Abstract
Purpose
The purpose of this study was to explore the characteristics of external auditory canal cholesteatoma (EACC) among children and to describe its radiological findings on high-resolution computed tomography (CT) of the temporal bone in order to improve the diagnostic accuracy of primary EACC.
Methods
The clinical records and CT imaging features of 44 patients who were diagnosed with EACC between January 2017 and May 2022 at Shenzhen Children’s Hospital were retrospectively reviewed. Clinical features, including external auditory canal wall findings, hearing damage, symptoms and physical examination findings, were analysed against the level of lesion involvement. The correlation between different types of EACC and the incidence of different clinical symptoms was analysed, and the degree of hearing impairment and the rate of bone wall destruction were examined using CT.
Results
The mean age at EACC onset was 9.02 ± 3.15 years, and the mean age at onset for EACC involving the right ear was older than that of EACC involving the left ear (P < 0.05). There were 44 patients (46 ears), including 10 ears with type I EACC, 23 ears with type II EACC, and 13 ears with type III EACC. Conductive hearing loss was the main type of hearing impairment observed among EACC patients. There were differences in types I, II and III EACC in terms of hearing impairment; specifically, there was a significant difference in moderate hearing impairment between type II and type III EACC patients (P < 0.05). The four most common symptoms were otorrhea, otalgia, itching and bleeding. The incidence of itching symptoms was greater in type I EACC than the incidence of otorrhea, and the incidence of otorrhea symptoms in type II and type III EACC was significantly greater than that in type I EACC(P < 0.05). There were no significant differences in the fracture rates of the anterior, posterior, superior or inferior walls of the external auditory canal within or between type II and type III EACC patients (P > 0.05). The failure rate of scute damage was significantly higher in type III EACC patients than in type II EACC patients (P < 0.05).
Conclusion
The presence of otorrhea and hearing loss as well as the identification of granulation tissue during otoscopy suggest the need for a temporal bone CT scan. This imaging modality can aid in the early detection and accurate classification of EACC, thereby guiding the selection of appropriate surgical interventions and greatly assisting in preventing further progression of hearing impairment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cholesteatoma is formed by stratified squamous epithelium that accumulates desquamated keratin debris and has the property of bone erosion [1]. The incidence of external auditory canal cholesteatoma (EACC) was first described by Toynbee in 1850 [2], but a more precise definition was provided by Piepergerdes et al. [3]. EACC can be divided into two distinct types based on its aetiology: primary EACC and secondary EACC. In primary EACC, there is no obvious aetiology. Chronic hypoxia may occur due to repeated minimal trauma and microvasculature [4]. Secondary EACC may be caused by previous trauma, surgery, radiation exposure, or chronic inflammation of the external auditory canal (EAC) [5].
The clinical symptoms of EACC include otorrhea, otalgia, itching and bleeding. Some patients may be asymptomatic, and other patients may experience complications. The nonspecific clinical findings of EACC often lead to a missed diagnosis or misdiagnosis. Recently, many studies of EACC have focused on adults, and only a few studies have focused on children. The missed diagnosis and misdiagnosis of EACC are likely due to a lack of published literature [1]. Paediatric EACC exhibits aggressive clinical behaviour, and if it is not detected early and properly managed, it can easily invade mastoid air cells [6].
The aim of this study was to analyse the characteristics of EACC in children by evaluating clinical and radiological features of this disease as well as to improve early diagnosis and accurate classification methods in order to optimize treatment.
Materials and methods
Case data
The clinical records of all patients who were diagnosed with EACC between January 2017 and May 2022 at Shenzhen Children’s Hospital were reviewed. Patients of all ages and sexes who visited the ear, nose and throat (ENT) inpatient department with a diagnosis of EACC were included in the study. The following data were extracted from clinical records: age, sex, side, symptoms, history of surgery, surgery records, endoscopy, pure tone audiogram, temporal bone CT images, and pathology. Three patients had a history of middle ear infection, with durations of 15 days, 1 month, and 3 months, separately. The symptoms were relieved with two weeks of antibiotic treatment and ear irrigation.
