Abstract
Background
External auditory canal cholesteatoma (EACC) is a rare pathological condition representing 0.1% of all new otologic cases. Bilaterality manifests in 10% of EACC cases. Similar disease processes include keratosis obturans (KO) and benign necrotizing otitis externa (BNOE). Diagnostic differentiation may not always be unexacting but does however influence management and eventual outcome. This study aims to briefly review the literature, describe an unusual case of synchronous bilateral primary EACC, and simplify the diagnostic challenges surrounding this disease.
Case presentation
A 66-year-old man with no relevant family history, no concurrent or intercurrent illnesses, having undergone no relevant surgery, and had visited the Department of Otorhinolaryngology regarding right-sided otorrhoea and aural pruritis 9 years ago. At the time, he was diagnosed clinically with KO. Nine years later after having defaulted follow-up, he presented again with the same symptoms. Oto-microscopy now revealed excavation of the posterior canal wall, keratinous desquamation, and an intact tympanic membrane. Oto-endoscopy demonstrated extension posteriorly into the mastoid segment of the temporal bone. Computed tomography evinced a soft tissue attenuating mass within the excavated sub-adjacent bone, with extension into the mastoid air cell system. The contralateral ear had a smaller epithelial defect of the canal floor with underlying tympanic plate erosion. A closed mastoidectomy with the reconstruction of the posterior canal wall was performed on the right ear. The left ear was managed conservatively with micro-suctioning and aural toilette. Six months post-surgery, however, the reconstructed posterior canal wall underwent necrotic breakdown. The contralateral lesion gradually progressed into a Naim et al. (2005) Stage III EACC.
Conclusion
This case illustrates the diagnostic challenge presented by EACC and is unusual regarding its bilaterality. The importance of diagnostic differentiation, both in the management of this disease and in the prevention of its progression, is highlighted. The idea of oto-endoscopy as a routine clinical tool is introduced, and endoscopic images are compared to high-definition computed tomography scans of the affected temporal bones.
Similar content being viewed by others
Background
External auditory canal cholesteatoma (EACC) is a rare disease with an estimated incidence proportion amongst new otologic patients of 0.1–0.5% [1, 2], and an annual incidence rate amongst the general population of 0.15 cases per 100,000 individuals [2], compared to 9.2 per 100,000 for middle-ear cholesteatoma [3].
EACC was first described by Toynbee in 1850 [4], who investigated a lesion of the external auditory canal (EAC) which he considered to be molluscum contagiosum. Schofield introduced the term EACC in 1893 [5]. In 1943, Altmann and Waltner concluded that the EACC results from the invasion of a sequestrated portion of the osseous EAC by keratinizing stratified squamous epithelium [6]. There remained confusion surrounding its relationship to other processes of the EAC, especially keratosis obturans (KO), until Piepergerdes et al. made the clinical distinction between the two in 1980 [7]. In 1984, Naiberg et al. demonstrated the histopathological differences between EACC and KO, the distinguishing feature of EACC being the focal invasion of necrotic bone by squamous epithelium, in comparison to the circumferential accumulation of desquamated keratin within a uniformly expanded EAC as seen in KO [8]. It is thus the focal nature of EACC that distinguishes it from KO [7]. For every case of EACC, there are four or five cases of KO [1].
Histopathology
The histopathology of EACC is characterised by hyperproliferative keratinizing squamous epithelium with an intact basement membrane and increasing accumulation of stromal inflammatory cells (mast cells, lymphocytes, monocytes) [9]. Periosteitis accompanies higher grades of EACC and results in dull otalgia. Invasion of EACC into deeper mesenchymal tissue results in necrotic osseous excavation. Standard haematoxylin and eosin staining demonstrate sporadic epithelial cones, which are extensions of the basal cell layer into the subepithelial connective tissue [9]. This osteonecrosis and overlying focal loss of epithelium are pathognomonic of the diagnosis [10]. Osseous erosion is thought to be caused by proteolytic enzymes produced within the matrix lining. This may be hastened by superimposed infection with ulceration and reactive granulation tissue formation [11]. The keratin sac contains sequestered bony fragments, and random keratin is found within the EAC lumen [8, 12]. Further extension may involve the middle ear, mastoid, sigmoid sinus, temporomandibular joint, or fallopian canal [1, 2, 13,14,15]. Albeit rare, posterior cranial fossa extension with resulting intracranial abscess has been reported [16].
