Abstract
In clinical trials, biochemical markers provide useful information on the drug’s mode of action, therapeutic response and side effect monitoring and can act as surrogate endpoints. In pharmacological intervention development for sarcopenia management, there is an urgent need to identify biomarkers to measure in clinical trials and that could be used in the future in clinical practice. The objective of the current consensus paper is to provide a clear list of biochemical markers of musculoskeletal health and aging that can be recommended to be measured in Phase II and Phase III clinical trials evaluating new chemical entities for sarcopenia treatment. A working group of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) proposed classifying biochemical markers into 2 series: biochemical markers evaluating musculoskeletal status and biochemical markers evaluating causal factors. For series 1, the group agreed on 4 biochemical markers that should be assessed in Phase II or Phase III trials (i.e., Myostatin-Follistatin, Brain Derived Neurotrophic Factor, N-terminal Type III Procollagen and Serum Creatinine to Serum Cystatin C Ratio – or the Sarcopenia Index). For series 2, the group agreed on 6 biochemical markers that should be assessed in Phase II trials (i.e., the hormones insulin-like growth factor-1 (IGF-I), dehydroepiandrosterone sulphate, and cortisol, and the inflammatory markers C-reactive protein (CRP), interleukin-6 and tumor necrosis factor-α), and 2 in Phase III trials (i.e., IGF-I and CRP). The group also proposed optional biochemical markers that may provide insights into the mode of action of pharmacological therapies. Further research and development of new methods for biochemical marker assays may lead to the evolution of these recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
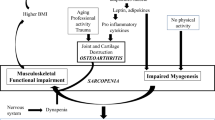
Sarcopenia is a progressive and generalized skeletal muscle disorder defined by low levels of measures of muscle strength, muscle quantity/quality and physical performance [1]. Sarcopenia is related to important negative clinical outcomes, with a series of delicate economic and social implications, including impaired mobility, loss of quality of life, institutionalization, hospitalization and death [2,3,4,5,6]. Given these critical consequences linked to sarcopenia, several pharmacological interventions have been studied in the past few years to understand whether they might be effective for sarcopenia. In April 2020, no fewer than 44 interventional trials studying pharmacological interventions for sarcopenia were indexed in the clinicaltrials.gov database [7]. However, to date, there are still no therapeutic indications for sarcopenia that are accepted by regulatory agencies in the US and Europe [8].
Nevertheless, in consideration of the continued rapid development of therapeutic strategies to slow down the development or reverse the sarcopenia process, it has become urgent to address issues related to the optimal conduct of clinical trials in this area, in particular, the identification of biomarkers to be measured in trials. A biomarker is defined as “a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention” [9]. Biomarkers can include soluble analytes measured in biospecimens such as blood or urine and anatomic biomarkers such as muscle mass or muscle strength measurements. This specific report will focus on soluble biomarkers, also called biochemical markers. In drug development and clinical trials, biochemical markers are useful, among other things, to increase knowledge about the mode of action of a drug, monitor the (early) therapeutic response of treatment, monitor side effects and act as potential surrogate endpoints [10]. Biochemical markers are also intended to be correlated with the evolution of “hard” clinical endpoints following treatment, such as loss of mobility and falls. Because of the complexity of sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP2), which provides the very latest consensus definition of sarcopenia, suggests the need to develop a panel of biomarkers, as it is unlikely that one single biomarker could be specific enough [1].
To address this topic, the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) organized a working group meeting including well-recognized key leaders. The working group members’ expertise and knowledge were shared to gain an understanding of the currently available biochemical markers that could be used in clinical trials of drugs for sarcopenia. The objective of the current consensus paper is to provide a clear list of biochemical markers of musculoskeletal status and the pathophysiological mechanisms of sarcopenia that are recommended in 2022 to be assessed in phase II and phase III clinical trials of drugs aimed at managing sarcopenia.
Methods
Literature Reviews and Meeting Consensus
The European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Disorders (ESCEO ASBL) jointly with the Centre Académique de Recherche et d’Expérimentation en Santé (CARES SRL) organized in September 2022, under the auspices of the World Health Organization Collaborating Center for Epidemiology of Musculoskeletal Health and Aging, a working group including scientists, specialists in laboratory medicine and clinician experts in the field of biochemical markers and sarcopenia as well as representatives of the regulatory bodies. The methodology employed in several other publications emerging from different ESCEO working groups was replicated [7, 11,12,13,14]. Three members of the present working group (EC, AL and AM) first prepared, before the meeting, a literature review to identify potential biochemical markers of sarcopenia to be assessed in clinical trials of drugs and possibly in clinical practice in the future. Literature reviews and original studies (either observational or interventional) published until September 2022 were searched on Medline and Scopus using a combination of the following MeSH terms and keywords: (Biomarkers (MeSH) OR [biologic* OR biochemical or laboratory or clinical or serum or surrogate) AND marker*] OR biomarker*) AND (Sarcopenia (MeSH) OR sarcopeni* OR EWGSOP). Additional studies were identified by a manual search of the bibliographies of relevant papers. Experts in the field were also requested to provide additional references. Finally, a free web search on Google Scholar was also performed.
The three members involved in this literature search were asked to prepare a presentation summarizing their findings and to make some preliminary recommendations to be discussed during the meeting.
Then, all experts met during a face-to-face meeting to discuss recommendations for the selection of biochemical markers of musculoskeletal status and the pathophysiological mechanisms of sarcopenia to be assessed in clinical trials of drugs aimed at the management of sarcopenia. The discussion ended when all experts reached a consensus and agreed on the conclusion of the manuscript. The general plan of the manuscript was also discussed and agreed upon by all. The core writing group (AL, CB, RR and EC) provided the first version of the manuscript, and all experts were invited to offer comments and corrections and ultimately approve its contents.
Clinical and Analytical Performances of Biochemical Markers
To be recommended in this paper, the biochemical markers examined needed to encounter clinical evidence as well as analytical performance. Among clinical evidence, the considered biochemical markers were required to be either increased or decreased in patients suffering from sarcopenia or either modified by non-pharmacological strategies aimed at sarcopenia management in older adults. Biochemical markers showing correlations with outcome that are available for phase II studies aimed at sarcopenia management as defined in our previous ESCEO recommendations [7] were also considered. Additionally, the selected biochemical markers were classified into one of the following four categories: useful for stratification of the disease, for monitoring of the disease, to assess response to treatment or to assess drug mode of action. As the current consensus paper did not aim to update the definition of sarcopenia, biochemical markers that only offer a diagnostic perspective of sarcopenia without any known physio-pathological explanation were not listed in the recommendations. Regarding analytical performances, to be selected for recommendations, the biomarkers should be measurable in an accurate and reproducible manner with a widely available method. The blood matrix was preferred over other matrices. Additional but not mandatory criteria were the existence of a standardized method, suitability for high-throughput analysis, feasibility of preanalytical conditions, existence of literature defining biological variation (available through https://biologicalvariation.eu) and other technical limitations.
Selection of Biochemical Markers
The group has identified two sets of biochemical markers to be assessed in phase II or phase III pharmacological trials on sarcopenia. The first category of biochemical markers evaluating musculoskeletal status comprises biochemical markers of muscle mass, neuro-muscular junction, muscle turnover and myokines. The second set comprises biochemical markers evaluating nonmuscle-specific pathophysiological mechanisms, also referred to hereafter as causal factors. This second set includes three subclasses: adipokines, hormones, and inflammatory biochemical markers. At least one biochemical marker per subclass for each set, except for muscle mass biochemical markers (see related section), should be selected if the pharmacological trial is a phase II or a phase III trial. Recommendations for the selection of these chemical biochemical markers, as well as their time point assessment, are summarized in Table 1.
Set 1. Musculoskeletal biochemical markers
This first set of biochemical markers is intended to evaluate the musculoskeletal status of sarcopenic patients. Ideally, biochemical markers included in this set should be highly specific to muscle, associated with muscle mass or strength, and sensitive to interventional trials. Additionally, the previous “ESCEO update on recommendations for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia” identified several biochemical markers of “muscle-bone interaction” as potentially applicable outcomes for phase II studies [7]. Major clinical evidence and analytical constraints for using this set of musculoskeletal biochemical markers in pharmacological trials are reported in Tables 2 and 3.
Biochemical Markers Specific to Muscle Mass
In addition to imaging techniques, a few biochemical tools, namely, the deuterium-labelled creatine (D3-Cr) dilution test and sarcopenia index (SI), were developed to evaluate muscle mass. In pharmacological trials, biochemical markers specific to muscle mass are expected to provide additional information that may help in patient risk stratification. Of note, given the poor specificity of SI to muscle mass, this ratio has been reclassified in the muscle turnover subclass.
The D3 creatine dilution test has been developed to ensure an analytical quantification of muscle mass, where imaging techniques are more considered to reflect fat-free mass or bone- and fat-free lean mass [15]. Thus, although well correlated, dual-energy X-ray absorptiometry (DXA) and D3-Cr dilution tests should not be considered equivalent [16]. The D3-Cr dilution test is based on the ingestion of an oral solution of deuterium-labelled creatine (D3-Cr) by a fasting patient followed by the measurement of both the labelled and total creatine and creatinine by liquid chromatography coupled with tandem mass spectrometry (LC‒MS/MS) in urine before and four days after ingestion [17]. As creatine directly enters the muscles where a certain amount is nonenzymatically converted to creatinine to be excreted in urine, an algorithm based on the ratio of D3-Cr to unlabelled creatine can provide an accurate measurement of muscle mass.
The D3-Cr dilution test has shown interesting clinical evidence, such as associations with “hard” clinical outcomes. Indeed, D3-Cr muscle mass appears to be an independent predictor of self-reported incident mobility disability together with walking speed [18] but also a predictor of self-reported disabilities in activities of daily living [19] and risk of hip fracture [20]. However, most of these observations have been made in the same cohort of older men (the osteoporotic fracture in men cohort) [18,19,20], and the D3-Cr dilution test was only evaluated in one cohort of postmenopausal women [21]. Furthermore, most often D3-Cr muscle mass was divided by body weight in the abovementioned studies, whereas body weight itself is associated with these difficult clinical outcomes.
From an analytical perspective, this method has some limitations that make the D3-Cr dilution test impractical for use in pharmacological trials. Indeed, it is only available in a few highly specialized laboratories without any external quality assessment or ring-test that allows lab-to-lab data comparison. Of note, this method requires a standardized procedure for D3-Cr ingestion and urine collection.