The inclusion criteria for patients were as follows: (1) had EACC diagnosed clinically by endoscopy, temporal bone CT, and operative and pathological findings; (2) inpatients; (3) clinical records were complete; (4) no previous ear infections treated by antibiotic therapy in the past six months; and (5) no history of ear surgery. The exclusion criteria were as follows: (1) incomplete medical records; (2) did not meet all of the inclusion criteria; (3) other ear disorders o r tumours; or (4) recurrent EACC. Outpatient follow-up was performed six mounts after surgery. If patients had clinical symptoms at follow-up, endoscopic and temporal bone CT images were obtained.
Equipment and scanning protocol
Patients were sedated using chloral hydrate 0.5% (0.5 ml/kg, < 10 ml).
The patients underwent CT imaging of the temporal bone using a 64-detector-row CT scanner (General Electric, Optima, USA) without intravenous contrast. Transverse scans were acquired parallel to the orbitomeatal plane in helical mode using the following parameters: 120 kV, 200 mA, detector width 20 mm, pitch 0.531:1, 512 × 512 matrix, 180 mm field of view (FOV), 0.625 mm slice thickness, 0.625 mm reconstruction interval, and HR reconstruction algorithm (FC 81). The standard acquisition was obtained with 0.625-mm-wide cuts in the axial plane and 0.625-mm-wide bone window reconstructions in the coronal plane.
Imaging analysis
Two radiologists who had five years of experience independently evaluated the cholesteatoma on each side of the temporal bone. Both specialists were blinded to the patients’ surgical data and findings. A paired t test was performed on the evaluation results. If no significant difference was found, the evaluations were found to be concordant; otherwise, the reviewers re-evaluated the data. The presence or absence of a cholesteatoma and bone destruction on each side of the temporal bone were evaluated, and the tympanic membrane, facial nerve canal and mastoid hive were examined.
The Udayabhanu HN [7] stage of EACC can be divided into three stages based on radiological evaluation and surgical confirmation of bone erosion and involvement of surrounding structures: stage I EACC, without bone erosion and middle ear extension; stage II EACC, with bone erosion and/or no middle ear extension; and stage III EACC, with bone erosion and extension to adjacent structures (a) without complications and (b) with complications.
Audiological assessment
Game audiometry was performed for patients younger than 6 years of age, while pure tone audiograms were used for all other patients. All of the audiological assessments were performed by an otorhinolaryngologist with three years of experience, and the mean values for frequencies of 0.5, 1, 2, and 4 kHz were calculated for each hearing test. Hearing was categorized as follows: ≤25dB indicates normal hearing, 26–40 dB indicates minor hearing impairment, 41–60 dB indicates moderate hearing loss, 61–80 dB indicates severe hearing loss, and ≥ 80 dB indicates epicophosis.
Statistical analysis
SPSS 26.0(Chicago, IL, USA)was used for data analysis. Measurement data are expressed as the x ± s. Rates were compared using the chi-square test (χ2), and a p-value < 0.05 was used to indicate statistical significance.
Results
Basic patient information
There were 44 paediatric patients (46 ears) who were diagnosed with EACC; 17 patients were girls, and 29 were boys. Fourteen patients had lesions on the left side, and 32 patients had lesions on the right side. There was a greater incidence of EACC on the right side (P = 0.008). Thirty-nine ears were idiopathic, and 7 ears had secondary EACC. The average age of the patients was 9.02 ± 3.15 years (range 4–15 years). There was no sex difference in EACC. The clinical characteristics (gender, side, average age) of the patients are shown in Table 1.
