Abstract
Background
Evaluation of disease activity in systemic lupus erythematosus (SLE) patients is important for modulating the therapeutic plan and decreasing organ damage. Autoantibodies are important serological biomarkers in SLE. We evaluated the effect of co-positivity of anti-dsDNA, anti-nucleosome, and anti-smith, autoantibodies on the SLEDAI score in SLE patients.
Results
Eighty adult SLE patients were included in this study. The correlations of the three autoantibodies with the SLEDAI score in addition to their sensitivity and specificity for the assessment of disease activity were analyzed. There was a highly significant difference between anti-dsDNA, anti-nucleosome, and anti-smith positive and negative groups as regards the SLEDAI score. Increased number of autoantibody positivity was associated with an increased mean rank of SLEDAI, and the three autoantibodies were positively correlated with each other and with the SLEDAI score. Roc curve analysis revealed that anti-smith has the highest sensitivity (90%) followed by anti-dsDNA and anti-nucleosome (85% for each). Moreover, anti-dsDNA had the highest specificity (88%) followed by anti-nucleosome (86%) then anti-smith (84%).
Conclusions
Anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies have a positive correlation with the SLEDAI score, and they may be considered as good serological biomarkers for the assessment of disease activity in SLE patients.
Similar content being viewed by others
Background
Systemic lupus erythematosus (SLE) is one of the autoimmune diseases which affects various body systems with a wide variety of clinical presentations [1]. The clinical manifestations consist of constitutional, mucocutaneous, musculoskeletal, hematologic, renal, and central nervous system manifestations [2]. Evaluation of disease activity in SLE patients is required for the follow-up and management, and it is also essential to modify the therapeutic plan and to predict the prognosis. The assessment of disease activity in SLE is important for distinguishing the disease flare from chronic damage, infection, and comorbidities [3]. Over the past 20 years, several indices have been developed to assess systemic lupus disease activity, and some of them have been validated. The most commonly used measures include the SLE Disease Activity Index (SLEDAI), the British Isles Lupus Assessment Group (BILAG) index, the Systemic Lupus Activity Measure (SLAM), the Lupus Activity Index (LAI), and the European Consensus Lupus Activity Measurement (ECLAM). All these measures are valid and have good reliability and responsiveness [4]. The SLEDAI is a scale that is specific to measure disease activity in adults with SLE. It can assess reversible manifestations of the underlying inflammatory disease process. The SLEDAI is ideal for the assessment of disease activity at an individual visit [5] and for organ/system assessment scales that assess disease activity in different organs [6].
Antinuclear antibodies (ANA) are autoantibodies that can recognize nuclear antigens and their complexes. ANA are considered as important biomarkers in the evaluation of several autoimmune diseases, most prominently SLE [7], and due to the role of immune complexes in the pathogenesis of the disease, some of these autoantibodies are considered as valuable laboratory findings to complete the clinical criteria of SLE [8]. The immune complexes which are formed from the interaction of ANA with antigens mediate disease pathogenesis by cytokine induction and tissue deposition. Some ANA can bind DNA and associated nucleosome proteins, while other ANA can bind RNA-binding proteins [9]. ANA testing and anti-extractable nuclear antigen (anti-ENA) are important serologic tests for SLE [10]. ANA can test positive in many autoimmune diseases and also in some normal individuals [8]. The effective care and follow-up of SLE patients depend on serological biomarkers. Serological tests are usually used for the assessment of disease activity and prediction of disease flare; one of these serological markers is the anti-dsDNA which increases with disease flare [11], but there are few serological biomarkers validated for mirroring lupus disease activity and prediction of flare from which the anti-dsDNA antibodies and complement, which can be deficient in some clinical situations as reliable markers for the assessment and follow-up of disease activity [12]. So, other autoantibodies like anti-nucleosome antibodies have been suggested as a specific marker for disease activity [8].