Given the analytical limitations of the D3-Cr dilution test, the group recommends not using the method in pharmacological trials at present. In addition to the need for a more widely available method, the group also identified that the method should be validated in other cohorts before any recommendation in clinical trials. Therefore, also taking into account that it is presently the only analytical test highly specific to muscle mass, the group concluded that muscle mass should be assessed according to the EWGSOP2 revised definition without additional biochemical markers
Myokines
Myokines are muscle-secreted small proteins (5–20 kDa) with autocrine, paracrine or endocrine effects. Myokines contribute to muscle maintenance, acting, for example, on metabolism, angiogenesis, and inflammation [22]. In aging, myokine secretion and the sensitization of the muscle to these myokines are altered, leading to a disturbance in the balance between anabolic and catabolic effects with consequent age-related muscle atrophy [23]. Therefore, the group agreed that myokines are interesting tools in pharmacological trials to decipher the drug mode of action and physio-pathological mechanisms of muscle protein turnover. Myokines might also be helpful in evaluating secondary outcomes for phase II studies. However, it is premature to determine whether myokines are useful for patient stratification, monitoring of therapies or monitoring side effects.
Myostatin, also called growth and differentiation factor 8 (GDF-8), is a member of the transforming growth factor-β (TGF-β) superfamily and is mostly seen as a muscle growth suppressor [22]. Indeed, when binding activin type IIA and IIB receptors or TGF-β receptors, myostatin suppresses mammalian target of rapamycin (mTOR)-mediated protein synthesis [24]. However, some argue that myostatin can promote muscle growth through different downstream pathways [22]. Myostatin is considered specific to skeletal muscle even if it is also expressed in adipose and cardiac tissues [22].
Myostatin is probably the most studied myokine. However, many studies have yielded conflicting data on the relationship between myostatin and its role in age-related muscle atrophy. Indeed, while most studies found an association between higher myostatin blood concentration and higher muscle mass, this association is not systematically observed [25]. Nevertheless, myostatin is a good predictor of one-year mortality as a “hard” clinical outcome in patients on hemodialysis [26]. Regarding the association between myostatin and muscle function, studies have shown either increased myostatin concentrations in participants with better muscle function or no (or only men-specific) association [25]. Part of these conflicting data may stem from a sex-dependent expression pattern [27, 28]. Regarding its variability with age, myostatin assessment through a highly specific LC‒MS/MS method showed a decline in myostatin concentration with aging in men but not in women [29]. However, many other studies have shown a relatively steady state or increased serum myostatin level with age [25, 30].
Another part of these discrepancies may come from the analytical heterogeneity of the assays. Indeed, myostatin is highly similar to its homologous growth and differentiation factor 11 (GDF-11) [29]. Thus, older kits are known to present significant cross-reactivity with several TGF-β superfamily members [27]. Therefore, whenever possible, LC‒MS/MS methods to measure myostatin should be favoured.
Follistatin is an antagonist of TGF-β ligands, including myostatin and activin A, by acting on myogenic transcription factors. Follistatin has been correlated with muscle mass and muscle function in women in a small number of studies [28, 31], but this association was not confirmed in men [28]. However, in mid- to long-term resistance training intervention trials, follistatin and/or follistatin/myostatin ratio were increased. The same observation was not consistently found for myostatin alone [32,33,34,35]. Analytically, several ELISA and RIA assays are easily available to measure follistatin, but preanalytical considerations and biological variability are not properly described [36].
The myostatin-follistatin system has long been considered a possible target for sarcopenia therapies, and several clinical trials are ongoing, as reviewed by Skrzypczak and colleagues [37]. Although it is unclear whether these proteins are good biochemical markers to monitor the disease or the drug efficacy, myostatin and follistatin should be considered as a couple that helps decipher the physio-pathological drug mode of action.
The group recommends that clinical trials with drugs directly targeting the myostatin/follistatin system should follow the two proteins in both phase II and phase III trials with a precise statistical analysis for men and women. To this end, the follistatin/myostatin ratio can be optionally calculated. However, when the target is an entirely different system, these biochemical markers should be measured in phase II and considered optional in phase III if no changes have been observed in the phase II study. Additionally, when physical training is associated with pharmacological therapies, the standardized procedure for blood collection should include a delay of at least 24 h between the last exercise and venepuncture. Indeed, myostatin and follistatin are acutely increased several hours after exercise but return to baseline after approximately 24 h [38]
Activin A is another member of the TGF-β superfamily that preferentially binds the activin type IIA receptor. Activin A is also considered a negative regulator of muscle growth, acting through the same pathways as myostatin. However, Activin A’s contribution to sarcopenia physiopathology is still a theoretical concept with an evident lack of cohort-based evidence.
The group considers that future research is required before any recommendation for using Activin A in clinical trials for sarcopenia can be made
Growth factor differentiation-15 (GDF-15) is also a member of the TGF-β superfamily, and its expression is induced by stress or myocardial infarction [39]. Several cohorts have shown increased GDF-15 expression in sarcopenic participants [40, 41]. GDF-15 was also sometimes, but not systematically, associated with handgrip strength, skeletal muscle index (SMI) [42,43,44] and physical performance tests [45, 46]. Myostatin and follistatin expression is induced upon acute physical exercise [47]. However, the few long-term interventional trials that tested GDF-15 expression upon resistance training in a sarcopenic cohort failed to observe any longitudinal change in GDF-15 expression [32, 35]. The GDF-15 could also not predict sarcopenia occurrence or evolution in a 2-year follow-up period [40, 48].
Several ELISAs are available on the market for GDF-15 measurement. Nevertheless, GDF-15 is increased with age and chronic kidney disease [49], and doubts have been raised regarding the existence of a circadian rhythm [39]. Given these analytical and clinical considerations, GDF-15 does not appear to be a good biomarker to assess response to treatment. However, adding GDF-15 concentrations to physical performance tests may have some added value to understanding the therapies' effects on physio-pathological mechanisms.
Given that GDF-15 has shown some interesting associations with muscle mass, muscle strength and performance tests but has failed thus far to show modifications in longitudinal follow-up, the group recommends the optional use of GDF-15 in phase II or phase III considering that baseline levels may help in stratifying patients. The standardized measurement procedure should include a 24 h free exercise period before venepuncture and a defined time for blood collection
Irisin is a myokine secreted by skeletal muscles under physical exercise, although it is not a member of the TGF-β superfamily. Irisin is responsible for the browning of white adipose tissue [50], and irisin injection in mice induces muscle hypertrophy with increased protein synthesis [51]. This mechanism might be at least partially regulated by the myostatin/follistatin couple, as irisin is increased in myostatin knock-out mice or after recombinant follistatin injection [50].
Irisin is decreased in sarcopenic persons in several cohorts with very few discordant data [52,53,54]. Irisin is also regularly associated with muscle strength or mass [55, 56]. Nevertheless, irisin has not been studied thus far either in non-pharmacological interventional trials or as a prognostic biomarker of outcomes. Analytically, the analytical precision of ELISA is not accurate enough, and physiological variation is poorly characterized [57].
The group considers it premature to recommend irisin use in clinical trials at present
Neuromuscular Junction
By including performance tests and muscle strength in the definition of sarcopenia, the EWGSOP group states that sarcopenia is “a multidimensional concept that not only involves muscles but also central and peripheral nervous function, including balance” [1]. Therefore, not only muscle-specific biochemical markers but also biochemical markers of the neuromuscular junction are required to evaluate muscle integrity, as any impairment to the neuromuscular junction could lead to decreased capacities in using muscles and producing volitional tasks. In pharmacological trials, any modification of these biochemical markers should be integrated with performance tests to understand potential drug modes of action. It is premature to determine whether these biochemical markers may help monitor the disease. However, it is conceivable that these biochemical markers could reflect positive effects on cognition or neurological side effects of therapies.
C-terminal agrin fragment (CAF), also called CAF-22 when referring to the smaller fragment of 22 kDa, is a byproduct of agrin released during the remodeling of the neuromuscular junction. CAF is increased in sarcopenic patients in various cohorts and is associated with the skeletal muscle index [58,59,60,61,62]. It has also been reported to be lower in older dancers than in their sedentary counterparts [63]. Clinically, this biomarker looks very promising. However, there is currently no commercially available assay for CAF.
Given that the appropriate technology is not available at the moment, the group does not currently recommend this biomarker in clinical trials. Nevertheless, as a clinically promising biomarker, developing a widely available and accurate method for CAF determination could modify this recommendation
Brain-derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factors (GDNF) are other biochemical markers of neuromuscular junctions and neuroinflammation. BDNF and GDNF are neurotrophic factors expressed by motor neurons [64]. BDNF and GDNF participate in motor axonal regeneration and neuronal plasticity through a paracrine effect [65]. BDNF and GDNF are lower in sarcopenia of hemodialyzed or kidney transplant patients [66, 67]. Both factors were shown to be as efficient as CAF for the diagnosis of sarcopenia in chronic obstructive pulmonary disease patients [68] and in Parkinson’s disease patients [69]. However, BDNF has been studied more in sarcopenia than GDNF.
BDNF is measurable through various ELISA kits. BDNF expression is controlled by sex hormones [70], and as a neuronal biomarker, BDNF expression is modified in many psychiatric disorders [71]. Thus, all these confounding factors should be included in the statistical analysis. Additionally, BDNF is increased upon acute exercise, but its level after long-term training still has to be determined [72, 73].
The group recommends BDNF measurement at baseline and follow-up in phase II and phase III studies to evaluate the neuromuscular part of the disease. For other biochemical markers that increase upon acute exercise, venepuncture should be performed at least 24 h after the last acute physical exercise
Other Biochemical Markers of Muscle Turnover
N-terminal type III procollagen (PIIINP) is a byproduct of the synthesis of type 3 collagen. Type III collagen is expressed in smooth muscles and in the endomysium of skeletal muscle to enhance tissue stretching properties [74, 75]. In sarcopenia, PIIINP has been associated with the skeletal muscle index and, to a lesser extent, physical performance but not muscle strength in several distinct cohorts [76,77,78,79]. Additionally, PIIINP is a biomarker of choice to measure muscle remodeling, as it reflects an anabolic response compared to steroid hormones, which are indicators of hormonal status [80]. Indeed, it appears to be a good biomarker of anabolic response to therapies with testosterone or growth hormone [81, 82]. PIIINP also shows a small-to-moderate increase in interventional trials on small cohorts of older participants [83, 84].