Naim et al. sought to classify the different stages of EACC [17] based on the depth of invasion of the lesion (Table 1). The evolutionary continuum begins with hyperproliferation of the epithelium, which becomes inflamed and results in periostitis, eventually denuding the underlying bone. Denudation leads to necrobiosis and excavation, leaving an osseous defect which collects keratinous debris, and can ultimately involve neighbouring anatomical structures.
Etiopathogenesis
The etiopathogenesis of EACC remains unclear [7,8,9, 18,19,20]. In keeping with earlier studies [6, 21], Sakamoto and Kitahara suggested that any disease of the osseous EAC could be a potential cause of EACC [22].
Holt divided EACC into five groups based on its etiopathogenesis. In order of prevalence, these are the following [13]: primary/spontaneous, postsurgical, posttraumatic, secondary to acquired EAC obstruction, and secondary to congenital EAC stenosis [23].
Although there have been cases reported in children and young adults [24, 25], primary/spontaneous EACC is generally regarded as a disease of the elderly. It results from a reduction in the migratory capacity of the EAC epithelium causing disruption to the self-cleansing mechanism and leading to retention of desquamated epithelium, i.e. ‘keratinization in situ’ [2, 13, 17, 26]. The accumulation of desquamated epithelium predisposes to inflammation, which in turn results in epithelial hyperproliferation [9]. Once this stage is established, active in-growth and bone resorption can occur [9, 23, 26].
Although recently challenged [27], this theory is supported by the findings of Makino and Amatsu, who found that the epithelial migratory rate in a normal ear is highest along the inferior EAC, at approximately 142 µm/day [1, 26]. These authors observed a markedly reduced migratory rate in this region of patients with EACC, likely attributable to a reduction in blood supply to this already tenuously perfused area [26]. In response to hypoxia, normal EAC skin may induce uncontrolled angiogenesis, predisposing to EACC formation [17, 26]. Hypoxic angiogenesis has been confirmed by studies of the angiogenic factors: hepatocyte growth factor/scatter factor (HGF/SF) and vascular endothelial growth factor (VEGF) and the tyrosine-kinase c-Met receptor, all of which are strongly expressed in specimens of EACC, induction of which results in an enhanced proliferation rate of these specimens [28, 29]. Adamczyk et al. also analysed the biological behaviour of EACC using the expression and distribution of the biomarkers transforming growth factor-α (TGF-α), epidermal growth factor-receptor (EGFR), and the proliferation marker MIB 1, in comparison to that of normal EAC skin [9]. It has long been established that TGF-α stimulates the growth of several types of epithelial tissues [30]. Mediated by TGF-α binding, the EGFR controls these cellular activities [31]. The authors demonstrated overexpression of EGFR and TGF-α as well as higher mitotic activity in the EACC epithelium, evidenced by a significantly higher number of MIB-1-positive cells, reflecting a hyperproliferative state. These results suggest that a chronic inflammatory process underlies EACC, potentially altering keratinocyte proliferation [9].
Surgery and trauma to the EAC may cause entrapment of squamous epithelium during the healing process, or predispose to periostitis, with subsequent invasion and proliferation of the squamous epithelium [23, 24, 32,33,34].
In some cases, the occurrence of EACC has been linked to branchial arch anomalies, which result in the retention of epithelial masses [19, 35].
Clinical features
A recent meta-analysis of published case series concluded that the most common presenting symptoms of idiopathic EACC are unilateral otorrhoea and mild to moderate otalgia [18]. The otorrhoea is thought to be related to an associated localised infection by a variety of organisms, most commonly Pseudomonas aeruginosa [13]. Bilaterality occurs in one-in-six to one-in-12 cases [1, 14], and synchronous bilateral cases have been reported [33].
Otoscopic findings include a localised area of epithelial erosion, an intact tympanic membrane, and a normal middle ear [9]. There is frequent osseous sequestration of the underlying EAC, and the defect is filled with keratin debris [9]. The majority of idiopathic EACCs occur along the inferior EAC [18], and most patients present with a Naim et al. Stage II EACC [17].
Epidemiological risk factors for the development of EACC include smoking, diabetes mellitus, and repeated micro-trauma (e.g. from cotton buds or hearing aids) [18, 19]. Smoking and diabetes are thought to result in microangiopathic changes in the EAC that potentially impair epithelial migration [18].
Radiographic features
Computed tomography (CT) of the temporal bones has become accepted as the gold standard for staging and preoperative planning in EACC [36]. Previous reports have shown that EACC is often more extensive than what is suggested by the clinical findings [37]. CT allows accurate evaluation of the extent of local bone erosion and examination for the involvement of adjacent landmark structures, namely the middle ear, tegmen tympani, mastoid, skull base, and fallopian canal. The involvement of these structures may determine management [13, 38].