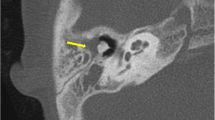
Type I EACC, 1 CT axial: soft tissue shadows in the right external auditory canal, no birth destruction; 2 A pure tone audiogram: mild hearing loss; 3 otoscope: white neoplasm; 4 pathological section: cholesteatoma. 5 type II EACC: tiny hyperdensities in the soft tissue of the right EAC, born destruction; 6 A pure tone audiogram: mild hearing loss; 7 otoscope: neoplasm; 8 HE staining; 9 type III EACC, CT axial: soft tissue and born destruction of the right EAC, involved middle ear; 10 A pure tone audiogram: mixed hearing loss; 11 otoscope: neoplasm; 12 HE staining: stratified squamous epithelium
Symptoms of different types of EACC
The main symptoms of EACC were otorrhea (48%, 22/46), otalgia (52%, 24/46), itching (9%, 4/46), bleeding (26%, 12/46), and other symptoms, such as tinnitus. The differences in symptoms among the three types of EACC patients are presented in Table 1. Itching was more frequent in type I EACC than in the other types, while otorrhea was more common in type II and type III EACC than in type I (P < 0.05). The rates of otalgia and bleeding were not different among the three types of EACC (P > 0.05).
Hearing loss over different stages of idiopathic disease
Sixteen patients had normal hearing, 13 patients had conductive hearing loss, 9 patients had mixed hearing loss, 7 patients had sensorineural hearing loss, and one patient had epicophosis. There was a difference in hearing loss among the three types of hearing loss; type III hearing loss was greater than type II hearing loss in mixed hearing loss (P < 0.05). Notably, type II EACC patients had a greater prevalence of sensorineural hearing loss than type III EACC patients (5:3). Among the patients with type II EACC and sensorineural hearing loss, two had congenital external auditory canal stenosis. It is hypothesized that the combination of external auditory canal stenosis and cholesteatoma can lead to obstruction of the ear canal, resulting in conductive hearing loss. Additionally, it should be acknowledged that the results may vary based on the sample size.
External auditory canal bone and tympanic membrane damage
All of the included patients had soft tissue lesions, and types II and III EACC patients had bony erosion of the external ear canal on temporal bone CT. The inferior wall of the external ear canal was most frequently involved in 32 (69.6%) ears, 22 (47.8%) ears had erosion of the posterior canal wall, 25 (54.3%) ears had erosion of the inferior canal wall, and 16 (34.8%) ears had erosion of the superior canal wall. More than one wall of the EAC was eroded in most of the cases – most commonly, the posterior and the inferior walls (Table 1). Tympanic membrane compression was more common than tympanic membrane perforation. There was a higher rate of tympanic membrane perforation in type III patients than in type II patients (P < 0.05). There were 10 cases of tympanic membrane perforation. Among them, 2 cases were absent due to external auditory canal stenosis, 3 cases occurred in the pars tensa, 2 cases occurred in the pars flaccida, and 3 cases involved a large perforation extending to the annulus. The lesions were in close proximity to the tympanic membrane, suggesting that the lesions invaded the tympanic membrane. There were no significant between- or within-group differences in bony erosion of the external ear canal, which contains the anterior wall, posterior wall, superior wall or inferior wall in type II and type III EACC patients (P > 0.05). The rate of scute damage in type III EACC patients was greater than that in type II EACC patients (P < 0.05).
Surgery and follow-up
Surgical methods vary across different types of EACC. For type I, the surgical method was canaloplasty ± reconstruction. For type II EACC, the surgical method was canaloplasty + reconstruction. For type III EACC, the surgical method was that EACC with bone erosion and extension to the tympanic cavity (canaloplasty + reconstruction), extension to mastoid air cells (canaloplasty + mastoidectomy, radical mastoidectomy), and extension to the extratemporal structure (subtotal petrosectomy) were performed. None of the patients received conservative treatment. In our study, the concordance rate of surgical modalities and classification was 96%. Two patients (one patients with type I and one patient with type II EACC) underwent extra reconstruction because of a history of middle ear cholesteatoma surgery.
