Abstract
Background
Thyroid nodules were widely encountered in the population, and the selection of thyroid nodules for fine needle aspiration cytology (FNAC) remains confusing. It is essential to investigate the risk factors associated with thyroid nodules.
Aim of work
This study aimed to evaluate the accuracy of the American College of Radiology-Thyroid Imaging Reporting and Data System (ACR-TIRADS) scoring system in distinguishing malignant thyroid nodules from benign ones and its association with cytological examination of the FNAC of the thyroid nodules. Additionally, we seek to investigate any potential association between thyroid nodules and some metabolic derangements.
Patients and methods
The study included 111 Egyptian patients with euthyroid nodules whom were subjected to history taking, clinical examination, and laboratory investigations including thyroid profile, fasting blood sugar (FBS), glycosylated hemoglobin A1c (HbA1c), and lipid profile. Thyroid ultrasound and FNAC were done for all patients. Categorization of each nodule was done according to the TIRADS. Cytopathological diagnosis was done by Bethesda system cytology classification.
Results
There were 19 malignant and 92 benign nodules. There was a statistically significant difference between benign and malignant nodules regarding TIRADS classification, taller-than-wide shape, solidity, border, presence of peripheral calcifications, or punctuate echogenic foci (p < 0.05). Taller-than-wide shape had the highest specificity followed by irregular margin (94.6% and 92.6%, respectively). Sensitivity, specificity, PPV, and NPV for ACR-TIRADS versus cytopathology were 73.7%, 57.6%, 26.4%, and 91.4% respectively with overall accuracy of 60.4%. The high sensitivity and NPV of the US-based TIRADS classification system have excellent utility for correctly classifying nodules as positive for malignant disease. As regards risks for thyroid nodules, results showed that most of the study population were obese [Body Mass Index (BMI) = 31.6 ± 6.3, Waist circumference (WC) = 107.4 ± 13.9]. TSH and hypercholesterolemia did not show a significant association with thyroid malignancy.
Conclusion
ACR-TIRADS classification is of high significant value in classifying nodules as positive for malignant disease and for predicting the absence of malignant disease, reducing unnecessary nodule FNAC. Hypercholesterolemia and TSH value were not significantly associated with malignant thyroid nodules.
Similar content being viewed by others
Introduction
Thyroid nodules are a common disorder, with a prevalence of 2–6% through palpation and 19–35% through ultrasound inspection in the general population. They are characterized as focal thyroid regions with radiologically distinguishable altered echogenicity [1]. While most thyroid nodules are benign, malignancy was found in only 5–15% of cases [2]. High-definition ultrasonography is recommended for clinically detected nodules in euthyroid individuals. Horvath et al. proposed a thyroid imaging recording and data system (TIRADS) to assess the risk of malignancy in thyroid nodules [3]. Fine needle aspiration cytology (FNAC) of the thyroid nodules offers a reasonable strategy for treatment and provides the appropriate surgical method if indicated [4, 5]. Bethesda’s cytological examination classification determines the patient’s eligibility for surgery or medical treatment. Using ultrasound-guided FNA allows proper localization of the thyroid nodule during aspiration [6]. With both benign and malignant thyroid nodules becoming more prevalent, it is crucial to organize thyroid cancer prevention strategies. This can be achieved by avoiding the risk factors associated with thyroid nodules, such as obesity, diabetes, and insulin resistance [7,8,9].
Aim of work
Determine whether cytological examination of the FNAC of the thyroid nodules correlates with the accuracy of the American College of Radiology-Thyroid Imaging Reporting and Data System (ACR-TIRADS) scoring system in differentiating malignant from benign thyroid nodules. Further, determine whether thyroid nodules are associated with a metabolic derangement.