Analytically, PIIINP is a serum biomarker measurable through several different techniques (ELISA, RIA, ECLIA). Additionally, although not described, preanalytical constraints are expected to be the same as for N-terminal type I procollagen (PINP).
The group recommends measuring PIIINP as a follow-up biomarker in phase II and phase III studies to evaluate overall muscle turnover. Sampling should be realized in fasting individuals as a precaution until further studies are assessing the preanalytical questions
The serum creatinine to serum cystatin C ratio, or sarcopenia index (SI), is a recent index first created to evaluate muscle mass [85]. Indeed, serum creatinine can be seen as a biomarker of muscle protein turnover [80]. However, its blood concentration is highly dependent on renal function. Thus, this ratio was developed to circumvent this limitation by normalizing creatinine with cystatin C, another biomarker of renal function. SI has shown a moderate correlation with CT muscle cross-sectional area, calf circumference and handgrip strength [85,86,87]. Nevertheless, SI is not sensitive and specific enough to be used as a biomarker of muscle mass [88]. However, SI has been shown to be associated with malnutrition in critically ill patients [89] and in patients suffering from cirrhosis [90]. Additionally, it was associated with clinical outcomes and especially hospitalization and/or mortality in hospitalized older patients [86], critically ill patients [85], cardiac patients [91, 92] and cancer patients [93, 94]. Thus, it is important to consider SI as a way of stratifying the risk of outcomes rather than a real muscle mass assessment. Given the lack of interventional studies thus far, its usefulness as a treatment follow-up is unclear.
Analytically, this index is easy to calculate from well-described biochemical markers in terms of physio-pathological and analytical variability, with widely available high-throughput methods. However, the use of an enzymatic method for creatinine should be preferred compared to a Jaffe method. Indeed, the enzymatic method is analytically more sensitive and specific than the Jaffe method [95]. Additionally, the creatinine assay should be traceable to isotope dilution mass spectrometry (IDMS), and cystatin C assays should be traceable to the ERM-DA471/IFCC reference material to ensure the consistency of the results.
Two formulas for SI coexist in the literature. The first one is the original one defined as [serum creatinine (mg/dL)/serum cystatin C (mg/L)] × 100. The second one emerged a few years later and is calculated as the serum creatinine X cystatin C-based glomerular filtration rate (eGFRcysC) [96]. Although this second formula has shown better correlation with muscle mass and handgrip strength [96, 97], we do not recommend its use in multifactorial statistical approaches. Indeed, eGFRcysC is calculated using the Chronic Kidney Disease Epidemiology Collaboration equation, which contains age and gender as variables [98]. Therefore, the use of this second formula introduces potential confounding factors in the index rather than as covariates in the statistical models.
The group recommends the use of the SI index with the formula (serum creatinine (mg/dL)/serum cystatin C (mg/L)) X 100 at least at baseline in both phase II and phase III studies to help in patient risk stratification. Longitudinal research is needed to define SI added value during follow-up
Set 2 Causal factor evaluation
As a multifactorial disease, sarcopenia-related loss of muscle mass and strength is related not only to muscle metabolism dysfunction but also to metabolic and endocrine abnormalities, including chronic low-grade inflammation. Thus, it is important to follow not only muscle-specific biochemical markers but also biochemical markers that reflect the patient’s metabolic, endocrine and inflammation status. Ideally, biochemical markers included in this set should be modified in sarcopenic persons compared to healthy controls, and their normalization should be considered beneficial for the patient. In pharmacological trials, measurement at baseline may help in patient risk stratification. Additionally, the improvement in these biochemical markers might be considered a secondary outcome in phase II and phase III studies. Major clinical evidence and analytical constraints for assessing these causal biochemical markers in pharmacological trials are reported in Tables 3 and 4.
Adipokines
Adipokines are adipocyte-secreted proteins involved in insulin resistance, glucose consumption by the muscle, lipolysis and inflammatory processes [99]. Muscle-fat cross talk is now well established, and measurement of adipokines partially evaluates metabolism abnormalities occurring in sarcopenia [23]. In pharmacological trials, the group agreed that adipokines are particularly relevant when therapies involve changes in body fat mass or insulin resistance to help decipher molecular modes of action. However, it is unclear whether adipokines are useful for patient risk stratification, monitoring of therapies or side effects.
Adiponectin is an adipokine involved in insulin resistance and inflammatory processes. The inflammatory function is partially achieved through the synthesis of cytokines such as interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) [100], while adiponectin-mediated insulin resistance occurs through the reduction of glucose uptake by modulating the interaction between adiponectin receptor AdopiR1 and adaptor protein containing pleckstrin homology domain (APPL1) [101]. In mice, adiponectin was shown to induce muscle regeneration by binding to T-cadherin [102].
Adiponectin has been inversely associated with appendicular lean mass [103, 104]. However, when body composition is considered a cofounding factor, this association disappears [103, 104]. Additionally, conflicting data coexist regarding the level of adiponectin in sarcopenic persons [103, 105, 106]. However, a recent meta-analysis concluded an increased level in sarcopenia regardless of body fat levels [107]. This finding is in line with the adiponectin paradox. In centenarians, adiponectin is higher and positively correlated with biochemical markers of low cardiovascular risk, such as HDL or reduced insulin resistance [108, 109]. However, in people > 65 years old, high levels of adiponectin are frequently associated with insulin resistance, metabolic disorders or mortality [110, 111]. Nevertheless, long-term physical interventions may increase adiponectin with a beneficial impact on muscle [23, 112]. Komici et al. have demonstrated that adiponectin increase in sarcopenia might represent a compensatory effect solicited by chronic inflammation and oxidative stress [107].
Analytically, adiponectin is easy to measure, and some automated methods are available. The physiological variations are well described and include age, BMI, fat mass and sex [113].
The group recommends the optional use of adiponectin during both phase II and phase III studies at baseline and during the whole follow-up to evaluate the contributing processes linking adipose tissue and sarcopenia. Changes in BMI, muscle density and any body composition measure should absolutely be integrated in the statistical analysis, as these changes can confound the association between adiponectin and sarcopenia factors [104]
Leptin is a pro-inflammatory adipokine that is often considered to have antagonistic effects on adiponectin, although it acts through different pathways [99]. By causing inflammation, exogenous leptin can cause muscle atrophy through protein synthesis reduction in myocytes [80]. However, inflammatory cytokines often do not correlate with leptin levels [114]. Leptin is higher in sarcopenic persons and often associated with lower appendicular lean mass [103, 106, 115]. However, for adiponectin, the strength of this association is reduced when fat mass is considered a cofounding factor [103]. Interestingly, leptin was reduced after exercise combined with nutritional intervention or exercise alone in a randomized controlled trial [116]. In this study, although not significant, leptin reduction tended to be smaller in the group with a smaller change in body fat mass.
Analytically, leptin can be measured by ELISA, but biological variation is not described. Nevertheless, leptin levels are higher in women even after adjustment for fat mass and decrease with age [117]. Obesity is also well known to influence leptin levels [118]. Hence, leptin is higher in sarcopenic obese patients than in normal sarcopenic hemodialyzed patients [119].
The group agrees that at least adiponectin or leptin should be measured in both phase II and phase III trials at baseline and during the whole follow-up. Nevertheless, because leptin appears to give more consistent results across the literature compared to adiponectin, leptin should be preferred to investigate the crosstalk between adipose tissue and muscle. The adiponectin-leptin ratio is another acceptable option. For both adiponectin and leptin, cofounding factors such as BMI, muscle density and any change to body composition or body fat mass should be adjusted for in the statistical analysis [23]
Hormones
Insulin-like growth factor-1 (IGF-1) is sometimes considered a growth factor or sometimes a myokine of low specificity to the muscle [22]. Nevertheless, IGF-1 has growth hormone (GH)-mediated anabolic properties, notably upon physical exercise [120]. Thus, IGF-1 behaviour in sarcopenia has been largely studied, and IGF-1 age-related decline is considered to be a causal factor of the disease [121]. Alone, IGF-1 specificity for the diagnosis of sarcopenia is low, although IGF-1 has shown promising features in diagnosing sarcopenia when combined with a panel of biochemical markers [122]. Nevertheless, studies have shown that IGF-1 is lower in sarcopenic persons [122,123,124], correlates with muscle mass, hand grip strength, and gait speed [30, 125] and is increased upon long-term resistance training [126].
Analytically, the total IGF-1 or IGF-1 bioactive form can be measured. Total IGF-1 is easily measurable through largely available automated methods, and thus, total measurement should be favoured. The major confounding factor that must be considered is age, as IGF-1 is well described to decrease with age. Importantly, the dosage of GH cannot be used to evaluate the GH/IGF-1 axis. Indeed, due to the pulsatile secretion of GH, the intraindividual biological variation of GH is high [127], and there are no preanalytical procedures that may reduce this variability.
The group recommends only using IGF-1 to study the GH/IGF-1 axis. Its measurement should be realized in both phase II and phase III trials at baseline and follow-up
Steroid hormones have long been studied in sarcopenia. Dehydroepiandrosterone sulphate (DHEAS) and testosterone age-related decline are often considered causal factors for muscle loss, as the aging phenotype shares common features with hypogonadism in younger men [128]. Cortisol, unlike testosterone and DHEAS, displays well-known catabolic properties, and higher cortisol levels are associated with frailty or sarcopenia [129, 130]. Nevertheless, as a biomarker of sarcopenia, DHEAS is the most studied steroid hormone. DHEAS is decreased in sarcopenia, while cortisol is increased, probably due to chronic inflammation [131, 132]. Both DHEAS and cortisol blood levels have been shown to be modified by nutritional or nutritional-exercise interventional trials [133]. Although testosterone has been tested as a drug in a wide variety of clinical trials, its usefulness as a biomarker is mainly based on concepts rather than cohort-based evidence [134, 135].
Analytically, the three parameters are widely measurable through automated methods. Obviously, as a steroid hormone, these parameters are age- and gender dependent. The circadian rhythm of cortisol includes a nadir at midnight and a morning peak [136]. A ratio between DHEAS and cortisol has also been proposed [131]. However, further data are needed before any recommendation can be made on this ratio.