Heilbrun et al. sought to define the CT features of EACC, as well as to determine which of these features impact its clinical management most prominently [13]. The authors concluded that the cardinal features of EACC are a soft tissue attenuating mass, most commonly involving the inferior-posterior portion of the EAC, with an erosion of the sub-adjacent bone [7, 37]. Bone fragments are often visible within the soft tissue mass as small, hyperattenuating flecks. The bone erosion adjacent to the soft tissue mass may be smooth, similar to a middle ear cholesteatoma, or irregular due to periostitis and osseous necrosis [13, 39].
Shin et al. classified EACC according to CT findings, with suggested management strategies for each stage of the disease (Table 2) [36].
Management
The current management of EACC is highly variable between centres [38]. Holt suggested dividing the treatment options for EACC into conservative and surgical, based on the pathological extent of the disease [23]. Treatment aims are disease eradication, preservation of normal structure and function, and the restoration of normal epithelial migration and thus a self-cleaning EAC [38].
Conservative therapy, with frequent debridement of the keratin debris and sequestered bone, is favoured in early-stage disease [18, 40]. Topical treatment with antibiotic and corticosteroid-impregnated gauze [41] or drops [1] may aid in expediting disease control. For refractory or more advanced cases (Naim et al. Stages II–IV) [17], surgical intervention is necessary [1, 7, 14, 38].
If the lesion does not extend into the mastoid air cell system, either a per-meatal or postauricular approach may be used. The postauricular approach is favoured for more adequate cholesteatoma extirpation, necrotic bone debridement, canaloplasty, and fascia/perichondrium/cartilage grafting to cover the canal defect [8, 17, 37, 42]. Garin et al. also suggested considering a split-thickness skin graft to cover the defect [37]. Tos recommended an inferiorly based subcutis-muscle-periosteum flap, harvested along the posterior edge of the postauricular incision, to obliterate the defect [41]. Jahnke and Lieberum suggested that the decision to place a graft must be based on the surgeon’s satisfaction that no epithelial fragments remain obscured in the osseous defect, lest these provoke recidivism [43].
Case presentation
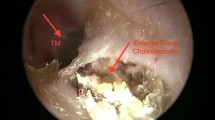
A 66-year-old man with no relevant family history, no concurrent or intercurrent illnesses, having undergone no relevant surgery, and visited the Department of Otorhinolaryngology regarding right-sided otorrhoea and aural pruritis 9 years ago. He was lost to follow-up after micro-debridement of what was clinically described at the time as KO. He now presented, 9 years later, with the same symptoms. Oto-microscopy of the right ear revealed irregular keratinous desquamation. Aural toileting uncovered a granulomatous excavation of the posterior EAC containing keratin and sequestered osseous fragments, against the backdrop of a normal tympanic membrane. Oto-endoscopy demonstrated extension posteriorly into the mastoid segment of the tympanic bone (Fig. 1).
Contralateral otoscopy disclosed a hyperkeratotic lesion of the inferior EAC. Computed tomography (CT) evinced soft tissue attenuation containing hyper-attenuating flecks within an excavated right-sided temporal bone. This excavation extended into the mastoid air cell system, making it a Stage III EACC as classified by Shin et al. [36]. The contralateral ear enclosed early tympanic plate erosion, making it a Stage I EACC according to the classification of Shin et al. [36]. The CT scan thus bespoke bilateral EACC [13], consistent with the suggestion by Holt (1992) that most of these lesions are idiopathic and therefore discovered incidentally (Fig. 2) [23].
Pure-tone audiometry showed an asymmetrical, bilateral, and pantonal sensorineural hearing loss. The right ear presented a moderate and the left ear a severe loss. This demonstrated poor correlation with disease severity between the respective ears. As such, it was decided that the hearing loss was most likely presbycusis, rather than being directly related to EACC.
A canaloplasty and canal wall-up mastoidectomy of the right ear were performed, as advocated by Shin, Shim, and Lee (2010) [36]. A retro-auricular incision was used for the surgical approach, which allowed harvesting of temporalis muscle fascia as well as cartilage of the auricular concha via the same incision. The tympanic membrane, being uninvolved by the disease process, was not operated upon. All squamous epithelium was removed from the EAC and mastoid air cell system, and the defect created by the disease was drilled smooth using a diamond burr, being careful not to spuriously open the mastoid segment of the fallopian canal of the facial nerve. The resultant cavity, which now connected the EAC and the mastoid air cell system, was irrigated with normal saline so as to remove all remaining squamous epithelial cells. The posterior wall of the EAC was then grafted with cartilage and fascia, the harvesting of which has been described above, in accordance with the recommendations of a variety of authors (Fig. 3) [8, 29, 37].