Materials and methods
It is a cross-sectional analytical prospective study which comprised 111 Egyptian patients (106 females and 5 males), their ages were from 25 to 70 years old, presenting by single or multiple thyroid nodules with a euthyroid state. They were referred to Endocrinology Clinic at Hospital of Cairo University, from January 2020 to December 2020. We excluded patients with hypothyroidism, hyperthyroidism, and previously known thyroid malignancy or with known bleeding diathesis. All patients underwent clinical examination including measuring waist circumference, body mass index (BMI), and blood pressure. The following tests were performed: serum thyroid stimulating hormone (TSH), free triiodothyronine (FT3), free thyroxine (FT4), fasting plasma glucose (FBG), 2-hour postprandial glucose (2HPP), glycosylated hemoglobin, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), and triglyceride (TG).
Thyroid ultrasound
Thyroid ultrasound examination was done, using a 7.5-MHZ transducer (Siemens, Erlangen, Germany scanner) including brightness B-mode, color-coded Doppler imaging, and transverse and longitudinal scanning of the thyroid gland for all cases. We use the TIRADS scoring to analyze the ultrasound findings of the nodules.
Image analysis
TIRADS scoring is determined from five distinct items of ultrasound findings. The cumulative score is proportional to the TR (TIRADS) category and malignancy rate. These radiologic items include composition of the thyroid nodule, the echogenicity, shape assessed on the transverse plane, margins, and echogenic foci with detected calcifications [10]. TIRADS scores 4 and 5 were considered positive for malignancy, while scores 1–3 were considered negative for malignancy.
One score is assigned from each of the following categories:
-
Composition
-
Cystic or completely cystic *: 0 points
-
Spongiform *: 0 points
-
Mixed cystic and solid: 1 point
-
Solid or almost completely solid: 2 points
-
-
Echogenicity
-
Anechoic: 0 points
-
Hyper- or isoechoic: 1 point
-
Hypoechoic: 2 points
-
Very hypoechoic: 3 points
-
-
Shape: (assessed on the transverse plane)
-
Wider than tall: 0 points
-
Taller than wide: 3 points
-
-
Margin
-
Smooth: 0 points
-
Ill-defined: 0 points
-
Lobulated/irregular: 2 points
-
Extra thyroidal extension: 3 points
-
-
Echogenic foci: (choose one or more)
-
None: 0 points
-
Large comet tail artifact: 0 points
-
Macro-calcifications: 1 point
-
Peripheral/rim calcifications: 2 points
-
Punctate echogenic foci: 3 points
-
*Predominantly cystic or spongiform nodules are inherently benign. If these features are present no further points will be added (automatically TR1, excluded from this study).
Scoring and classification
TR 1 | TR2 | TR3 | TR4 | TR5 |
|---|---|---|---|---|
0 points, benign | 2 points, not suspicious | 3 points, mildly suspicious | 4–6 points, moderately suspicious | ≥ 7 points, highly suspicious |
Ultrasound (U/S) guided FNAC of thyroid nodules
Patients underwent FNA of the thyroid nodules, after their consent. The ultrasound scanner utilized to find the nodule served as a guide for the FNA of the thyroid nodules. A 21-G needle connected to a plastic syringe was used during the procedure, which was carried out under strictly aseptic settings. At least two distinct passes sample the aimed nodule. Smears were fixed by 70% ethyl alcohol spray, then transported in slide containers to Cytopathology Unit, Pathology Department, Faculty of Medicine, Cairo University. For complex nodule, cyst content aspiration and any solid area samples were performed. The cyst content aspirate was sent for centrifuge in the cytopathology unit. FNAC were done for all the thyroid nodules from TIRADS score 2 to score 5, we excluded spongiform nodules and simple cysts.
Cytopathology
Smears of the FNAC were stained by modified Papanicolaou as well as hematoxylin and eosin (H&E) stains. The smears for each case were examined by a Leica microscope, then diagnosed using The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) after fulfilling the adequacy criteria [6]. Bethesda IV, V, and VI groups were accepted as potentially malignant; on the other side, Bethesda II and III groups were accepted as benign samples. TIRADS sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy were calculated based on cytology results.