No complications were observed herein. Four patients had otorrhea and otalgia symptoms after surgery at 1 (2 ears), 2 (1 ear), and 4 (1 ear) years. EACC recurrence was diagnosed by endoscopic and temporal bone CT images.
Discussion
While most cholesteatomas are found in the middle ear and mastoid, external auditory canal cholesteatoma (EACC) is rare. However, its low occurrence may be related to underreporting. The incidence of external auditory canal cholesteatoma is 1 per 1,000 new patients visiting an otology clinic [8]. In children, the incidence of EACC is 1.6 per 1,000 new otology patients [9]. In contrast to adults [1], children have an onset age of 2–15 years [10]. In our study, the average age at onset for EACC was 9.02 years. Primary EACC has been epidemiologically linked with microtrauma (cotton-tipped applicators, hearing aids) and smoking, leading to microangiopathy in the ear canal, which causes keratin deposition due to poor blood supply [1, 5]. In this study, there were no differences between idiopathic and secondary EACC patients. 11% (5/46 ears) of patients used cotton-tipped applicators, but no one used hearing aids. In contrast to previous reports [11], we found that the incidence of EACC between the left and right ears in children was obviously different, and EACC in the right ear was more common than that in the left ear; these findings were the same as those in paediatric EACC patients reported by Yeo-Hoon et al. [9]. and other reports [12,13,14]. The predisposing factors identified were cotton-tipped applicators (microtraumas) [1, 5]. We hypothesize that this may be related to the cotton-tipped applicators used by the right hand leading to microangiopathy in the ear canal.
Our study revealed significant differences between hearing loss and EACC types in children. Conductive hearing loss is the most common audiologic finding in EACC patients. The level of conductive hearing loss differed among types I, II, and III EACC. In the early stage, hearing loss is mild in severity, possibly because of the accumulation of squamous keratinized material in the external ear canal [15, 16]. Gradual impairment of hearing loss develops with disease progression [17]. The hearing loss in type III patients was more serious than that in type II patients, and this difference may be related to tympanic membrane perforation, ossicular chain continuity loss, ossicular defects and fixation with the progression of EACC [18]. Therefore, early diagnosis and active treatment of EACC in children are essential for hearing.
The clinical symptoms commonly observed are otorrhea, otalgia, itching, and bleeding [1, 5]. In our study, the most common complaints were otorrhea and otalgia, which are associated with localized infection by various bacteria, such as Pseudomonas aeruginosa [1, 16]. Twelve patients had middle ear (mastoid) inflammation, with 9 patients having no previous history of middle ear infection. Only 3 patients had a history of middle ear infection, with durations of otorrhea and ear pain lasting for 15 days, 1 month, and 3 months, respectively. Symptoms were relieved with two weeks of antibiotic treatment and ear irrigation. Otalgia presents at the early stages and decreases with disease progression [19]. One study reported that otorrhea in the presence of an ear canal less than 2 mm should increase suspicion of cholesteatoma and at least prompt a temporal bone CT study [18].
In this study, CT of all EACC patients revealed soft tissue shadows in the external auditory canal. The most important feature is the presence of tiny hyperdensities within the soft tissue, and the complication rate can reach more than 50%, probably representing bony fragments or sequestra [1, 12, 16, 20]. We found that 22 (48%) ears had high density in the soft tissue, 12 (26%) ears had hyperdensities, and 10 (22%) ears had apparent bone fragments and sequestra. Therefore, identification of these fragments is crucial for a confident radiological diagnosis of EACC, and temporal bone CT has an important role in the diagnosis of EACC. Based on our findings, we recommend performing a temporal bone CT scan when there are symptoms of otorrhea, hearing loss, and the presence of granulation tissue observed during otoscopy.