Several previous studies tried to explore the relationship between the clinical features of SLE and anti-ENA antibodies. Anti-dsDNA and anti-smith are autoantibodies against DNA with high specificity for SLE, and they are included in the classification criteria for SLE [13, 14]. Anti-dsDNA autoantibodies are highly specific for SLE; they are present in about 70% of cases, whereas they appear in only 0.5% of people without SLE [15]. Also, anti-dsDNA antibodies have been used as a marker for disease activity [16]. There is great interest in the identification of other biomarkers that can be used in the diagnosis and assessment of disease activity in SLE patients [17].
The nucleosome is the main element of chromatin, and it consists of about 170–200 base pairs of DNA wrapped around the two histone octamer [18]. It was suggested that the nucleosome is one of the important antigens playing an important role in the pathophysiology of lupus, and the presence of anti-nucleosome autoantibodies may contribute to organ damage [19]. Nucleosomes have been reported to have more strong immunogenicity than DNA or histones which induces a powerful T-helper cell response [20].
Assessment of anti-chromatin antibodies including anti-histone and anti-nucleosome may be important for identifying the high-risk patients for proliferative lupus nephritis (LN) [21]. Anti-nucleosome autoantibodies have nearly equal specificity and higher sensitivity than anti-dsDNA antibodies in LN patients [22]. So, it is important to evaluate the effect of anti-nucleosome autoantibody positivity on SLEDAI score [23] particularly in patients with renal involvement [24]. Anti-nucleosome antibodies were suggested as important markers and as complementary to anti-dsDNA antibodies for SLE diagnosis, and they should be included in the criteria for the diagnosis of SLE [25]. Recently, it has been suggested that combined positivity for anti-nucleosome and anti-dsDNA antibodies may have a prognostic value especially for renal affection, and this supports the routine assessment of these autoantibodies [22]. Despite previous studies reported increased anti-nucleosome antibodies in SLE patients particularly with increased disease activity, few data are available showing their suitability for monitoring disease activity [26].
Anti-smith antibodies are autoantibodies against seven proteins that consist of a core of small nuclear ribonucleoprotein [27]. The specificity of anti-smith autoantibodies for classification of SLE reached 90% in a previous study [28]. Despite the very high specificity of the anti-smith antibodies for SLE, its significance is still unclear [13]. It is reported that patients who are positive for anti-smith antibodies are more liable to the renal and central nervous system involvement [8]. The identification of active SLE patients depends on clinical symptoms and signs in addition to the serological abnormalities. So, there is a high demand for the presence of a serological biomarker with high sensitivity and specificity for the assessment of SLE disease activity, and as anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies are present with a high frequency in SLE patients especially those with organ damage and they have high specificity for the diagnosis of SLE we aimed to evaluate the effect of co-positivity of these three autoantibodies on SLEDAI score and to determine their sensitivity and specificity for the assessment of disease activity in SLE patients.
Methods
The data from 80 adult SLE patients (75 females (93.7%) and 5 males (6.3%)) were collected from the Rheumatology and Rehabilitation Department, from October 2019 to November 2020. The patients were classified as SLE according to the 2012 SLICC classification criteria for SLE [14]. Patients with other autoimmune diseases, systemic diseases, hematological disorders, malignancy, and pregnancy were excluded. Routine laboratory investigations were performed in the form of CBC, ESR, CRP, urine analysis, protein/creatinine ratio (P/C), 24 h urinary proteins, and kidney function tests, in addition to ANA and ANA profile. The disease activity in our patients was assessed by the SLEDAI score which consists of 24 “weighted” items grouped into 9 domains. The final score is the sum of all weighted attributed scores: 0 means no activity, 1–5 means mild activity, 6–10 means moderate activity, 11–19 means high activity, and more than 20 is considered as very high activity [29].