The group considers that DHEAS and cortisol should be monitored in phase II pharmacological trials. In phase III trials, the use of these biochemical markers should be regarded as mandatory in case any changes have been observed during phase II trials. Otherwise, DHEAS and cortisol are optional biochemical markers for phase III trials. Given that DHEAS is a testosterone precursor, the group recommends using testosterone only in trials where testosterone is part of the therapy mechanism. For the three parameters, blood compared to salivary and total hormone compared to free hormone should be favoured. For cortisol, the time of collection should be standardized
Inflammatory Biochemical Markers
Chronic inflammation has long been described as a fundamental mechanism in sarcopenia [137]. Many cytokines have been proposed to be dysregulated in sarcopenia, and a “core cytokinome” in sarcopenia and frail persons has been published [138]. Based on the investigation of 27 inflammatory molecules, the authors showed that C-reactive protein (CRP) is increased, while myeloperoxidase, interleukin-8 (IL-8), monocyte chemoattractant protein-1 and platelet-derived growth factor BB are decreased. Additionally, a recent meta-analysis found that among 168 articles studying the links between inflammatory molecules and muscle strength or mass, the three more studied inflammatory molecules are CRP, IL-6 and TNF-α [139].
CRP, IL-6, and TNF-α have been associated with a decline in muscle mass and muscle strength in a meta-analysis [139]. Nevertheless, in a second meta-analysis, CRP, but not IL-6 or TNF-α, was shown to be increased in sarcopenia [140]. Moreover, higher CRP and IL-6 are associated with a future decline in muscle strength [141]. Additionally, several nutritional intervention trials in older adults or in sarcopenic patients showed marked reduction of these biochemical markers after several weeks of intervention [135, 142]. Obviously, the nature of the intervention and the provided compounds are linked to the strength of the observed effect [143]. Furthermore, a meta-analysis on resistance training concluded a beneficial effect of resistance training on CRP but not on IL-6 and TNF-α [144], while acute training is known to show a short-term increase in these biomarkers [145]. Thus, clinically, CRP seems to be the most interesting biomarker of chronic inflammation in sarcopenia. Furthermore, analytically, methods for measuring CRP are the most automated and widely available compared to methods for IL-6 and TNF-α measurement.
The group recommends at least the use of CRP at baseline and follow-up as a biochemical marker of chronic inflammation in both phase II and phase III clinical trials, whereas IL-6 and TNF-α should only be considered optional biochemical markers. Of note, only the ultrasensitive method for CRP measurement should be used because the “classical” method for CRP detection might not be sensitive enough to detect any clinically relevant changes
Analytical Recommendations
Several analytical and preanalytical considerations should be followed to ensure the accuracy of the measurements. First, a standardized collection procedure per biomarker should be established, and the type of collection tube, time for collection, time for coagulation, need for a fasting or an exercise-free period, centrifugation process and decantation process should be defined. Major specific preanalytical constraints per biochemical marker are listed in Table 3. It is especially important to define whether fasting or a 24 h exercise-free period are needed. The storage procedure should also be standardized, and freeze‒thaw cycles should be avoided. Additionally, it is important to ensure a centralized measurement procedure of biochemical markers in a certified and experienced laboratory. The laboratory should subscribe to external quality evaluations whenever available. Regarding the validation procedure, all methods should be appropriately validated according to ISO 15189 guidelines. This validation should at least include an establishment of the coefficient of variation (CV) of the dosage through repeatability and reproducibility studies and the definition of the lower limit of quantification. Reference ranges should be checked as well. Whenever feasible, the method should be compared to another laboratory method allowing the measurement of the same biomarker. Regarding the measurement procedure, all the analyses should be performed in a batchwise manner to limit lot-to-lot variations. The staff should be adequately trained, and traceability ensured. Finally, statistical analysis should include careful adjustments for potential confounders, including physical activity level and body fat, when investigating the association between biochemical markers and muscle parameters, physical function or hard clinical endpoints.
Future Research Needs
Overall, few meta-analyses are available in the literature regarding the biochemical markers for sarcopenia, and specific studies should be dedicated to this task. Additionally, two primary pathophysiological mechanisms in sarcopenia are uncovered by the recommended panel of biochemical markers: nutritional status and oxidative stress. For nutritional status, the “update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults” recommends evaluating the nutritional status of all participants at inclusion and at least on each time point assessment using tools such as European Society of Clinical Nutrition and Metabolism (ESPEN) criteria or Global Leadership Initiative of Malnutrition (GLIM) criteria [7]. Because these criteria are free of biochemical markers, the present recommendation does not advise additional use of biochemical markers.
Many studies have been conducted on oxidative stress with inconsistent biomarker reporting [146,147,148]. A meta-analysis identifying the most studied and accurate biochemical markers of oxidative stress in sarcopenia is urgently needed. It is, therefore, premature to recommend some specific biochemical markers of oxidative stress to be monitored in sarcopenia.
Finally, several promising biomarkers with interesting clinical features have been identified. First, we already discussed the D3-Cr dilution test and CAF measurement for which clinical data are convincing, but we do not have the appropriate technology to ensure widely available and accurate measurement for pharmacological trials. Additionally, apelin [149] and fibroblast growth factor-21 (FGF-21) [150] as musculoskeletal markers, together with neurofilament light chains (NfL) for the neuromuscular junction [151], have been identified as promising biochemical markers with clinically relevant data but with a too restricted number of studies to recommend their use in clinical trials. Finally, in the future, innovative approaches such as miRNA panels [152] and microbiome analysis [153, 154] will probably find a place in the biochemical marker field, but it is certainly premature to define their clinical utility at the present time in sarcopenia.
Conclusions
In this consensus report, experts from the ESCEO working group proposed a clear list of biochemical markers of musculoskeletal status and the pathophysiological mechanisms of sarcopenia that are recommended to be assessed in any phase II and phase III clinical trials of drugs aimed at the management of sarcopenia. Based on the analytical and clinical properties of biochemical markers, the group agreed on four mandatory biochemical markers evaluating musculoskeletal status that should be assessed in any new Phase II or Phase III trial, namely, the myostatin-follistatin couple, BDNF, PIIINP and the Sarcopenia Index [using the formula (serum creatinine (mg/dL)/serum cystatin C (mg/L)) ×100]. In addition, experts also agreed on six mandatory biochemical markers evaluating nonmuscle-specific physio-pathological mechanisms (i.e., causal factors) that should be assessed in any Phase II trials, namely, IGF-1, DHEAS, Cortisol, CRP, IL6, and TNF-α. IGF-1 and CRP are also recommended to be measured in any phase III trials. The recommendations made in this consensual report are based on the available evidence and the availability of proper methodologies for biomarker assessment. Further research and development of new methods of biochemical marker dosage may lead to the evolution of these recommendations.
Change history
24 July 2023
A Correction to this paper has been published: https://doi.org/10.1007/s00223-023-01114-y
References
Cruz-Jentoft AJ, Bahat G, Bauer J et al (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31. https://doi.org/10.1093/ageing/afy169
Bruyère O, Beaudart C, Ethgen O et al (2019) The health economics burden of sarcopenia: a systematic review. Maturitas 119:61–69. https://doi.org/10.1016/j.maturitas.2018.11.003
Beaudart C, Zaaria M, Pasleau F et al (2017) Health outcomes of sarcopenia: a systematic review and meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0169548
Veronese N, Demurtas J, Soysal P et al (2019) Sarcopenia and health-related outcomes: an umbrella review of observational studies. Eur Geriatr Med 10:853–862. https://doi.org/10.1007/s41999-019-00233-w
Fernandes LV, Paiva AEG, Silva ACB et al (2022) Prevalence of sarcopenia according to EWGSOP1 and EWGSOP2 in older adults and their associations with unfavorable health outcomes: a systematic review. Aging Clin Exp Res 34:505–514. https://doi.org/10.1007/s40520-021-01951-7
Beaudart C, Reginster J, Bruyère O, Geerinck A (2021) Quality of Life and Sarcopenia. In: Cruz-Jentoft AJ, Morley JE (eds) Sarcopenia. Wiley, New York, pp 279–304
Reginster JY, Beaudart C, Al-Daghri N et al (2021) Update on the ESCEO recommendation for the conduct of clinical trials for drugs aiming at the treatment of sarcopenia in older adults. Aging Clin Exp Res 33:3–17. https://doi.org/10.1007/s40520-020-01663-4