-
TBSRTC Category 1: Non-diagnostic or unsatisfactory (excluded in this study)
A smear was categorized as non-diagnostic if it did not fulfill the adequacy criteria laid down by the Bethesda system.
-
TBSRTC Category 2: Benign
Smears were interpreted as benign if they showed the cytomorphological features of colloid goiter/adenomatoid goiter or thyroiditis.
-
TBSRTC Category 3: Atypia of undetermined significance/follicular lesion of undetermined significance
Aspirates which had some features of atypia but could not be categorized definitely into either of the benign, SFN, SM, or malignancy categories.
-
TBSRTC Category 4: follicular neoplasm ((FN)/suspicious for a follicular neoplasm
Aspirates with cytomorphologic features of moderate to high cellularity, scant or absent colloid, with predominantly microfollicular or trabecular configuration of follicular cells in repetitive pattern. Aspirates with cytomorphologic features of Hurthle cell neoplasm were also placed in this category.
-
TBSRTC Category 5: Suspicious for malignancy
Aspirates that had cytological features suggestive of, but not definitive of, papillary carcinoma, medullary carcinoma, or others.
-
TBSRTC Category 6: Malignant
Aspirates that appeared unequivocally malignant were placed in this category.
Histopathology
Cases that undergone surgical resection of the thyroid gland were retrieved. Tissue sections of thyroidectomy were diagnosed according to WHO Classification of Tumors of Endocrine Organs, 5th Edition (Baloch et al., 2022). Required auxiliary immune stains were performed to assure diagnosis (BRAF v600E for NIFTP and chromogranin for medullary thyroid carcinoma). Serial sections of formalin-fixed paraffin-embedded tissue blocks were cut, then placed over adhesive-coated glass slides. Avidin-Biotin immunoperoxidase system was used. Sections were stained by using primary antibodies (rabbit monoclonal BRAF V600E, Ventana Medical Systems, Tucson, AZ, USA) and (monoclonal anti-chromogranin A clone DAK-A3, Dako, Santa Clara, CA) with positive and negative controls.
Statistics
Statistical Package for Social Sciences (SPSS) version 25 was used for data management and analysis. Proper means, standard deviations, medians, and/or ranges summarize the numerical data. Numbers and percentages were used to represent a categorical set of data. The percentages and figures were used to estimate the frequency. Using the Shapiro-Wilk test and the Kolmogrov-Smirnov test, numerical data were examined for normality. To compare two independent percentages and determine the association between categorical variables, the chi-square test or the Fisher exact was used. For comparisons between the two groups, the Student’s t-test and Mann-Whitney U test were used. Significance represented probability (p-value) of 0.05.
Results
Our case study embraced 111 patients with thyroid gland nodules. The age range was 25 to 70 years (mean = 44 ± 12). Females represent the majority of cases (n = 106, 95.5%). Figures 1, 2, and 3 and Table 1 illustrate the ultrasound results of cases.
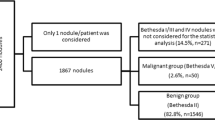
Samples were categorized by The Bethesda System for Reporting Thyroid Cytopathology (TBSRTC) of thyroid nodules. TBSRTC III is considered indeterminate and can carry a small proportion of malignant potentiality, but guidelines do not necessitate surgery in this case. TBSRTC IV represents neoplastic category with both benign tumors as follicular adenomas and malignant ones as follicular carcinomas (invasion could not be documented in FNAC). We had to discriminate nodules which are cytological malignant or suspicious for malignancy, with operational guidelines, from the other nodules. Therefore, Bethesda IV (n = 7), V (n = 10), and VI (n = 2) groups were accepted as potentially malignant (n = 19); on the other side, Bethesda II (n = 75) and III (n = 17) groups were accepted as benign samples (n = 92). Subsequently, cytological findings for studied nodules will be denoted by benign and malignant / potentially malignant terminology (Table 2). TBSRTC I nodules were dismissed (n = 9).