In anticipation of disease control, the left ear was managed conservatively with regular debridement and topical antibiotics, congruent with the suggestions of Tos (1997) [41], as well as those of a recent meta-analysis by Dubach, Mantokoudis, and Caversaccio (2010) [18].
Six months post-surgery, however, the fascial graft underwent necrotic breakdown. The resultant defect was small and was allowed to heal by primary intention. The contralateral lesion gradually progressed into a Naim et al. (2005) Stage III EACC (Fig. 4) [29].
Discussion
This case illustrates the diagnostic challenge presented by EACC and is unusual regarding its synchronous bilateralism [1, 14, 33].
The hallmark of EACC is focal osteo-necrotic epithelial invasion, in comparison to KO which features circumferential, geometrically lamellar keratinous accumulation within a uniformly expanded EAC. Differentiation between these diseases influences both management and outcome. The significance of early diagnostic differentiation is emphasised, considering EACC’s indolent yet erosive nature which allows unperceived extension beyond the EAC [38]. Expansive visualisation, as is afforded by endoscopy, would thus facilitate timeous diagnosis.
Computed tomography is the accepted gold standard for staging and surgical planning regarding EACC [36]. Current management, however, remains variable between centres [38]. We believe that oto-endoscopy may aid with decision-making in this regard.
We speculate that the treatment-refractory nature of the lesions in this report may be a feature of their position within the EAC. Makino and Amatsu (1986) demonstrated that the epithelial migratory rate, normally highest along the inferior EAC (142 µm/day), diminishes most markedly in this region with age [26]. Migratory curtailment, predisposing to recalcitrant lesions, could result from ischemia to an already tenuously perfused area. Such conditions would prompt secondary hypoxemic angiogenesis, altering keratinocyte proliferation in favour of EACC formation, as suggested by Adamczyk, Sudhoff and Jahnke (2003) [9], and Naim et al. (2004; 2005) [28, 29] in their research into the biomolecular behaviour of EACC.
Another possible explanation for surgical failure is that of incomplete disease extirpation. Jahnke and Lieberum (1995) suggested that the decision to graft should be based on the surgeon’s satisfaction that there remain no occult epithelial fragments, lest these provoke recidivism [43].
Conclusion
This case illustrates aberrations in the clinical presentation and treatment response of EACC. Attention is drawn to the significance of accurate diagnosis with regard to appropriate disease management. Garin, Degols, and Delos (1997) demonstrated that EACC is often more extensive than what is suggested clinically [37]. We, therefore, emphasise the value of combining visualisation techniques towards an enhanced understanding of disease idiosyncrasies.
Availability of data and materials
Not applicable.
Abbreviations
- EACC:
-
External auditory canal cholesteatoma
- EAC:
-
External auditory canal
- KO:
-
Keratosis obturans
- HGF/SF:
-
Hepatocyte growth factor/scatter factor
- VEGF:
-
Vascular endothelial growth factor
- TGF-α:
-
Transforming growth factor-α
- EGFR:
-
Epidermal growth factor-receptor
- MIB1:
-
MIB1 gene encoded E3 ubiquitin-protein ligase MIB1 enzyme
- CT:
-
Computed tomography
References
Anthony PF, Anthony WP (1982) Surgical treatment of external auditory canal cholesteatoma. Laryngoscope 92(1):70–75. https://doi.org/10.1288/00005537-198201000-00016
Owen HH, Rosborg J, Gaihede M (2006) Cholesteatoma of the external ear canal: etiological factors, symptoms and clinical findings in a series of 48 cases. BMC Ear Nose Throat Disord 6:16. https://doi.org/10.1186/1472-6815-6-16
Kemppainen HO, Puhakka HJ, Laippala PJ, Sipilä MM, Manninen MP, Karma PH (1999) Epidemiology and aetiology of middle ear cholesteatoma. Acta Otolaryngol 119(5):568–572. https://doi.org/10.1080/00016489950180801