As reported in previous studies, the posterior canal wall is most commonly involved in paediatric EACC, whereas the inferior wall is most commonly involved in adult EACC [9, 11, 21, 22]. This is probably related to the poor blood supply and reduced migratory capacity of the canal epithelium [4, 5]. Some patients experienced no symptoms if only the inferior wall was involved because the inferior wall is thicker. Heilbrun et al. reported that EACC granulations most often involve multiple walls of the external auditory canal, including circumferential involvement [10, 16]. In our study, the inferior half of the external auditory canal, including the inferior, posterior, and anterior wall areas, was most commonly involved, with granulations usually involving more than one wall. These findings were consistent with earlier studies. Kim et al. [6] reported that paediatric EACC developed most commonly in the posterior canal wall; therefore, paediatric EACC may easily invade the mastoid air cell if it is not detected early and properly managed. In our study, the between- and within-group differences in bony erosion of the external ear canal were not statistically significant. It is possible that our sample size was small and limited to a single centre. We found that the rate of scute damage in type III patients was greater than that in type II patients, suggesting that cholesteatomas invade the middle ear and mastoid cells through the broken tympanic membrane. Other studies have proposed that posterior and anterior lesions are more common and may easily invade the middle and mastoid air [22,23,24].
Compared with previous studies, our study had the largest sample size and used statistical data instead of statistical data, and the results were more accurate. Limitations of the present study include its retrospective nature, incomplete tympanic membrane perforation details, the small sample size and the lack of a control group. Further studies may be performed to compare the differences between the external auditory canal bone and tympanic membrane damage and hearing loss of cholesteatomas of the EAC and those of the middle ear and the mastoid.
Conclusion
Paediatric EACC is a rare condition, but its low occurrence may be related to underreporting. In our study, otorrhea and otalgia were the most common presenting symptoms. However, patients may have variable clinical findings, which could lead to misdiagnosis. For irreversible hearing damage due to EACC, early diagnosis and active treatment are important. In this study, the most common finding on CT of the temporal bone was soft-tissue density in the EAC, with bony erosion and small bone samples in the soft tissues. However, this was a retrospective study with a small sample size, which has certain limitations. Therefore, further prospective controlled studies are needed. CT of the temporal bone can both aid in early diagnosis and aid in the diagnosis of EACC, which can influence surgical methods. Therefore, temporal bone CT in the early stage has important implications for the early diagnosis and prevention of progression in paediatric patients with EACC.
Data availability
Cannot be shared openly.
Data, materials and/or code availability
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
References
DONGOL K, SHADIYAH H, GYAWALI B R et al (2022) External Auditory Canal Cholesteatoma: clinical and radiological features [J]. Int Arch Otorhinolaryngol 26(2):e213–e8
TOYNBEE J (1850) On the structure of the Membrana Tympani in the human ear [J]. Provincial Med Surg J 14(16):429–430
PIEPERGERDES M C, KRAMER B M, BEHNKE EE (1980) Keratosis obturans and external auditory canal cholesteatoma [J]. Laryngoscope 90(3):383–391
DUBACH P, MANTOKOUDIS G, CAVERSACCIO M (2010) Ear canal cholesteatoma: meta-analysis of clinical characteristics with update on classification, staging and treatment [J]. Curr Opin Otolaryngol Head Neck Surg 18(5):369–376
DUBACH P, HäUSLER R (2008) External auditory canal cholesteatoma: reassessment of and amendments to its categorization, pathogenesis, and treatment in 34 patients [J]. Otol Neurotol 29(7):941–948