Follow-up of patients revealed only 27 cases who underwent thyroidectomy (24.32%), with detected seven malignant cases by histopathology. Six cases of them were categorized as TBSRTC V and VI (6/7 cases), with one FNA-missed incidental micro-carcinoma. There were 4 cases diagnosed as papillary thyroid carcinoma, one case diagnosed as medullary thyroid carcinoma (which was confirmed by positive chromogranin expression), and one case of follicular carcinoma. Non-invasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) has been documented in one thyroidectomy specimen, confirmed by negative expression to BRAF-600E to exclude non-invasive follicular variant of papillary thyroid carcinoma (Figs. 4, 5, 6, and 7).
NIFTP (non-invasive follicular thyroid neoplasm with papillary nuclear features). A and B Smears of follicular cells arranged in syncytia, showing copious cytoplasm, enlarged overlapping nuclei and focal nuclear pseudoinclusion (H&E stain, low and high powers). C, D, and E Tissue sections revealed defined fibrous nodule capsule, predominate follicular arrangement with focal abortive hyperplastic papillae. Nuclear score 3 (enlargement, crowding / overlapping, elongation, grooves, pseudoinclusions, and chromatin clearing). (H&E stain, low and high powers). F Negative BRAF-V600E immune stain
Oncocytic cell adenoma. A and B Cells in microfollicular pattern showing abundant eosinophilic cytoplasm, round nuclei with focal enlargement. C and D Tissue section showing capsulated nodule with focal site aspiration changes. The cells showed abundant cytoplasm. The nuclei showed focal prominent nucleoli. (H&E stain, low and high powers)
Medullary carcinoma. A and B Follicular cells arranged singly and focal microfollicles. The cells exhibit eccentric nuclei, comet-shaped cytoplasmic extensions. C Amyloid deposit (H&E and pap stains, smear high powers). D and E Tissue sections showing neoplastic cells arranged in trabeculae with amyloid eosinophilic material (H&E, low & high powers). F Tumor cells showed positive chromogranin immune reaction (chromogranin immune stain, low power)
Papillary thyroid carcinoma. A and B Follicular cells show malignant nuclear features: enlargement, overlapping, powdery chromatin, nuclear grooving, and inclusion (Pap stain, smears, low and high powers). C and D Tissue sections of papillary thyroid carcinoma. The cells showed malignant nuclear features (H&E stain, low and high powers)
Table 3 summarizes the results of the analysis of ultrasound findings with thyroid potential malignancy. Our results show that solid composition, taller than wide, irregular nodules, peripheral calcifications, and punctuate echogenic foci showed significant associations with thyroid malignancy (p < 0.05). In contrast, echogenicity and macro-calcifications showed no significant association.
Correlating TIRADS with Bethesda system (Fig. 8), we find that:
-
Out of the 23 TIRADS 2 nodules, none turned out to be Bethesda IV or higher (100% concordance).
-
From the 35 nodules labeled as TIRADS 3, 30 nodules were Bethesda II and III (benign) and 4 nodules were Bethesda IV, only one nodule was Bethesda V and none was Bethesda VI (85.7% concordance).
-
On the contrary, out of the 40 TIRADS 4 (moderately suspicious) nodules, only 8 nodules eventually were Bethesda IV or higher. Five nodules out of the 13 classified as TIRADS 5 (highly suspicious) came to be Bethesda IV or higher with low concordance 20% and 38.5%, respectively.
Table 4 shows calculated rates of malignancy in TIRADS categories compared with malignancy risk recommended by the ACR-TIRADS Committee. Along with the higher risk categories, the malignancy rates tended to rise.
The diagnostic accuracy of each evaluated ultrasound finding to identify malignancy is summarized in Table 5. “Taller-than-wide shape” category had the highest specificity (94.6%) but low sensitivity (21.1%). Irregular margin had high specificity (92.4%) and low sensitivity (26.3%). On the contrary, solid composition and the presence of echogenic foci had relatively higher sensitivity values (63.2% for both).