Feike Y, Zhijie L, Wei C (2021) Advances in research on pharmacotherapy of sarcopenia. Aging Med 4:221–233. https://doi.org/10.1002/agm2.12168
Atkinson AJ, Colburn WA, DeGruttola VG et al (2001) Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin Pharmacol Ther 69:89–95. https://doi.org/10.1067/mcp.2001.113989
Selleck MJ, Senthil M, Wall NR (2017) Making meaningful clinical use of biomarkers. Biomark Insights 12:1177271917715236. https://doi.org/10.1177/1177271917715236
Honvo G, Bannuru RR, Bruyère O et al (2019) Recommendations for the reporting of harms in manuscripts on clinical trials assessing Osteoarthritis drugs: a consensus statement from the European society for clinical and economic aspects of Osteoporosis, Osteoarthritis and musculoskeletal diseases (ESCEO). Drugs Aging 36:145–159. https://doi.org/10.1007/s40266-019-00667-8
Diez-Perez A, Brandi ML, Al-Daghri N et al (2019) Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: state of the art—outcomes of an expert consensus meeting organized by the European society for clinical and economic aspects of Osteoporosis, Osteoarthritis and Mus. Aging Clin Exp Res 31:1375–1389. https://doi.org/10.1007/s40520-019-01294-4
Beaudart C, Rolland Y, Cruz-Jentoft AJ et al (2019) Assessment of muscle function and physical performance in daily clinical practice: a position paper endorsed by the European society for clinical and economic aspects of Osteoporosis, Osteoarthritis and Musculoskeletal diseases (ESCEO). Calcif Tissue Int 105(1):1–14
Buckinx F, Landi F, Cesari M et al (2018) Pitfalls in the measurement of muscle mass: a need for a reference standard. J Cachexia Sarcopenia Muscle 9:269–272. https://doi.org/10.1002/jcsm.12268
Evans WJ, Hellerstein M, Orwoll E et al (2019) D 3 -Creatine dilution and the importance of accuracy in the assessment of skeletal muscle mass. J Cachexia Sarcopenia Muscle 10:14–21. https://doi.org/10.1002/jcsm.12390
Buehring B, Siglinsky E, Krueger D et al (2018) Comparison of muscle/lean mass measurement methods: correlation with functional and biochemical testing. Osteoporos Int 29:675–683. https://doi.org/10.1007/s00198-017-4315-6
Shankaran M, Czerwieniec G, Fessler C et al (2018) Dilution of oral D 3 -Creatine to measure creatine pool size and estimate skeletal muscle mass: development of a correction algorithm. J Cachexia Sarcopenia Muscle 9:540–546. https://doi.org/10.1002/jcsm.12278
Zanker J, Patel S, Blackwell T et al (2020) Walking speed and muscle mass estimated by the D3-creatine dilution method are important components of sarcopenia associated with incident mobility disability in older men: a classification and regression tree analysis. J Am Med Dir Assoc 21:1997–2002. https://doi.org/10.1016/j.jamda.2020.03.017
Cawthon PM, Blackwell T, Cummings SR et al (2020) Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J Gerontol Ser A Biol Sci Med Sci 76:123–130. https://doi.org/10.1093/GERONA/GLAA111
Cawthon PM, Peters KE, Cummings SR et al (2022) Association between muscle mass determined by D3-creatine dilution and incident fractures in a prospective cohort study of older men. J Bone Miner Res 37:1213–1220. https://doi.org/10.1002/jbmr.4505
Zhu K, Wactawski-Wende J, Ochs-Balcom HM et al (2021) The association of muscle mass measured by D3-Creatine dilution method with dual-energy x-ray absorptiometry and Physical function in postmenopausal women. J Gerontol Ser A Biol Sci Med Sci 76:1591–1599. https://doi.org/10.1093/gerona/glab020
Mancinelli R, Checcaglini F, Coscia F et al (2021) Biological aspects of selected myokines in skeletal muscle: focus on aging. Int J Mol Sci 22:8520. https://doi.org/10.3390/ijms22168520
Paris MT, Bell KE, Mourtzakis M (2020) Myokines and adipokines in sarcopenia: understanding cross-talk between skeletal muscle and adipose tissue and the role of exercise. Curr Opin Pharmacol 52:61–66. https://doi.org/10.1016/j.coph.2020.06.003
White TA, Lebrasseur NK (2014) Myostatin and sarcopenia: opportunities and challenges - a mini-review. Gerontology 60:289–293. https://doi.org/10.1159/000356740
Baczek J, Silkiewicz M, Wojszel ZB (2020) Myostatin as a biomarker of muscle wasting and other pathologies-state of the art and knowledge gaps. Nutrients 12:2401. https://doi.org/10.3390/nu12082401
Delanaye P, Bataille S, Quinonez K et al (2019) Myostatin and insulin-like growth factor 1 are biomarkers of muscle strength, muscle mass, and mortality in patients on Hemodialysis. J Ren Nutr 29:511–520. https://doi.org/10.1053/j.jrn.2018.11.010
Bergen HR, Farr JN, Vanderboom PM et al (2015) Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: insights using a new mass spectrometry-based assay. Skelet Muscle 5:21. https://doi.org/10.1186/s13395-015-0047-5
Fife E, Kostka J, Kroc Ł et al (2018) Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr 18:200. https://doi.org/10.1186/s12877-018-0888-y
Schafer MJ, Atkinson EJ, Vanderboom PM et al (2016) Quantification of GDF11 and myostatin in human aging and cardiovascular disease. Cell Metab 23:1207–1215. https://doi.org/10.1016/j.cmet.2016.05.023
Moriwaki K, Matsumoto H, Tanishima S et al (2019) Association of serum bone- and muscle-derived factors with age, sex, body composition, and physical function in community-dwelling middle-aged and elderly adults: a cross-sectional study. BMC Musculoskelet Disord 20:276. https://doi.org/10.1186/s12891-019-2650-9
Du Y, Xu C, Shi H et al (2021) Serum concentrations of oxytocin, DHEA and follistatin are associated with osteoporosis or sarcopenia in community-dwelling postmenopausal women. BMC Geriatr 21(1):1–10. https://doi.org/10.1186/s12877-021-02481-7
Hofmann M, Schober-Halper B, Oesen S et al (2016) Effects of elastic band resistance training and nutritional supplementation on muscle quality and circulating muscle growth and degradation factors of institutionalized elderly women: the Vienna Active Ageing Study (VAAS). Eur J Appl Physiol 116:885–897. https://doi.org/10.1007/s00421-016-3344-8
Bagheri R, Moghadam BH, Church DD et al (2020) The effects of concurrent training order on body composition and serum concentrations of follistatin, myostatin and GDF11 in sarcopenic elderly men. Exp Gerontol 133:110869. https://doi.org/10.1016/j.exger.2020.110869
Mafi F, Biglari S, Afousi AG, Gaeini AA (2019) Improvement in skeletal muscle strength and plasma levels of follistatin and myostatin induced by an 8-week resistance training and epicatechin supplementation in sarcopenic older adults. J Aging Phys Act 27:384–391. https://doi.org/10.1123/japa.2017-0389
Seo MW, Jung SW, Kim SW et al (2021) Effects of 16 weeks of resistance training on muscle quality and muscle growth factors in older adult women with sarcopenia: a randomized controlled trial. Int J Environ Res Public Health 18:6762. https://doi.org/10.3390/ijerph18136762
Evans LW, Muttukrishna S, Groome NP (1998) Development, validation and application of an ultra-sensitive two-site enzyme immunoassay for human follistatin. J Endocrinol 156:275–282. https://doi.org/10.1677/joe.0.1560275
Skrzypczak D, Skrzypczak-Zielińska M, Ratajczak AE et al (2021) Myostatin and follistatin—new kids on the block in the diagnosis of sarcopenia in IBD and possible therapeutic implications. Biomedicines 9:1301. https://doi.org/10.3390/biomedicines9101301
He Z, Tian Y, Valenzuela PL et al (2018) Myokine response to high-intensity interval vs resistance exercise: an individual approach. Front Physiol 9:1735. https://doi.org/10.3389/fphys.2018.01735
Johann K, Kleinert M, Klaus S (2021) The role of gdf15 as a myomitokine. Cells 10:2990. https://doi.org/10.3390/cells10112990
Kim M, Walston JD, Won CW (2022) Associations between elevated growth differentiation factor-15 and sarcopenia among community-dwelling older adults. J Gerontol Ser A 77:770–780. https://doi.org/10.1093/gerona/glab201
Patel MS, Lee J, Baz M et al (2016) Growth differentiation factor-15 is associated with muscle mass in chronic obstructive pulmonary disease and promotes muscle wasting in vivo. J Cachexia Sarcopenia Muscle 7:436–448. https://doi.org/10.1002/jcsm.12096
Yamamoto H, Takeshima F, Haraguchi M et al (2022) High serum concentrations of growth differentiation factor-15 and their association with Crohn’s disease and a low skeletal muscle index. Sci Rep 12:6591. https://doi.org/10.1038/s41598-022-10587-0
Oba K, Ishikawa J, Tamura Y et al (2020) Serum growth differentiation factor 15 level is associated with muscle strength and lower extremity function in older patients with cardiometabolic disease. Geriatr Gerontol Int 20:980–987. https://doi.org/10.1111/ggi.14021
Nishikawa R, Fukuda T, Haruyama A et al (2022) Association between serum GDF-15, myostatin, and sarcopenia in cardiovascular surgery patients. IJC Hear Vasc 42:101114. https://doi.org/10.1016/j.ijcha.2022.101114
Semba RD, Gonzalez-Freire M, Tanaka T et al (2020) Elevated plasma growth and differentiation factor 15 is associated with slower gait speed and lower physical performance in healthy community-dwelling adults. J Gerontol Ser A Biol Sci Med Sci 75:175–180. https://doi.org/10.1093/gerona/glz071
Alcazar J, Frandsen U, Prokhorova T et al (2021) Changes in systemic GDF15 across the adult lifespan and their impact on maximal muscle power: the Copenhagen Sarcopenia study. J Cachexia Sarcopenia Muscle 12:1418–1427. https://doi.org/10.1002/jcsm.12823
Klein AB, Nicolaisen TS, Ørtenblad N et al (2021) Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Nat Commun 12:1041. https://doi.org/10.1038/s41467-021-21309-x
Sanchez-Sánchez JL, He L, Virecoulon Giudici K et al (2022) Circulating levels of Apelin, GDF-15 and Sarcopenia: lack of association in the MAPT Study. J Nutr Heal Aging 26:564–570. https://doi.org/10.1007/s12603-022-1800-1