Toynbee JA (1850) Specimen of molluscum contagiosum developed in the external auditory meatus. Lond Med Gazette 46:811
Schofield RE (1893) Cholesteatoma of auditory canal caused by a bug. Lancet 2:929
Altmann F, Waltner JG (1943) Cholesteatoma of the external auditory meatus. Arch Otolaryngol 38(3):236–240. https://doi.org/10.1001/archotol.1943.00670040249005
Piepergerdes MC, Kramer BM, Behnke EE (1980) Keratosis obturans and external auditory canal cholesteatoma. Laryngoscope 90(3):383–391. https://doi.org/10.1002/lary.5540900303
Naiberg J, Berger G, Hawke M (1984) The pathological features of keratosis obturans and cholesteatoma of the external auditory canal. Arch Otolaryngol 110(10):690–693. https://doi.org/10.1001/archotol.1984.00800360062016
Adamczyk M, Sudhoff H, Jahnke K (2003) Immunohistochemical investigation on external auditory canal cholesteatomas. Otol Neurotol 24(5):705–708. https://doi.org/10.1097/00129492-200309000-00001
Persaud RAP, Hajioff D, Thevasagayam MS, Wareing MJ, Wright A (2004) Keratosis obturans and external ear canal cholesteatoma: how and why we should distinguish between these conditions. Clin Otolaryngol Allied Sci 29(6):577–581. https://doi.org/10.1111/j.1365-2273.2004.00898.x
Applebaum EL, Duff BE (2001) Ear and temporal bone, I: clinical considerations for non-neoplastic lesions of the ear and temporal bone. In: Fu YS, Wenig BM, Abemayor E, Wenig BL (eds) Head and neck pathology with clinical correlations. Churchill Livingstone, Philadelphia
Lesser THJ (2018) Keratosis obturans. In: Watkinson JC, Clarke RW (eds) Scott-Brown’s Otorhinolaryngology, 8th edn. Chapman and Hall/CRC, Boca Raton
Heilbrun ME, Salzman KL, Glastonbury CM, Harnsberger HR, Kennedy RJ, Shelton C (2003) External auditory canal cholesteatoma: clinical and imaging spectrum. Am J Neuroradiol 24(4):751–756
Vrabec JT, Chaljub G (2000) External canal cholesteatoma. Am J Otol 21(5):608–614
Naim R, Linthicum FH Jr (2004) External auditory canal cholesteatoma. Otol Neurotol 25(3):412–413. https://doi.org/10.1097/00129492-200405000-00035
Martin DW, Selenick SH, Parisier SC (1999) External auditory canal cholesteatoma with erosion into the mastoid. Otolaryngol Head Neck Surg 121(3):298–300. https://doi.org/10.1016/S0194-5998(99)70187-7
Naim R, Linthicum F, Shen T, Bran G, Hormann K (2005) Classification of the external auditory canal cholesteatoma. Laryngoscope 115(3):455–460. https://doi.org/10.1097/01.mlg.0000157847.70907.42
Dubach P, Mantokoudis G, Caversaccio M (2010) Ear canal cholesteatoma: meta-analysis of clinical characteristics with update on classification, staging and treatment. Curr Opin Otolaryngol Head Neck Surg 18(5):369–376. https://doi.org/10.1097/MOO.0b013e32833da84e
Dubach P, Hausler R (2008) External auditory canal cholesteatoma: reassessment of and amendments to its categorization, pathogenesis, and treatment in 34 patients. Otol Neurotol 29(7):941–948. https://doi.org/10.1097/MAo.0b013e318185fb20
Shire JR, Donegan JO (1986) Cholesteatoma of the external auditory canal and keratosis obturans. Am J Otol 7(5):361–364
Mayer O, Frazer JS (1936) Pathologic changes in late congenital syphilis. J Laryngol Otol 51(11):683–714. https://doi.org/10.1017/S0022215100043139
Sakamoto M, Kitahara N (2002) Spontaneous external auditory canal cholesteatoma complicated by rheumatoid arthritis: case report and review of the literature. Auris Nasus Larynx 29(2):191–194. https://doi.org/10.1016/s0385-8146(01)00141-9
Holt JJ (1992) Ear canal cholesteatoma. Laryngoscope 102(6):608–613. https://doi.org/10.1288/00005537-199206000-00004
Negreiros J, Oliveira HF, Neves CA, Oliveira CA (2009) External auditory canal cholesteatoma case report. Int Adv Otol 5(3):391–393
Cheng YF, Shiao AS, Lien CF (2005) Pediatric external canal cholesteatoma with extensive invasion into the mastoid cavity. Int J Pediatr Otorhinolaryngol 69(4):561–566. https://doi.org/10.1016/j.ijporl.2004.10.019