Regarding the clinical and laboratory data collected in this study, no difference was significantly found between benign and malignant groups (Table 6).
Discussion
A noticeable increase in thyroid carcinoma incidence has been observed, possibly due to the wide use of neck ultrasonography and the surveillance of sonar-guided FNAC of thyroid nodules [1]. While attempting to identify malignant nodules requiring surgery, it was a challenge to achieve the balance between avoiding overdiagnosis of unnecessary FNA and not missing the diagnosis of malignant thyroid nodules. The ACR-TIRADS system has standardized the language used to communicate thyroid ultrasound findings between clinicians and provided valuable insight into the management plan [3, 11, 12]. FNAC is crucial in triaging patients into operative and non-operative groups amid the increased awareness of thyroid diseases [13]. While radioactive exposure, improper iodine intake, and family history are recognized risk factors for thyroid cancer, they do not illustrate the whole picture. The prevalence of thyroid carcinoma is higher in the high-income lifestyles of the USA and China, where socioeconomic status significantly influences thyroid status. Therefore, many studies focused on modifiable risk factors such as overnutrition, obesity, and dyslipidemia [14, 15]. Despite females having a higher incidence of thyroid nodules than males, men face a higher risk of malignancy [16]. In this study, females represented 95.5% of cases with thyroid nodules, consistent with findings in two other studies conducted in our endemic region [17, 18]. The risk of malignancy indicated is > 20% in the TR-5 group of ACR-TIRADS. In our study, we found a rate of 38.5% in TR-5 of ACR-TIRADS. However, rates for TR-3 and TR-4 groups were 14.3% and 22.5%, respectively, higher than the indicated risk in guidelines (5% for TR3 nodules, 5 to 20% for TR4) [10]. Barbosa reported a thyroid malignancy rate of 23.3% in patients with TR3 [19]. Another Indian study by De et al. found that TR-3 and TR-4 exhibited 22% and 29% rates of malignancy, respectively [20]. Discrepancies in these results may arise from the challenge of distinguishing between calcification and comet tail artifacts during sonographic examination, directly impacting the nodule’s ultimate score. Additionally, macrocalcification and hyperechoicity did not indicate malignancy in earlier systems. Different tools were used in the cytology and histopathology diagnoses. Sonographic evaluation of thyroid nodules was aimed at ascertaining the likelihood of malignancy in conjunction with the results of the FNAC; this would pave the way for the best choice of further therapy. According to the sonographic features of the nodule, firmness, lobulation, or irregularity of the border, taller-than-wide shapes, peripheral calcifications, and punctate echogenic patches within the nodule were the most prevalent sonographic characteristics of malignant nodules. In agreement with our results, studies by Kwak and colleagues found that solid components, hypoechogenicity, margin irregularity, microcalcifications, and taller-than-wide shapes were significantly associated with malignancy [12]. Many studies acknowledged these features as potential signs of malignancy [18, 21,22,23,24].