Antinuclear antibody (ANA) was measured by the indirect immunofluorescence technique (IIF). Using ANAFLUOR, Stillwater, DiaSorin, MN 55082-0285, USA, kit following the instructions in the manufacturing protocol. The patient serum was diluted and then dropped onto HEP-2 cells which are fixed as separated dots on the slide as a source of nuclei, incubated with patient serum for 30 min then washed, and secondary antibodies which are conjugated with FITC were added and incubated for 30 min followed by washing the slides then the fluorescence microscope was used for detecting the fluorescence [30], the positive ANA test is reported as both a pattern and a titer. Anti-dsDNA, Anti-nucleosome, and anti-smith autoantibody positivity were recorded from the ANA profile. The ANA profile was done by the EIA technique, using Blue Diver Quantrix ANA IgG kit (Code: ANA19Q-24) for BlueDiver Instrument (BDI), according to the manufacturer’s protocol [31]. All patients gave their informed consent before their inclusion in the study.
Statistical analysis
Data were analyzed statistically using SPSS, version 20 (IBM Corp., Armonk, USA). The results were shown as mean ± SD in normally distributed data. Qualitative data were shown as percentages and numbers. The correlations were done between the autoantibodies using Kendall’s tau-b. For normally distributed variables, an independent-sample t test was used. To compare the three groups for the not normally distributed data, the Kruskal-Wallis test was used followed by the Mann-Whitney U test to compare between every two groups. ROC curve analysis was done to calculate the sensitivity, specificity, and area under the curve for the three autoantibodies.
Results
Our study included 80 adult SLE patients (75females (93.7%) and 5males (6.3%). Table 1 shows the demographic and clinical manifestations of the patients. The most frequent clinical manifestations were mucocutaneous and musculoskeletal manifestations (92.5% for each). Followed by hematological (58.75%), LN (46.25%), and constitutional manifestations (31.25%). Table 2 shows the laboratory findings and medication characteristics of the patients; ANA was positive in 97.5% of the patients. Regarding the medications, steroids and hydroxychloroquine were used by 100% of the patients, azathioprine was used by (60%) of the patients, cyclophosphamide was used by (43.75%) of the patients, and methotrexate was used by (27.5%) of the patients.
Distribution of the three autoantibodies in different grades of SLEDAI
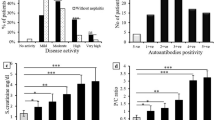
According to the SLEDAI score, 7.5% of our patients had no activity, 10% had mild activity, 20% had moderate activity, 35% had high activity, and 27.5% had very high disease activity. The anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies were present with high frequency in the high disease activity group (38.58%, 32.26 %, and 35.72%, respectively), and also in the very high disease activity group (25.72%, 25.8%, and 28.58%, respectively) and present with low frequency in patients with mild disease activity (10%, 14.52, and 10.71% respectively) and no disease activity (4.28%, 6.45%, and 7.14% respectively), and the three autoantibodies showed significant differences between low and high disease activity patients as shown in Fig. 1.
Effect of anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies on SLEDAI score
Seventy patients (87.5%) were positive for anti-dsDNA, 62 (77.5%) for anti-nucleosome, and 34 (42.5%) for anti-smith. By comparing the SLEDAI scores between the positive and negative groups for each autoantibody, there was a highly significant difference between anti-dsDNA, anti-nucleosome, and anti-smith positive and negative groups (p < 0.001, p < 0.001, p = 0.005, respectively), as shown in Table 3.
Co-positivity of the three autoantibodies in SLE patients and their effect on disease activity
Six (7.5%) of our patients were negative for the three autoantibodies, eleven (13.75%) were positive for one autoantibody, forty-one (51.25%) were positive for two autoantibodies, and twenty-two (27.5%) were positive for the three autoantibodies. By comparing the mean rank of SLEDAI, in the patients according to the autoantibody positivity, the mean rank of SLEDAI was significantly higher (p = 0.001) in patients with two and three positive autoantibodies than in patients with three negative autoantibodies, and by increasing the number of positivity, the mean rank of SLEDAI increased in all groups except in one positive when compared with three negatives where the mean rank of SLEDAI was high but insignificant as shown in Table 4.
Correlations of the three autoantibodies with the SLEDAI score
Regarding the correlation of the three autoantibodies with the SLEDAI score, we found that the anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies were positively correlated with each other and with the SLEDAI score, as shown in Table 5.