Yazawa H, Fukuda T, Kaneda H et al (2020) Association of serum growth differentiation factor-15 with eGFR and hemoglobin in healthy older females. IJC Hear Vasc 31:100651. https://doi.org/10.1016/j.ijcha.2020.100651
Shan T, Liang X, Bi P, Kuang S (2013) Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1-Fndc5 pathway in muscle. FASEB J 27:1981–1989. https://doi.org/10.1096/fj.12-225755
Reza MM, Subramaniyam N, Sim CM et al (2017) Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat Commun 8:1104. https://doi.org/10.1038/s41467-017-01131-0
Zhao M, Zhou X, Yuan C et al (2020) Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: a cross-sectional study. Sci Rep 10:16093. https://doi.org/10.1038/s41598-020-73176-z
Qaisar R, Karim A, Muhammad T et al (2021) Prediction of sarcopenia using a battery of circulating biomarkers. Sci Rep 11:8632. https://doi.org/10.1038/s41598-021-87974-6
Alsaawi TA, Aldisi D, Abulmeaty MMA et al (2022) Screening for Sarcopenia among elderly Arab females: influence of body composition, lifestyle, irisin, and vitamin D. Nutrients 14:1855. https://doi.org/10.3390/nu14091855
Chang JS, Kim TH, Nguyen TT et al (2017) Circulating irisin levels as a predictive biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int 17:2266–2273. https://doi.org/10.1111/ggi.13030
Park HS, Kim HC, Zhang D et al (2019) The novel myokine irisin: clinical implications and potential role as a biomarker for sarcopenia in postmenopausal women. Endocrine 64:341–348. https://doi.org/10.1007/s12020-018-1814-y
Cavalier E, Beaudart C, Buckinx F et al (2016) Critical analytical evaluation of promising markers for sarcopenia. Eur Geriatr Med 7:239–242. https://doi.org/10.1016/j.eurger.2015.11.002
Pratt J, De Vito G, Narici M et al (2021) Plasma C-terminal agrin fragment as an early biomarker for sarcopenia: results from the GenoFit study. J Gerontol - Ser A Biol Sci Med Sci 76:2090–2096. https://doi.org/10.1093/gerona/glab139
Landi F, Calvani R, Lorenzi M et al (2016) Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older multimorbid community-dwellers: Results from the ilSIRENTE study. Exp Gerontol 79:31–36. https://doi.org/10.1016/j.exger.2016.03.012
Marzetti E, Calvani R, Lorenzi M et al (2014) Serum levels of C-terminal agrin fragment (CAF) are associated with sarcopenia in older hip fractured patients. Exp Gerontol 60:79–82. https://doi.org/10.1016/j.exger.2014.10.003
Drey M, Sieber CC, Bauer JM et al (2013) C-terminal Agrin Fragment as a potential marker for sarcopenia caused by degeneration of the neuromuscular junction. Exp Gerontol 48:76–80. https://doi.org/10.1016/j.exger.2012.05.021
Steinbeck L, Ebner N, Valentova M et al (2015) Detection of muscle wasting in patients with chronic heart failure using C -terminal agrin fragment: results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur J Heart Fail 17:1283–1293. https://doi.org/10.1002/ejhf.400
Marcolin G, Franchi MV, Monti E et al (2021) Active older dancers have lower C-terminal Agrin fragment concentration, better balance and gait performance than sedentary peers. Exp Gerontol 153:111469. https://doi.org/10.1016/j.exger.2021.111469
Pratt J, De Vito G, Narici M, Boreham C (2021) Neuromuscular junction aging: a role for biomarkers and exercise. J Gerontol Ser A Biol Sci Med Sci 76:576–585
Boyd JG, Gordon T (2003) Glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor sustain the axonal regeneration of chronically axotomized motoneurons in vivo. Exp Neurol 183:610–619. https://doi.org/10.1016/S0014-4886(03)00183-3
Miyazaki S, Iino N, Koda R et al (2021) Brain-derived neurotrophic factor is associated with sarcopenia and frailty in Japanese hemodialysis patients. Geriatr Gerontol Int 21:27–33. https://doi.org/10.1111/ggi.14089
Koito Y, Yanishi M, Kimura Y et al (2021) Serum brain-derived neurotrophic factor and myostatin levels are associated With skeletal muscle mass in kidney transplant recipients. Transplant Proc 53:1939–1944. https://doi.org/10.1016/j.transproceed.2021.04.021
Karim A, Muhammad T, Qaisar R (2021) Prediction of sarcopenia using multiple biomarkers of neuromuscular junction degeneration in chronic obstructive pulmonary disease. J Pers Med 11:919. https://doi.org/10.3390/jpm11090919
Karim A, Iqbal MS, Muhammad T, Qaisar R (2022) Evaluation of Sarcopenia using biomarkers of the neuromuscular junction in Parkinson’s disease. J Mol Neurosci 72:820–829. https://doi.org/10.1007/s12031-022-01970-7
Wei Y-C, Wang S-R, Xu X-H (2017) Sex differences in brain-derived neurotrophic factor signaling: functions and implications. J Neurosci Res 95:336–344. https://doi.org/10.1002/jnr.23897
Lima Giacobbo B, Doorduin J, Klein HC et al (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56:3295–3312. https://doi.org/10.1007/s12035-018-1283-6
Håkansson K, Ledreux A, Daffner K et al (2017) BDNF responses in Healthy older persons to 35 minutes of physical exercise, cognitive training, and mindfulness: associations with working memory function. J Alzheimer’s Dis 55:645–657. https://doi.org/10.3233/JAD-160593
Heyman E, Gamelin FX, Goekint M et al (2012) Intense exercise increases circulating endocannabinoid and BDNF levels in humans-possible implications for reward and depression. Psychoneuroendocrinology 37:844–851. https://doi.org/10.1016/j.psyneuen.2011.09.017
Kuivaniemi H, Tromp G (2019) Type III collagen (COL3A1): Gene and protein structure, tissue distribution, and associated diseases. Gene 707:151–171. https://doi.org/10.1016/j.gene.2019.05.003
Mackey AL, Donnelly AE, Roper HP (2005) Muscle connective tissue content of endurance-trained and inactive individuals. Scand J Med Sci Sport 15:402–408. https://doi.org/10.1111/j.1600-0838.2005.00449.x
Chen YY, Chiu YL, Kao TW et al (2021) Cross-sectional associations among P3NP, HtrA, Hsp70, Apelin and sarcopenia in Taiwanese population. BMC Geriatr 21:192. https://doi.org/10.1186/s12877-021-02146-5
Shin HE, Kim M, Won CW (2021) Association between plasma procollagen type III N-terminal peptide (P3NP) levels and physical performance in elderly men: The Korean Frailty and Aging Cohort Study (KFACS). Exp Gerontol 154:111523. https://doi.org/10.1016/j.exger.2021.111523
Berry SD, Ramachandran VS, Cawthon PM et al (2013) Procollagen type III N-terminal peptide (P3NP) and lean mass: a cross-sectional study. J Frailty Aging 2:129–134. https://doi.org/10.14283/jfa.2013.19
Santanasto AJ, Cvejkus RK, Wojczynski MK et al (2021) Circulating procollagen type III N-terminal peptide and physical Function in adults from the long life family study. J Gerontol A Biol Sci Med Sci 76:1273–1279. https://doi.org/10.1093/gerona/glaa197
Curcio F, Ferro G, Basile C et al (2016) Biomarkers in sarcopenia: a multifactorial approach. Exp Gerontol 85:1–8. https://doi.org/10.1016/j.exger.2016.09.007
Bhasin S, He EJ, Kawakubo M et al (2009) N-terminal propeptide of type III procollagen as a biomarker of anabolic response to recombinant human GH and testosterone. J Clin Endocrinol Metab 94:4224–4233. https://doi.org/10.1210/jc.2009-1434
Chen F, Lam R, Shaywitz D et al (2011) Evaluation of early biomarkers of muscle anabolic response to testosterone. J Cachexia Sarcopenia Muscle 2:45–56. https://doi.org/10.1007/s13539-011-0021-y
Fragala MS, Jajtner AR, Beyer KS et al (2014) Biomarkers of muscle quality: N-terminal propeptide of type III procollagen and C-terminal agrin fragment responses to resistance exercise training in older adults. J Cachexia Sarcopenia Muscle 5:139–148. https://doi.org/10.1007/s13539-013-0120-z
Kargaran A, Abedinpour A, Saadatmehr Z et al (2021) Effects of dual-task training with blood flow restriction on cognitive functions, muscle quality, and circulatory biomarkers in elderly women. Physiol Behav 239:113500. https://doi.org/10.1016/j.physbeh.2021.113500
Kashani KB, Frazee EN, Kukrálová L et al (2017) Evaluating muscle mass by using markers of kidney function: development of the sarcopenia index. Crit Care Med 45:e23–e29. https://doi.org/10.1097/CCM.0000000000002013
Tang T, Zhuo Y, Xie L et al (2020) Sarcopenia index based on serum creatinine and cystatin C is associated with 3-year mortality in hospitalized older patients. Sci Rep 10:1260. https://doi.org/10.1038/s41598-020-58304-z
Ren C, Su H, Tao J et al (2022) Sarcopenia Index based on serum creatinine and cystatin C is associated with mortality, nutritional risk/malnutrition and sarcopenia in older patients. Clin Interv Aging 17:211–221. https://doi.org/10.2147/CIA.S351068
He Q, Jiang J, Xie L et al (2018) A sarcopenia index based on serum creatinine and cystatin C cannot accurately detect either low muscle mass or sarcopenia in urban community-dwelling older people. Sci Rep 8:11534. https://doi.org/10.1038/s41598-018-29808-6
Barreto EF, Kanderi T, DiCecco SR et al (2019) Sarcopenia index is a simple objective screening tool for malnutrition in the critically Ill. J Parenter Enter Nutr 43:780–788. https://doi.org/10.1002/jpen.1492
Wu YK, Li M, Zhang YC et al (2022) The sarcopenia index is an effective predictor for malnutrition in patients with liver cirrhosis. Nutr Diet. https://doi.org/10.1111/1747-0080.12738
Lee HS, Park KW, Kang J et al (2020) Sarcopenia index as a predictor of clinical outcomes in older patients with coronary artery disease. J Clin Med 9:3121. https://doi.org/10.3390/jcm9103121
Romeo FJ, Chiabrando JG, Seropian IM et al (2021) Sarcopenia index as a predictor of clinical outcomes in older patients undergoing transcatheter aortic valve replacement. Catheter Cardiovasc Interv 98:E889–E896. https://doi.org/10.1002/ccd.29799
Chen X, Hou L, Shen Y et al (2021) The role of baseline Sarcopenia index in predicting chemotherapy-induced undesirable effects and mortality in older people with stage III or IV non-small cell lung cancer. J Nutr Heal Aging 25:878–882. https://doi.org/10.1007/s12603-021-1633-3