KIM C W, BAEK S H, LEE S H et al (2014) Clinical characteristics of spontaneous cholesteatoma of the external auditory canal in children comparing with cholesteatoma in adults [J]. Eur Arch Otorhinolaryngol 271(12):3179–3185
HN U, PRASAD S C RUSSOA et al (2018) Cholesteatoma of the External Auditory Canal: review of staging and Surgical Strategy [J]. Otol Neurotol 39(10):e1026–e33
ANTHONY P F, ANTHONY WP (1982) Surgical treatment of external auditory canal cholesteatoma [J]. Laryngoscope 92(1):70–75
YOON Y H, PARK C H, KIM E H et al (2008) Clinical characteristics of external auditory canal cholesteatoma in children [J]. Otolaryngol Head Neck Surg 139(5):661–664
HE G, XU Y, ZHU Z (2019) Clinical analysis of pediatric primary external auditory canal cholesteatoma [J]. Int J Pediatr Otorhinolaryngol 118:25–30
OWEN H H, ROSBORG J, GAIHEDE M (2006) Cholesteatoma of the external ear canal: etiological factors, symptoms and clinical findings in a series of 48 cases [J]. BMC Ear Nose Throat Disord 6:16
LI CL, CHEN Y, CHEN Y Z et al (2016) Congenital aural stenosis: clinical features and long-term outcomes [J]. Sci Rep 6:27063
jing HUANG, Nan WANG, Tao PENG, Peng MA, Bo FENG (2022) Clinical features of External Auditory Canal Cholesteatoma with different levels of involvement [J]. Chin J Otology 20(1):6
Xiayu SUN, Dekun GAO, Yuyu HUANG, Yang YANG, Zhengyu LIN, Shuna LI, YANG Jun (2022). Clinical characteristics of Pediatric External Auditory Canal Cholesteatoma [J]. Chin J Otology, (002):020
NAIM R, SHEN T, RIEDEL F et al (2005) Regulation of apoptosis in external auditory canal cholesteatoma by hepatocyte growth factor/scatter factor [J]. its Relat Specialties 67(1):45–50ORL; journal for oto-rhino-laryngology
HEILBRUN ME, SALZMAN K L, GLASTONBURY C M et al (2003) External auditory canal cholesteatoma: clinical and imaging spectrum [J]. AJNR Am J Neuroradiol 24(4):751–756
MORITA S, NAKAMARU Y, FUKUDA A et al (2018) Clinical characteristics and treatment outcomes for patients with External Auditory Canal Cholesteatoma [J]. Otol Neurotol 39(2):189–195
CASALE G, NICHOLAS B D, KESSER BW (2014) Acquired ear canal cholesteatoma in congenital aural atresia/stenosis [J]. Otol Neurotol 35(8):1474–1479
KONISHI M, IWAI H, TOMODA K (2016) Reexamination of etiology and Surgical Outcome in Patient with Advanced External Auditory Canal Cholesteatoma [J]. Otol Neurotol 37(6):728–734
CHAWLA A, EZHIL BOSCO J I, LIM T C et al (2015) Computed tomography features of External Auditory Canal Cholesteatoma: a pictorial review [J]. Curr Probl Diagn Radiol 44(6):511–516
HOLT JJ (1992) Ear canal cholesteatoma [J]. Laryngoscope 102(6):608–613
SHIN S H, SHIM J H, LEE HK (2010) Classification of external auditory canal cholesteatoma by computed tomography [J]. Clin Exp Otorhinolaryngol 3(1):24–26
DARR E A, LINSTROM CJ (2010) Conservative management of advanced external auditory canal cholesteatoma [J]. Otolaryngol Head Neck Surg 142(2):278–280
Yan LIU, Ruiming XIA, Lisheng YU (2014) Management of ear canal cholesteatoma with Otitis Media [J]. Chin J Otology 12(4):3
Funding
This work was supported by the Sanming Project of Medicine in Shenzhen (NO. SZSM202011005), Guangdong High-level Hospital Construction Fund (NO. ynkt-2022-zz41), Natural Science Foundation of Shenzhen City (NO. JCYJ20220530155607018) .
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Ke Wei, Yongchao Chen, Zebin Wu, Juan Cao and Weiguo Cao. The first draft of the manuscript was written by Ya Zhang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics approval
The studies involving human participants were reviewed and approved by Medical Ethics Committee of the Shenzhen Children’s Hospital (NO.202308502).
Informed consent
The images involved in our study do not require informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/.
About this article
Cite this article
Zhang, Y., Wei, K., Chen, Y. et al. External auditory canal cholesteatoma in children: clinical manifestations. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08892-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08892-7