As regards the ultrasound characteristics of the thyroid nodule, we found that a taller-than-wide shape had the highest specificity of 94.6% but a low sensitivity of 21%. Similarly, irregular margins had high specificity (92.4%) but a low sensitivity (26.3%). Our findings align with a meta-analysis study by Remonti, which found that a taller-than-wide shape and irregular margins had the highest specificities of 96% and 83%, respectively, but were not sensitive (26% and 50%) [25, 26]. Similarly, De D and his colleagues calculated the specificity values for irregular margins and taller-than-wider shapes at 89% and 92%, respectively [20]. Regarding hypoechogenicity, our study showed a sensitivity of 36% and a specificity of 57%. However, the results of a meta-analysis showed better specificity (62.3%) and sensitivity (62.7%) [2]. In other studies, the sensitivity levels ranged from 26.5 to 87.2% [24, 27]. Peripheral calcifications and punctate echogenic foci in our study correlated with thyroid malignancy, consistent with previous studies [18, 23]. In our results, macrocalcification showed no significant association with the risk of malignant thyroid nodules, which agrees with some studies [22, 28, 29] but conflicts with other studies [27, 30]. So, macrocalcification played a controversial role in the risk of malignancy. Our study demonstrated 100% concordance between TIRADS 2 and TBSRTC, while TIRADS 3 had 85.7% concordance. TIRADS 4 and TIRADS 5 had concordance at 20% and 38.5%, respectively. Singaporewalla reported similar results for the TIRADS II and TIRADS III groups, with a concordance of 100% and 81% to cytology, respectively. However, the TIRADS 4 group nodules had a 33.3% higher concordance than in our study, and the TIRADS 5 group nodules showed even higher concordance between TIRADS and cytology at 60% [31]. In contrast, Pandya found that the TIRADS 2 and TIRADS 3 groups showed high concordance between TIRADS and cytology (93.7% and 95%, respectively) but low concordance in the TIRADS 4 and TIRADS 5 groups (11.2% and 23%, respectively).
In this study, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for ACR-TIRADS versus cytology were 73.7%, 57.6%, 26.4%, and 91.4%, respectively, with an accuracy of 60.4%. These findings agree with some studies [32, 33], although others showed higher specificity values [29, 31]. Mistry compared the TIRADS with the American Thyroid Association (ATA) guidelines and found that the TIRADS was superior in sensitivity, whereas the ATA guidelines were superior in specificity and PPV. TIRADS had a median sensitivity, specificity, PPV, and NPV of 90.0%, 57.4%, 49.0%, and 91.0%, respectively [34]. The present study observed no significant differences between benign and malignant groups in BMI, waist circumference, lipid profile, or HbA1C levels. These results align with De Siqueira’s findings, in which thyroid nodules were more frequent in obese individuals, and parenchymal hypoechogenicity was more pronounced in obese than non-obese individuals. However, no significant differences were observed in terms of the risk of malignancy between obese and non-obese individuals [35]. Grimmichova did not observe an increased risk of thyroid cancer in diabetes or prediabetic patients compared to controls [36,37,38]. On the contrary, he found that BMI was positively correlated with the risk of thyroid cancer [39]. The association between hypercholesterolemia and thyroid cancer remains unclear. Our study found that serum cholesterol levels had no significant association with malignancy. The rate of malignancy in hypercholesterolemic patients (16.7%) nearly corresponded to those with normal levels (15%). These findings contrast with a Chinese study that concluded that hypercholesterolemia was a risk factor for thyroid cancer [40]. Our study observed no significant difference in serum TSH levels between benign and malignant nodules, consistent with Hrafnkelsson’s finding that serum levels of TSH and thyroxine did not differ between cases of thyroid malignancy and controls [41].
On the contrary, an extensive meta-analysis in China in 2020 revealed a significant association between higher TSH levels and the risk of thyroid malignancy [42, 43]. Finally, we concluded that the ACR-TIRADS classification, compared to the cytological findings of the ultrasound-guided FNAC of the thyroid nodules, holds significant value in discriminating between benign and malignant thyroid nodules. The TIRADS scoring system can be relied upon to avoid unnecessary FNAC or surgical treatment. Study limitations included the relatively small number of patients and the small number of males, potentially affecting the statistical power and reproducibility of the results. Moreover, the study performed the postoperative histopathological examination for a limited number of patients included in the study group. Further, extensive studies involving both genders in multiple centers across Egypt are recommended.
Availability of data and materials
The data used to support the findings of this study are available from the corresponding author upon reasonable request.