ROC curve analysis for the detection of autoantibodies sensitivity and specificity for disease activity
ROC curve analysis revealed that anti-smith had the highest AUC (0.851) with 90% sensitivity and 84% specificity at a cutoff of 9.5U/ml followed by anti-dsDNA with AUC 0.818, 85% sensitivity, and 88% specificity at cut off 49U/ml then anti-nucleosome with AUC 0.812, 85% sensitivity and 86% specificity at a cutoff 42U/ml as shown in Fig. 2.
Discussion
Autoantibodies represent the hallmarks of SLE. Ninety-eight percent of the autoantibodies detected in the sera of SLE patients bind to cell nucleus antigens, like ANA [32]. Autoantibodies induce tissue injury through several mechanisms including the formation of immune complexes, binding to the cell surface and cytotoxicity, reactivity with autoantigens which are expressed on activated or apoptotic cell surfaces, penetration of living cells, and conjugation with cross-reactive extra-cellular molecules [32]. The immune complexes bind FC receptors with modulation of the innate and adaptive immune responses [33]. Genetic variants of FC receptors were associated with susceptibility to SLE and with the disease severity [34]. Autoantibodies are essential biomarkers for autoimmune diseases, and they have important diagnostic and prognostic values in SLE [22]. Recently, there is a huge interest towards the use of ANA as a predictor for disease progression in SLE, aiming to decrease morbidity and mortality [33].
Our findings suggested that the mean SLEDAI score increased with the increased number of autoantibodies positivity, and this was obvious in comparing the mean rank of SLEDAI of patients who are positive for two or three autoantibodies with those who are negative for all autoantibodies (p = 0.001) and also on comparing one positive with two positives, one positive with three positives and two positives with three positives (p = 0.016, p = 0.007, p = 0.001, respectively). This result is near to that of Elsayed and Mohafez, who found that when the autoantibodies positivity increased, the mean rank of SLEDAI increased [22].
Our study showed a highly significant difference between positive and negative groups for anti-dsDNA, anti-nucleosome, and anti-smith regarding the SLEDAI score (p = 0.001, p = 0.005, p = 0.001, respectively). Our findings revealed that the three autoantibodies were positively correlated with each other and with the SLEDAI score. This agrees with several previous studies that focused on the role of individual ANA autoantibodies in SLE. Anti-dsDNA antibody titer is known to be correlated with disease activity, so it is considered as a good marker for disease activity in SLE [26]. A previous study suggested that anti-dsDNA antibodies positively correlate with lupus disease activity, and serial measurements of anti-dsDNA antibodies are important for follow-up and prediction of disease flare [35]. A significant association was reported between anti-dsDNA antibodies and lupus disease activity [23]. Mahmoudi et al. showed that the positive correlation between the SLEDAI score and anti-dsDNA was expected because anti-dsDNA is one of the items included in the calculation of the SLEDAI score [36]. Suleiman et al. and Abdalla et al. reported that there was a significant correlation between anti-nucleosome antibodies and the disease activity indices [23, 25]. Several previous studies reported a correlation between anti-nucleosome antibodies and SLEDAI [5, 25, 37]. Thus, serial measurements of anti-nucleosome antibodies may provide a good reflection for the assessment of disease activity [8]. Elsayed and Mohafez found that anti-nucleosome and anti-dsDNA both show a positive correlation with SLEDAI, but anti-dsDNA antibodies show a stronger correlation with SLEDAI than anti-nucleosome antibodies [22]. Živković et al. found a significant correlation between anti-dsDNA and anti-nucleosome antibodies, which suggested a link between ds-DNA-specific immune response and nucleosomes in patients with SLE. This serologic overlap may be because these autoantibodies share the same antigenic structure and reactivities [38]. Hung et al. reported a high frequency of anti-dsDNA positivity in patients who are anti-nucleosome positive [39]. Elsayed and Mohafez found that anti-nucleosome and anti-dsDNA antibodies were correlated with each other and with the SLEDAI score [22]. Ahn et al. found a significant correlation between anti-smith antibody titer and SLEDAI score, and anti-dsDNA titer and alterations in anti-smith antibody titer may reflect alterations in lupus disease activity so, it can be used as a serological marker for the assessment of disease activity in SLE patients [13]; also, Emad et al. found that anti-smith autoantibodies were positively correlated with disease activity in SLE patients [40]. Anti-smith antibodies were found to be negatively correlated with complement level and as the activation of complement is an important pathway in SLE pathogenesis, so activation of complement by anti-smith antibodies may be one of the mechanisms explaining the relation between anti-smith antibodies and disease activity in lupus [13].