Zheng C, Wang E, Li JS et al (2022) Serum creatinine/cystatin C ratio as a screening tool for sarcopenia and prognostic indicator for patients with esophageal cancer. BMC Geriatr 22:207. https://doi.org/10.1186/s12877-022-02925-8
Delanaye P, Cavalier E, Pottel H (2017) Serum creatinine: not so simple! Nephron 136:302–308. https://doi.org/10.1159/000469669
Yang J, Zhang T, Feng D et al (2019) A new diagnostic index for sarcopenia and its association with short-term postoperative complications in patients undergoing surgery for colorectal cancer. Color Dis 21:538–547. https://doi.org/10.1111/codi.14558
Fu X, Tian Z, Wen S et al (2021) A new index based on serum creatinine and cystatin C is useful for assessing sarcopenia in patients with advanced cancer. Nutrition 82:111032. https://doi.org/10.1016/j.nut.2020.111032
Inker LA, Schmid CH, Tighiouart H et al (2012) Estimating Glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367:20–29. https://doi.org/10.1056/nejmoa1114248
VanSaun MN (2013) Molecular pathways: adiponectin and leptin signaling in cancer. Clin Cancer Res 19:1926–1932. https://doi.org/10.1158/1078-0432.CCR-12-0930
Hajri T, Tao H, Wattacheril J et al (2011) Regulation of adiponectin production by insulin: Interactions with tumor necrosis factor-α and interleukin-6. Am J Physiol - Endocrinol Metab 300:239–242. https://doi.org/10.1152/ajpendo.00307.2010
Mao X, Kikani CK, Riojas RA et al (2006) APPL1 binds to adiponectin receptors and mediates adiponectin signalling and function. Nat Cell Biol 8:516–523. https://doi.org/10.1038/ncb1404
Tanaka Y, Kita S, Nishizawa H et al (2019) Adiponectin promotes muscle regeneration through binding to T-cadherin. Sci Rep 9:1–12. https://doi.org/10.1038/s41598-018-37115-3
Rossi FE, Lira FS, Silva BSA et al (2019) Influence of skeletal muscle mass and fat mass on the metabolic and inflammatory profile in sarcopenic and non-sarcopenic overfat elderly. Aging Clin Exp Res 31:629–635. https://doi.org/10.1007/s40520-018-1029-3
Baker JF, Newman AB, Kanaya A et al (2019) The Adiponectin Paradox in the elderly: associations with body composition, Physical functioning, and mortality. J Gerontol Ser A Biol Sci Med Sci 74:247–253. https://doi.org/10.1093/gerona/gly017
Harada H, Kai H, Shibata R et al (2017) New diagnostic index for sarcopenia in patients with cardiovascular diseases. PLoS ONE 12:e0178123. https://doi.org/10.1371/journal.pone.0178123
Li C, wei, Yu K, Shyh-Chang N, et al (2019) Circulating factors associated with sarcopenia during ageing and after intensive lifestyle intervention. J Cachexia Sarcopenia Muscle 10:586–600. https://doi.org/10.1002/jcsm.12417
Komici K, Dello Iacono A, De Luca A et al (2021) Adiponectin and Sarcopenia: a systematic review with meta-analysis. Front Endocrinol 12:329. https://doi.org/10.3389/fendo.2021.576619
Bik W, Baranowska-Bik A, Wolinska-Witort E et al (2013) Assessment of adiponectin and its isoforms in polish centenarians. Exp Gerontol 48:401–407. https://doi.org/10.1016/j.exger.2013.01.015
Atzmon G, Pollin TI, Crandall J et al (2008) Adiponectin levels and genotype: a potential regulator of life span in humans. J Gerontol Ser A Biol Sci Med Sci 63:447–453. https://doi.org/10.1093/gerona/63.5.447
Menzaghi C, Trischitta V (2018) The adiponectin paradox for all-cause and cardiovascular mortality. Diabetes 67:12–22. https://doi.org/10.2337/dbi17-0016
Kistorp C, Faber J, Galatius S et al (2005) Plasma adiponectin, body mass index, and mortality in patients with chronic heart failure. Circulation 112:1756–1762. https://doi.org/10.1161/CIRCULATIONAHA.104.530972
Inoue A, Cheng XW, Huang Z et al (2017) Exercise restores muscle stem cell mobilization, regenerative capacity and muscle metabolic alterations via adiponectin/AdipoR1 activation in SAMP10 mice. J Cachexia Sarcopenia Muscle 8:370–385. https://doi.org/10.1002/jcsm.12166
Cnop M, Havel PJ, Utzschneider KM et al (2003) Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia 46:459–469. https://doi.org/10.1007/s00125-003-1074-z
Santoro A, Guidarelli G, Ostan R et al (2019) Gender-specific association of body composition with inflammatory and adipose-related markers in healthy elderly Europeans from the NU-AGE study. Eur Radiol 29:4968–4979. https://doi.org/10.1007/s00330-018-5973-2
Waters DL, Qualls CR, Dorin RI et al (2008) Altered growth hormone, cortisol, and leptin secretion in healthy elderly persons with sarcopenia and mixed body composition phenotypes. J Gerontol Ser A Biol Sci Med Sci 63:536–541. https://doi.org/10.1093/gerona/63.5.536
Kim H, Kim M, Kojima N et al (2016) Exercise and nutritional supplementation on community-dwelling elderly Japanese women with sarcopenic obesity: a randomized controlled trial. J Am Med Dir Assoc 17:1011–1019. https://doi.org/10.1016/j.jamda.2016.06.016
Ostlund RE, Yang JW, Klein S, Gingerich R (1996) Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab 81:3909–3913. https://doi.org/10.1210/jcem.81.11.8923837
Havel PJ, Kasim-Karakas S, Mueller W et al (1996) Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab 81:4406–4413. https://doi.org/10.1210/jcem.81.12.8954050
Beberashvili I, Azar A, Khatib A et al (2022) Sarcopenic obesity versus nonobese Sarcopenia in hemodialysis patients: differences in nutritional status, quality of life, and clinical outcomes. J Ren Nutr S1051–2276:00089–00099. https://doi.org/10.1053/j.jrn.2022.05.003
Frystyk J (2010) Exercise and the growth hormone-insulin-like growth factor axis. Med Sci Sports Exerc 42:58–66. https://doi.org/10.1249/MSS.0b013e3181b07d2d
Giovannini S, Marzetti E, Borst SE, Leeuwenburgh C (2008) Modulation of GH/IGF-1 axis: Potential strategies to counteract sarcopenia in older adults. Mech Ageing Dev 129:593–601. https://doi.org/10.1016/j.mad.2008.08.001
Kwak JY, Hwang H, Kim SK et al (2018) Prediction of sarcopenia using a combination of multiple serum biomarkers. Sci Rep 8:8574. https://doi.org/10.1038/s41598-018-26617-9
Xu B, Guo Z, Jiang B et al (2022) Factors affecting sarcopenia in older patients with chronic diseases. Ann Palliat Med. 11:972–983. https://doi.org/10.21037/apm-22-201
Jiang J, jin, Chen S min, Chen J, et al (2022) Serum IGF-1 levels are associated with sarcopenia in elderly men but not in elderly women. Aging Clin Exp Res. https://doi.org/10.1007/s40520-022-02180-2
Hofmann M, Halper B, Oesen S et al (2015) Serum concentrations of insulin-like growth factor-1, members of the TGF-beta superfamily and follistatin do not reflect different stages of dynapenia and sarcopenia in elderly women. Exp Gerontol 64:35–45. https://doi.org/10.1016/j.exger.2015.02.008
Amiri N, Fathei M, Mosaferi Ziaaldini M (2021) Effects of resistance training on muscle strength, insulin-like growth factor-1, and insulin-like growth factor–binding protein-3 in healthy elderly subjects: a systematic review and meta-analysis of randomized controlled trials. Hormones 20:247–257. https://doi.org/10.1007/s42000-020-00250-6
Veldhuis JD, Bowers CY (2003) Human GH pulsatility: an ensemble property regulated by age and gender. J Endocrinol Invest 26:799–813. https://doi.org/10.1007/BF03345229
Maggio M, Cattabiani C, Lauretani F et al (2010) The concept of multiple hormonal dysregulation. Acta Biomed 81:19–29
Varadhan R, Walston J, Cappola AR et al (2008) Higher levels and blunted diurnal variation of cortisol in frail older women. J Gerontol Ser A Biol Sci Med Sci 63:190–195. https://doi.org/10.1093/gerona/63.2.190
Gonzalez Rodriguez E, Marques-Vidal P, Aubry-Rozier B et al (2021) Diurnal salivary cortisol in sarcopenic postmenopausal women: the OsteoLaus cohort. Calcif Tissue Int 109:499–509. https://doi.org/10.1007/s00223-021-00863-y
Yanagita I, Fujihara Y, Kitajima Y et al (2019) A high serum Cortisol/DHEA-S ratio is a risk factor for sarcopenia in elderly diabetic patients. J Endocr Soc 3:801–813. https://doi.org/10.1210/js.2018-00271
Du Y, Xu C, Shi H et al (2021) Serum concentrations of oxytocin, DHEA and follistatin are associated with osteoporosis or sarcopenia in community-dwelling postmenopausal women. BMC Geriatr 21:542. https://doi.org/10.1186/s12877-021-02481-7
Yamada M, Nishiguchi S, Fukutani N et al (2015) Mail-based intervention for sarcopenia prevention increased anabolic hormone and skeletal muscle mass in community-dwelling Japanese older adults: the INE (Intervention by Nutrition and Exercise) Study. J Am Med Dir Assoc 16:654–660. https://doi.org/10.1016/j.jamda.2015.02.017
Maggio M, Lauretani F, Ceda GP (2013) Sex hormones and sarcopenia in older persons. Curr Opin Clin Nutr Metab Care 16:3–13. https://doi.org/10.1097/MCO.0b013e32835b6044
Lu Y, Niti M, Yap KB et al (2021) Effects of multi-domain lifestyle interventions on sarcopenia measures and blood biomarkers: secondary analysis of a randomized controlled trial of community-dwelling pre-frail and frail older adults. Aging 13:9330–9347. https://doi.org/10.18632/aging.202705
Chan S, Debono M (2010) Review: replication of cortisol circadian rhythm: new advances in hydrocortisone replacement therapy. Ther Adv Endocrinol Metab 1:129–138. https://doi.org/10.1177/2042018810380214
Tournadre A, Vial G, Capel F et al (2019) Sarcopenia. Jt bone Spine 86:309–314. https://doi.org/10.1016/j.jbspin.2018.08.001
Marzetti E, Picca A, Marini F et al (2019) Inflammatory signatures in older persons with physical frailty and sarcopenia: the frailty “cytokinome” at its core. Exp Gerontol 122:129–138. https://doi.org/10.1016/j.exger.2019.04.019
Tuttle CSL, Thang LAN, Maier AB (2020) Markers of inflammation and their association with muscle strength and mass: a systematic review and meta-analysis. Ageing Res Rev 64:101185
Bano G, Trevisan C, Carraro S et al (2017) Inflammation and sarcopenia: a systematic review and meta-analysis. Maturitas 96:10–15. https://doi.org/10.1016/j.maturitas.2016.11.006