References
Niedziela M (2014) Thyroid nodules. Best Pract Res Clin Endocrinol Metab 28(2)
Haugen BR, Alexander EK, Bible KC et al (2016) American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Horvath E, Majlis S, Rossi R et al (2009) An ultrasonogram reporting system for thyroid nodules stratifying cancer risk for clinical management. J Clin Endocrinol Metab 94(5):1748–1751
Yoon JH, Lee HS, Kim EK et al (2015) Thyroid nodules: nondiagnostic cytologic results according to thyroid imaging reporting and data system before and after application of the Bethesda system. Radiology 276(2):579–587
Ospina NS, Brito JP, Maraka S et al (2016) Diagnostic accuracy of ultrasound-guided fine needle aspiration biopsy for thyroid malignancy: systematic review and meta-analysis. Endocrine 53(3):651–661
Cibas ES, Ali SZ (2017) The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 27(11):1341–1346
Davies L, Welch HG (2014) Current thyroid cancer trends in the United States. JAMA Otolaryngol Head Neck Surg 140(4):317–322
Balkan F, Onal ED, Usluogullari A et al (2014) Is there any association between insulin resistance and thyroid cancer?: a case control study. Endocrine 45(1):55–60
Shih SR, Chiu WY, Chang TC et al (2012) Diabetes and thyroid cancer risk: literature review. Exp Diabetes Res 2012
Tessler FN, Middleton WD, Grant EG et al (2017) ACR thyroid imaging, reporting and data system (TI-RADS): white paper of the ACR TI-RADS committee. J Am Coll Radiol 14(5):587–595
Soto GD, Halperin I, Squarcia M et al (2010) Update in thyroid imaging. The expanding world of thyroid imaging and its translation to clinical practice. Hormones 9(4):287–298
Kwak JY, Han KH, Yoon JH et al (2011) Thyroid imaging reporting and data system for US features of nodules: a step in establishing better stratification of cancer risk. Radiology 260(3):892–899
Lin J-D, Chao T-C, Huang B-Y et al (2009) Thyroid cancer in the thyroid nodules evaluated by ultrasonography and fine-needle aspiration cytology. Thyroid 15(7):708–717
Boscoe FP, Henry KA, Sherman RL et al (2016) The relationship between cancer incidence, stage and poverty in the United States. Int J Cancer 139(3):607–612
Zheng R, Zeng H, Zhang S et al (2017) Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer 36(1):1–6
Rahbari R, Zhang L, Kebebew E (2010) Thyroid cancer gender disparity. Future Oncol 6(11):1771–1779
Ibrahim MMES, Omar W, Elhofy A (2019) Prospective study evaluating malignancy in solitary thyroid nodule. Egyptian J Surg 38(3):411
Okasha HH, Mansor M, Sheriba N et al (2020) Role of elastography strain ratio and TIRADS score in predicting malignant thyroid nodule. Arch Endocrinol Metab 64(6):735–742
Barbosa TLM, Junior COM, Graf H et al (2019) ACR TI-RADS and ATA US scores are helpful for the management of thyroid nodules with indeterminate cytology. BMC Endocr Disord 19(1):1–11
De D, Dutta S, Tarafdar S et al (2020) Comparison between sonographic features and fine needle aspiration cytology with histopathology in the diagnosis of solitary thyroid nodule. Indian J Endocrinol Metab 24(4):349
Tae HJ, Lim DJ, Baek KH et al (2007) Diagnostic value of ultrasonography to distinguish between benign and malignant lesions in the management of thyroid nodules. Thyroid 17(5):461–466
Rios A, Torregrosa B, Rodríguez JM et al (2016) Ultrasonographic risk factors of malignancy in thyroid nodules. Langenbecks Arch Surg 401(6):839–849
Koc AM, Adıbelli ZH, Erkul Z et al (2020) Comparison of diagnostic accuracy of ACR-TIRADS, American Thyroid Association (ATA), and EU-TIRADS guidelines in detecting thyroid malignancy. Eur J Radiol 133:109390