ROC curve analysis revealed that anti-dsDNA had the highest specificity for SLE disease activity followed by anti-nucleosome then anti-smith, while anti-smith had the highest sensitivity, followed by anti-dsDNA and anti-nucleosome. Our data suppose that anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies have high sensitivity and specificity for SLE disease activity and co-positivity of these three autoantibodies markedly affects the SLEDAI score; thus, they may be considered as valuable serological biomarkers for assessment of disease activity in SLE patients.
Conclusions
Anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies have a positive correlation with the SLEDAI score, and they may be considered as good serological biomarkers for the assessment of disease activity in SLE patients.
Availability of data and materials
The data of the current study are available from the corresponding author on reasonable request.
Abbreviations
- SLE:
-
Systemic lupus erythematosus
- ROC:
-
Receiver operating characteristic
- ANA:
-
Anti-nuclear antibodies
- SLEDAI:
-
Systemic Lupus Erythematosus Disease Activity Index
- AUC:
-
Area under the curve
References
Tsokos GC (2011) Systemic lupus erythematosus. N Engl J Med 365:2110–2121
Merola JF, Bermas B, Lu B, Karlson E, Massarotti E, Schur PH, Costenbader KH (2014) Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus 23:778–784
Thanou A, Jupe E, Purushothaman M, Niewold TB, Munroe ME (2021) Clinical disease activity and flare in SLE: current concepts and novel biomarkers. J Autoimmun 119:102615
Griffiths B, Mosca M, Gordon C (2005) Assessment of patients with systemic lupus erythematosus and the use of lupus disease activity indices. Best Pract Res Clin Rheumatol 19:685–708
Ibañez D, Gladman D, Urowitz M (2007) Summarizing disease features over time: II. Variability measures of SLEDAI-2K. J Rheumatol 34:336–340
Romero-Diaz J, Isenberg D, Ramsey-Goldman R (2011) Measures of adult systemic lupus erythematosus: updated version of British Isles Lupus Assessment Group (BILAG 2004), European Consensus Lupus Activity Measurements (ECLAM), Systemic Lupus Activity Measure, Revised (SLAM-R), Systemic Lupus Activity Questionnaire for Population Studies (SLAQ), Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K), and Systemic Lupus International Collaborating Clinics/American College of Rheumatology Damage Index (SDI). Arthritis Care Res 63:S37–S46
Pisetsky DS (2017) Antinuclear antibody testing-misunderstood or misbegotten? Nat Rev Rheumatol 13:495–502
Arroyo-Ávila M, Santiago-Casas Y, McGwin G, Cantor RS, Petri M, Ramsey-Goldman R, Reveille JD, Kimberly RP, Alarcón GS, Vilá LM (2015) Clinical associations of anti-Smith antibodies in PROFILE: a multi-ethnic lupus cohort. Clin Rheumatol 34:1217–1223
Pisetsky DS, Lipsky PE (2020) New insights into the role of antinuclear antibodies in systemic lupus erythematosus. Nat Rev Rheumatol 16:565–579
Pons-Estel GJ, Alarcon GS, Scofield L, Reinlib L, Cooper GS (2010) Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum 39:257–268
Steiman AJ, Urowitz MB, Ibañez D, Li TT, Gladman DD, Wither J (2015) Anti-dsDNA and antichromatin antibody isotypes in serologically active clinically quiescent systemic lupus erythematosus. J Rheumatol 42:810–816
Akhter E, Burlingame R, Seaman A, Magder L, Petri M (2011) Anti-C1q antibodies have higher correlation with flares of lupus nephritis than other serum markers. Lupus 20:1267–1274