Makino K, Amatsu M (1986) Epithelial migration on the tympanic membrane and external canal. Arch Otorhinolaryngol 243(1):39–42. https://doi.org/10.1007/BF00457906
Bonding P, Ravn T (2008) Primary cholesteatoma of the external auditory canal: is the epithelial migration defective? Otol Neurotol 29(3):334–338. https://doi.org/10.1097/MAO.0b013e31816569ad
Naim R, Riedel F, Gotte K et al (2004) Co-expression of different angiogenic factors in external auditory canal cholesteatoma. Acta Otolaryngol 124(5):563–568. https://doi.org/10.1080/00016480310015254
Naim R, Sadick H, Schafer C et al (2005) HGF/SF induces VEGF in human external auditory canal cholesteatoma (EACC) cell culture. Int J Mol Med 15(1):67–71
Derynck R (2004) Transforming growth factor-alpha: structure and biological activities. J Cell Biochem 32(4):293–304. https://doi.org/10.1002/jcb.240320406
Hunter T (1984) The epidermal growth factor receptor gene and its product. Nature 311(5985):414–416. https://doi.org/10.1038/311414a0
Bharadwaj VK, Walling KE, Rees J et al (1984) Necrosis and sequestration in the tympanic part of the temporal bone. J Otolaryngol 13(5):299–304
Persaud R, Singh A, Georgalas C, Kirsch C, Wareing M (2006) A new case of synchronous primary external ear canal cholesteatoma. Otolaryngol Head Neck Surg 134(6):1055–1056. https://doi.org/10.1016/j.otohns.2005.03.031
Park K, Chun YM, Park HJ, Lee YD (1999) Immunohistochemical study of cell proliferation using BrdU labelling on tympanic membrane, external auditory canal and induced cholesteatoma in Mongolian gerbils. Acta Otolaryngol 119(8):874–879. https://doi.org/10.1080/00016489950180207
Hickey SA, Scott GA, Traub P (1994) Defects of the first branchial cleft. J Laryngol Otol 108(3):240–243. https://doi.org/10.1017/s0022215100126404
Shin SH, Shim JH, Lee HK (2010) Classification of external auditory canal cholesteatoma by computed tomography. Clin Exp Otorhinolaryngol 3(1):24–26. https://doi.org/10.3342/ceo.2010.3.1.24
Garin P, Degols JC, Delos M (1997) External auditory canal cholesteatoma. Arch Otolaryngol Head Neck Surg 123(1):62–65. https://doi.org/10.1001/archotol.1997.01900010072010
Sayles M, Kamel HA, Fahmy FF (2013) Operative management of external auditory canal cholesteatoma: case series and literature review. J Laryngol Otol 127(9):859–866. https://doi.org/10.1017/S0022215113001850
Swartz JD, Harnsberger HR (1998) The external auditory canal. In: Swartz JD, Harnsberger HR (eds) Imaging of the temporal bone, 3rd edn. Thieme, New York
Belcadhi M, Chahed H, Mani R, Bouzouita K (2010) Therapeutic approaches to complicated cholesteatoma of the external auditory canal: a case of associated facial paresis. Ear Nose Throat J 89(8):E1-6. https://doi.org/10.1177/014556131008900801
Tos M (1997). In: Koos WT, Spetzler RF, Lang J (eds) Manual of Middle Ear Surgery, 1st edn. Georg Thieme Verlag, New York
Paparella MM, Goycoolea MV (1981) Canalplasty for chronic intractable external otitis and keratosis obturans. Otolaryngol Head Neck Surg 89(3 Pt 1):440–443. https://doi.org/10.1177/019459988108900317
Jahnke K, Lieberum B (1995) Surgery of cholesteatoma of the ear canal. Laryngorhinootologie 74(1):46–9. German. https://doi.org/10.1055/s-2007-997686
Acknowledgements
Not applicable.
Funding
There is no funding to declare.
Author information
Authors and Affiliations
Contributions
GJK was responsible for researching and writing the case report, while CF oversaw said research and writing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable. This case report was performed in accordance with the Declaration of Helsinki.
Consent for publication
Written consent for publication was obtained from the patient in question.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Klopper, G.J., Favara, C. Synchronous bilateral idiopathic external auditory canal cholesteatoma: a case report and review of the literature. Egypt J Otolaryngol 39, 98 (2023). https://doi.org/10.1186/s43163-023-00459-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43163-023-00459-3