–667. https://doi.org/10.1016/j.cell.2005.08.021
Bartke A (2019) Growth hormone and aging: updated review. World J Mens Health 37(1):19–30
Sataranatarajan K, Qaisar R, Davis C, Sakellariou GK, Vasilaki A, Zhang Y, Liu Y, Bhaskaran S, McArdle A, Jackson M et al (2015) Neuron specific reduction in CuZnSOD is not sufficient to initiate a full sarcopenia phenotype. Redox Biol 5:140–148
Marzetti E, Calvani R, Lorenzi M, Marini F, D’Angelo E, Martone AM, Celi M, Tosato M, Bernabei R, Landi F (2014) Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol 60:79–82
Hettwer S, Dahinden P, Kucsera S, Farina C, Ahmed S, Fariello R, Drey M, Sieber CC, Vrijbloed JW (2013) Elevated levels of a C-terminal agrin fragment identifies a new subset of sarcopenia patients. Exp Gerontol 48:69–75. https://doi.org/10.1016/j.exger.2012.03.002
Qaisar R, Karim A, Muhammad T, Shah I (2020) Circulating biomarkers of accelerated sarcopenia in respiratory diseases. Biology 9:322
Adav SS, Wang Y (2021) Metabolomics signatures of aging: recent advances. Aging Dis 12(2):646–661
Calvani R, Picca A, Marini F, Biancolillo A, Gervasoni J, Persichilli S, Primiano A, Coelho-Junior HJ, Bossola M, Urbani A, Landi F, Bernabei R, Marzetti E (2018) A distinct pattern of circulating amino acids characterizes older persons with physical frailty and sarcopenia: results from the biosphere study. Nutrients 10(11):1691
Li G, Jin B, Fan Z (2022) Mechanisms involved in gut microbiota regulation of skeletal muscle. Oxid Med Cell Longev 18(2022):2151191
Alsegiani AS, Shah ZA (2022) The influence of gut microbiota alteration on age-related neuroinflammation and cognitive decline. Neural Regen Res 17(11):2407–2412
Huang WC, Chen YH, Chuang HL, Chiu CC, Huang CC (2019) Investigation of the effects of microbiota on exercise physiological adaption, performance, and energy utilization using a gnotobiotic animal model. Front Microbiol 20(10):1906
Blanton LV, Charbonneau MR, Salih T, Barratt MJ, Venkatesh S, Ilkaveya O, Subramanian S, Manary MJ, Trehan I, Jorgensen JM, Fan YM, Henrissat B, Leyn SA, Rodionov DA, Osterman AL, Maleta KM, Newgard CB, Ashorn P, Dewey KG, Gordon JI (2016) Gut bacteria that prevent growth impairments transmitted by microbiota from malnourished children. Science 351(6275):aad3311. https://doi.org/10.1126/science.aad3311
De Spiegeleer A, Elewaut D, Van Den Noortgate N, Janssens Y, Debunne N, Van Langenhove S, Govindarajan S, De Spiegeleer B, Wynendaele E (2020) Quorum sensing molecules as a novel microbial factor impacting muscle cells. Biochim Biophys Acta Mol Basis Dis 1866(3):165646
Chung H, Jo Y, Ryu D, Jeong C, Choe SK, Lee J (2021) Artificial-intelligence-driven discovery of prognostic biomarker for sarcopenia. J Cachexia Sarcopenia Muscle 12(6):2220–2230
Blanc-Durand P, Schiratti JB, Schutte K et al (2020) Abdominal musculature segmentation and surface prediction from CT using deep learning for sarcopenia assessment. Diagn Interv Imaging 101(12):789–794
Paris MT, Tandon P, Heyland DK et al (2020) Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr 39(10):3049–3055
Burns JE, Yao J, Chalhoub D, Chen JJ, Summers RM (2020) A machine learning algorithm to estimate sarcopenia on abdominal CT. Acad Radiol 27(3):311–320
Feng Z, Rong P, Luo M, Sun X, Wang W (2019) Influence of methods used to establish sarcopenia cutoff values for skeletal muscle measures using unenhanced and contrast-enhanced computed tomography images. JPEN J Parenter Enteral Nutr 43(8):1028–1036
Dong Q (2020) Fully-automated segmentation of muscle measurement on CT in detecting central sarcopenia: a trend of standardization. Acad Radiol 27(3):321–322
Acknowledgements
Not applicable.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
All authors shared in writing and reviewing the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’ s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El-Sebaie, M., Elwakil, W. Biomarkers of sarcopenia: an unmet need. Egypt Rheumatol Rehabil 50, 45 (2023). https://doi.org/10.1186/s43166-023-00213-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-023-00213-w