Ahn SS, Jung SM, Yoo J, Lee S-W, Song JJ, Park Y-B (2019) Anti-Smith antibody is associated with disease activity in patients with new-onset systemic lupus erythematosus. Rheumatol Int 39:1937–1944
Petri M, Orbai AM, Alarcon GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O (2012) Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 64:2677–2686
Rahman A, Isenberg DA (2008) Systemic lupus erythematosus. N Engl J Med 358:929–939
Muller S, Dieker J, Tincani A, Meroni P (2008) Pathogenic anti-nucleosome antibodies. Lupus 17:431–436
Wandstrat AE, Carr-Johnson F, Branch V, Gray H, Fairhurst A-M, Reimold A, Karp D, Wakeland EK, Olsen NJ (2006) Autoantibody profiling to identify individuals at risk for systemic lupus erythematosus. J Autoimmun 27:153–160
McGinty RK, Tan S (2015) Nucleosome structure and function. Chem Rev 115:2255–2273
Rodriguez-Jimenez NA, Perez-Guerrero EE, Gamez-Nava JI, Sanchez-Mosco DI, Saldaña-Cruz AM, Alcaraz-Lopez MF, Fajardo-Robledo NS, Muñoz-Valle JF, Bonilla-Lara D, Diaz-Rizo V (2020) Anti-nucleosome antibodies increase the risk of renal relapse in a prospective cohort of patients with clinically inactive systemic lupus erythematosus. Sci Rep 10:1–10
Ghiggeri GM, D’Alessandro M, Bartolomeo D, Degl’Innocenti ML, Magnasco A, Lugani F, Prunotto M, Bruschi M (2019) An update on antibodies to necleosome components as biomarkers of sistemic lupus erythematosus and of lupus flares. Int J Mol Sci 20:5799
Sui M, Lin Q, Xu Z, Han X, Xie R, Jia X, Guo X, Zhang W, Guan X, Ren H (2013) Simultaneous positivity for anti-DNA, anti-nucleosome and anti-histone antibodies is a marker for more severe lupus nephritis. J Clin Immunol 33:378–387
Elsayed SA, Mohafez OMM (2020) Autoantibodies spectrum in lupus nephritis in a cohort of Egyptian patients: relation to disease activity and prognostic value. Egypt Rheumatol Rehabil 47:1–10
Abdalla MA, Elmofty SA, Elmaghraby AA, Khalifa RH (2018) Anti-nucleosome antibodies in systemic lupus erythematosus patients: relation to anti-double stranded deoxyribonucleic acid and disease activity. Egypt Rheumatol 40:29–33
Simon J, Cabiedes J, Ortiz E, Alcocer-Varela J, Sanchez-Guerrero J (2004) Anti-nucleosome antibodies in patients with systemic lupus erythematosus of recent onset. Potential utility as a diagnostic tool and disease activity marker. Rheumatology 43:220–224
Suleiman S, Kamaliah D, Nadeem A, Naing NN, Che Maraina CH (2009) Anti-nucleosome antibodies as a disease activity marker in patients with systemic lupus erythematosus. Int J Rheum Dis 12:100–106
Shang X, Ren L, Sun G, Yu T, Yao Y, Wang L, Liu F, Zhang L, He X, Liu M (2021) Anti-dsDNA, anti-nucleosome, anti-C1q, and anti-histone antibodies as markers of active lupus nephritis and systemic lupus erythematosus disease activity. Immun Inflamm Dis 9:407–418
Migliorini P, Baldini C, Rocchi V, Bombardieri S (2005) Anti-Sm and anti-RNP antibodies. Autoimmunity 38:47–54
Flechsig A, Rose T, Barkhudarova F, Strauss R, Klotsche J, Dähnrich C, Schlumberger W, Enghard P, Burmester G-R, Hiepe F (2017) What is the clinical significance of anti-Sm antibodies in systemic lupus erythematosus? A comparison with anti-dsDNA antibodies and C3. Clin Exp Rheumatol 35:598–606
Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH, Austin A, Bell A, Bloch DA, Corey PN, Decker JL (1992) Derivation of the SLEDAI. A disease activity index for lupus patients. Arthritis Rheum 35:630–640
Peng X, Tang J, Wu Y, Yang B, Hu J (2014) Novel method for ANA quantitation using IIF imaging system. J Immunol Methods 404:52–58
Kern P, Kron M, Hiesche K (2000) Measurement of antinuclear antibodies: assessment of different test systems. Clin Diagn Lab Immunol 7:72–78
Rekvig OP, Putterman C, Casu C, Gao H-X, Ghirardello A, Mortensen ES, Tincani A, Doria A (2012) Autoantibodies in lupus: culprits or passive bystanders? Autoimmun Rev 11:596–603
Li X, Kimberly RP (2014) Targeting the Fc receptor in autoimmune disease. Expert Opin Ther Targets 18:335–350
Crispín JC, Hedrich CM, Tsokos GC (2013) Gene-function studies in systemic lupus erythematosus. Nat Rev Rheumatol 9:476–484
Narayanan K, Marwaha V, Shanmuganandan K, Shankar S (2010) Correlation between systemic lupus erythematosus disease activity index, C3, C4 and anti-dsDNA antibodies. Med J Armed Forces India 66:102–107
Mahmoudi M, Rastin M, Sahebari M, Zamani S, Tabasi N (2017) Autoantibody profile, disease activity and organ involvement in Iranian systemic lupus erythematosus patients. Rheumatol Res 2:11–16
Mok CC, Ho LY, Leung HW, Wong LG (2010) Performance of anti-C1q, antinucleosome, and anti-dsDNA antibodies for detecting concurrent disease activity of systemic lupus erythematosus. Transl Res 156:320–325
Živković V, Stanković A, Cvetković T, Mitić B, Kostić S, Nedović J, Stamenković B (2014) Anti-dsDNA, anti-nucleosome and anti-C1q antibodies as disease activity markers in patients with systemic lupus erythematosus. Srpski arhiv za celokupno lekarstvo 142:431–436
Hung W, Chen Y, Lan J, Chen H, Chen Y, Chen D, Hsieh C, Wen M (2011) Antinucleosome antibodies as a potential biomarker for the evaluation of renal pathological activity in patients with proliferative lupus nephritis. Lupus 20:1404–1410
Emad Y, Gheita T, Darweesh H, Klooster P, Gamal R, Fathi H, El-Shaarawy N, Gamil M, Hawass M, El-Refai R (2018) Antibodies to extractable nuclear antigens (ENAS) in systemic lupus erythematosus patients: correlations with clinical manifestations and disease activity. Reumatismo 70:85–91
Acknowledgements
None
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
The conceptualization of this study and selection and diagnosis of patients were performed by S.A.E. and M.A.E. The collection of the data was performed by H.M.K. The manuscript was written and revised by S.A.E. and H.M.K. The data interpretation was performed by S.A.E. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was carried out in accordance with the ethical standards laid down in the Helsinki Declaration of 1975 and its later amendments in 2000 and approved by the Medical Research Ethics Committee, Faculty of Medicine, Sohag University, under the reference number Soh-Med-8-9-19. All patients included in this study gave written informed consent to participate in this research.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elsayed, S.AR., Kamaly, H.M. & Esmail, M.A. Co-positivity of anti-dsDNA, anti-nucleosome, and anti-smith autoantibodies as serological biomarkers for disease activity in systemic lupus erythematosus. Egypt Rheumatol Rehabil 49, 8 (2022). https://doi.org/10.1186/s43166-021-00110-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43166-021-00110-0