Schaap LA, Pluijm SMF, Deeg DJH, Visser M (2006) Inflammatory markers and loss of muscle mass (Sarcopenia) and strength. Am J Med. https://doi.org/10.1016/j.amjmed.2005.10.049
Kumar P, Liu C, Hsu JW et al (2021) Glycine and N-acetylcysteine (GlyNAC) supplementation in older adults improves glutathione deficiency, oxidative stress, mitochondrial dysfunction, inflammation, insulin resistance, endothelial dysfunction, genotoxicity, muscle strength, and cognition: Re. Clin Transl Med 11:e372. https://doi.org/10.1002/ctm2.372
Custodero C, Mankowski RT, Lee SA et al (2018) Evidence-based nutritional and pharmacological interventions targeting chronic low-grade inflammation in middle-age and older adults: a systematic review and meta-analysis. Ageing Res Rev 46:42–59. https://doi.org/10.1016/j.arr.2018.05.004
Sardeli AV, Tomeleri CM, Cyrino ES et al (2018) Effect of resistance training on inflammatory markers of older adults: a meta-analysis. Exp Gerontol 111:188–196. https://doi.org/10.1016/j.exger.2018.07.021
Le Goff C, Laurent T, Kaux JFCJ (2012) Intense physical exercise related to the emergent generation of cardio-vascular risk markers: a review. Biol Sport 29:11–16
Delrieu L, Martin A, Touillaud M et al (2021) Sarcopenia and serum biomarkers of oxidative stress after a 6-month physical activity intervention in women with metastatic breast cancer: results from the ABLE feasibility trial. Breast Cancer Res Treat 188:601–613. https://doi.org/10.1007/s10549-021-06238-z
da Lage VK, S, de Paula FA, dos Santos JM, et al (2022) Are oxidative stress biomarkers and respiratory muscles strength associated with COPD-related sarcopenia in older adults? Exp Gerontol 157:111630. https://doi.org/10.1016/j.exger.2021.111630
Jones RL, Paul L, Steultjens MPM, Smith SL (2022) Biomarkers associated with lower limb muscle function in individuals with sarcopenia: a systematic review. J Cachexia Sarcopenia Muscle. https://doi.org/10.1002/jcsm.13064
Bae JH, Kwak SE, Lee JH et al (2019) Does exercise-induced apelin affect sarcopenia? a systematic review and meta-analysis. Hormones 18:383–393. https://doi.org/10.1007/s42000-019-00157-x
Jung HW, Park JH, Kim DA et al (2021) Association between serum FGF21 level and sarcopenia in older adults. Bone 145:115877. https://doi.org/10.1016/j.bone.2021.115877
Pratt J, De Vito G, Segurado R et al (2022) Plasma neurofilament light levels associate with muscle mass and strength in middle-aged and older adults: findings from GenoFit. J Cachexia Sarcopenia Muscle 13:1811–1820. https://doi.org/10.1002/jcsm.12979
Javanmardifard Z, Shahrbanian S, Mowla SJ (2021) MicroRNAs associated with signaling pathways and exercise adaptation in sarcopenia. Life Sci 285:119926. https://doi.org/10.1016/j.lfs.2021.119926
Yamamoto K, Ishizu Y, Honda T et al (2022) Patients with low muscle mass have characteristic microbiome with low potential for amino acid synthesis in chronic liver disease. Sci Rep 12:3674. https://doi.org/10.1038/s41598-022-07810-3
Ticinesi A, Nouvenne A, Cerundolo N et al (2019) Gut microbiota, muscle mass and function in aging: a focus on physical frailty and sarcopenia. Nutrients 11:1633. https://doi.org/10.3390/nu11071633
Rodriguez-Mañas L, Araujo de Carvalho I, Bhasin S et al (2020) ICFSR task force perspective on biomarkers for sarcopenia and frailty. J Frailty Aging 9:4–8. https://doi.org/10.14283/jfa.2019.32
Cawthon PM, Orwoll ES, Peters KE et al (2019) Strong relation between muscle mass determined by d3-creatine dilution, physical performance, and incidence of falls and mobility limitations in a prospective cohort of older men. J Gerontol Ser A Biol Sci Med Sci 74:844–852. https://doi.org/10.1093/gerona/gly129
Calvani R, Marini F, Cesari M et al (2017) Biomarkers for physical frailty and sarcopenia. Aging Clin Exp Res 29:29–34. https://doi.org/10.1007/s40520-016-0708-1
Yamada S, Tsuruya K, Yoshida H et al (2016) Factors associated with the serum myostatin level in patients undergoing peritoneal dialysis: potential effects of skeletal muscle mass and vitamin D receptor activator use. Calcif Tissue Int 99:13–22. https://doi.org/10.1007/s00223-016-0118-6
Zhou Y, Hellberg M, Hellmark T et al (2021) Muscle mass and plasma myostatin after exercise training: a substudy of renal exercise (RENEXC) — a randomized controlled trial. Nephrol Dial Transplant 36:95–103. https://doi.org/10.1093/NDT/GFZ210
Skladany L, Koller T, Molcan P et al (2019) Prognostic usefulness of serum myostatin in advanced chronic liver disease: Its relation to gender and correlation with inflammatory status. J Physiol Pharmacol 70:357–368. https://doi.org/10.26402/jpp.2019.3.03
Han DS, Chang KV, Li CM et al (2016) Skeletal muscle mass adjusted by height correlated better with muscular functions than that adjusted by body weight in defining sarcopenia. Sci Rep 6:1–8. https://doi.org/10.1038/srep19457
Gruson D, Ahn SA, Ketelslegers JM, Rousseau MF (2011) Increased plasma myostatin in heart failure. Eur J Heart Fail 13:734–736. https://doi.org/10.1093/eurjhf/hfr024
Kalinkovich A, Livshits G (2015) Sarcopenia—the search for emerging biomarkers. Ageing Res Rev 22:58–71. https://doi.org/10.1016/j.arr.2015.05.001
Chang J (2017) Circulating irisin levels as a predictiv biomarker for sarcopenia: a cross-sectional community-based study. Geriatr Gerontol Int 17:2266–2273
Willoughby DS, Beretich KN, Chen M, Funderburk LLK (2020) Decreased serum levels of C-terminal agrin in postmenopausal women following resistance training. J Aging Phys Act 28:73–80. https://doi.org/10.1123/japa.2019-0066
Lopez-Ruiz A, Kashani K (2020) Assessment of muscle mass in critically ill patients: role of the sarcopenia index and images studies. Curr Opin Clin Nutr Metab Care 23:302–311. https://doi.org/10.1097/MCO.0000000000000673
Huang C, Tomata Y, Kakizaki M et al (2015) High circulating adiponectin levels predict decreased muscle strength among older adults aged 70 years and over: a prospective cohort study. Nutr Metab Cardiovasc Dis 25:594–601. https://doi.org/10.1016/j.numecd.2015.03.010
Hui X, Lam KS, Vanhoutte PM, Xu A (2012) Adiponectin and cardiovascular health: an update. Br J Pharmacol 165:574–590. https://doi.org/10.1111/j.1476-5381.2011.01395.x
Galbreath M, Campbell B, Labounty P et al (2018) Effects of adherence to a higher protein diet on weight loss, markers of health, and functional capacity in older women participating in a resistance-based exercise program. Nutrients. https://doi.org/10.3390/nu10081070
Adamek A, Kasprzak A (2018) Insulin-like growth factor (IGF) system in liver diseases. Int J Mol Sci 19:1308. https://doi.org/10.3390/ijms19051308
Vitale G, Cesari M, Mari D (2016) Aging of the endocrine system and its potential impact on sarcopenia. Eur J Intern Med 35:10–15. https://doi.org/10.1016/j.ejim.2016.07.017
Westbury LD, Fuggle NR, Syddall HE et al (2018) Relationships between markers of inflammation and muscle mass, strength and function: findings from the Hertfordshire cohort study. Calcif Tissue Int 102:287–295. https://doi.org/10.1007/s00223-017-0354-4
Funding
The ESCEO Working Group was funded by ESCEO. The ESCEO receives Unrestricted Education Grants to support its educational and scientific activities from non-governmental organisations, not-for-profit organisations, non-commercial or corporate partners. The choice of topics, participants, content and agenda of the Working Group as well as the writing, editing, submission and reviewing of the manuscript are under the sole responsibility of the ESCEO, without any influence from third parties.
Author information
Authors and Affiliations
Contributions
JYR: organized the meeting. AL, EC and AM: performed the literature review. AL, EC, CB: have drafted the manuscript. All authors have taken part in the discussion and meeting and have critically revised, and given inputs to the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The views expressed in this article are the personal views of the authors and may not be understood or quoted as being made on behalf of or reflecting the position of the agencies or organizations with which the authors are affiliated. C. Beaudart reports to be a shareholder of SARQOL SRL, a spin-off of the University of Liege O. Bruyere reports grants or fees from AMGEN, APTISSEN, BIOPHYTIS, IBSA, MEDA, SANOFI, SERVIER, SMB, THERAMEX and UCB outside the submitted work. He also a shareholder of SARQOL SRL, a spin-off of the University of Liege. J-Y. Reginster reports consulting fees or paid advisory boards (IBSA-GENEVRIER, MYLAN, RADIUS HEALTH, PIERRE FABRE, ECHOLIGHT, TEVA), lecture fees when speaking at the invitation of sponsor (IBSA-GENEVRIER, MYLAN, CNIEL, DAIRY RESEARCH COUNCIL (DRC), TEVA), grant Support from Industry (All through Institution) (IBSA-GENEVRIER, MYLAN, CNIEL, RADIUS HEALTH). He also a shareholder of SARQOL SRL, a spin-off of the University of Liege as well as the CARES SRL, former spin-off of the University of Liège. AJ Cruz-Jentoft reports consulting fees or paid advisory boards from REJUVENATE BIOMED, RENEO PHARMACEUTICASL and AKROS PHARMA on the development of drugs to treat sarcopenia. Other authors reported no conflicts of interest in relation to this work.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ladang, A., Beaudart, C., Reginster, JY. et al. Biochemical Markers of Musculoskeletal Health and Aging to be Assessed in Clinical Trials of Drugs Aiming at the Treatment of Sarcopenia: Consensus Paper from an Expert Group Meeting Organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the Centre Académique de Recherche et d'Expérimentation en Santé (CARES SPRL), Under the Auspices of the World Health Organization Collaborating Center for the Epidemiology of Musculoskeletal Conditions and Aging. Calcif Tissue Int 112, 197–217 (2023). https://doi.org/10.1007/s00223-022-01054-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01054-z