Remonti LR, Kramer CK, Leitao CB et al (2015) Thyroid ultrasoundfeatures and risk of carcinoma: a systematic review and meta-analysis of observational studies. Thyroid 25(5)
Kim EK, Park CS, Chung WY et al (2002) New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol 178(3):687–691
Okasha HH, Mansor M, Sheriba N et al (2023) Value of TI-RADS and elastography strain ratio in predicting malignant thyroid nodules: experience from a single center in Egypt. Egypt J Intern Med 35:1
Moon WJ, Jung SL, Lee JH et al (2008) Benign and malignant thyroid nodules: US differentiation—multicenter retrospective study. Radiology 247(3):762–770
Na DG, Baek JH, Sung JY et al (2016) Thyroid imaging reporting and data system risk stratification of thyroid nodules: categorization based on solidity and echogenicity. Thyroid 26(4):562–572
Wettasinghe MC, Rosairo S, Ratnatunga N et al (2019) Diagnostic accuracy of ultrasound characteristics in the identification of malignant thyroid nodules. BMC Res Notes 12(1):193
Arpaci D, Ozdemir D, Cuhaci N et al (2014) Evaluation of cytopathological findings in thyroid nodules with macrocalcification: macrocalcification is not innocent as it seems. Arq Bras Endocrinol Metabol 58(9):939–945
Singaporewalla RM, Hwee J, Lang TU et al (2017) Clinico-pathological correlation of thyroid nodule ultrasound and cytology using the TIRADS and Bethesda classifications. World J Surg 41(7):1807–1811
Pandya A, Caoili EM, Jawad-Makki F et al (2020) Retrospective cohort study of 1947 thyroid nodules: a comparison of the 2017 American College of Radiology TI-RADS and the 2015 American Thyroid Association classifications. AJR Am J Roentgenol 214(4):900–906
Clark TJ, McKinney K, Jensen A et al (2019) Risk threshold algorithm for thyroid nodule management demonstrates increased specificity and diagnostic accuracy as compared with American College of Radiology Thyroid Imaging, reporting and data system; Society of Radiologists in ultrasound; and American Thyroid Association Management Guidelines. Ultrasound Q 35(3):224–227
Mistry R, Hillyar C, Nibber A et al (2020) Ultrasound classification of thyroid nodules: a systematic review. Cureus 12(3)
De Siqueira RA, Rodrigues APDS, Miamae LM et al (2020) Thyroid nodules in severely obese patients: frequency and risk of malignancy on ultrasonography. Endocr Res 45(1):9–16
Grimmichova T, Haluzik M, Vondra K et al (2020) Relation of prediabetes and type 2 diabetes mellitus to thyroid cancer. Endocr Connec 9(7):607–616
Fussey JM, Beaumont RN, Wood AR et al (2020) Does obesity cause thyroid cancer? A Mendelian randomization study. J Clin Endocrinol Metab 105(7):e2398–e2407
Handelsman RS, Alvarez AL, Picado O et al (2019) Inverse relationship of BMI to TSH and risk of papillary thyroid cancer in surgical patients. J Surg Res 244:96–101
He Q, Sun H, Li F et al (2019) Obesity and risk of differentiated thyroid cancer: a large-scale case-control study. Clin Endocrinol 91(6):869–878
Zhao J, Tian Y, Yao J et al (2020) Hypercholesterolemia is an associated factor for risk of differentiated thyroid cancer in Chinese population. Front Oncol 10
Hrafnkelsson J, Tulinius H, Kjeld M et al (2000) Serum thyroglobulin as a risk factor for thyroid carcinoma. Acta Oncol 39(8):973–977
Zhang X, Zhang X, Chang Z et al (2018) Correlation analyses of thyroid-stimulating hormone and thyroid autoantibodies with differentiated thyroid cancer. J BUON 23(5):1467–1471
Su A, Zhao W, Wu W et al (2020) The association of preoperative thyroid-stimulating hormone level and the risk of differentiated thyroid cancer in patients with thyroid nodules: a systematic review and meta-analysis. Am J Surg 220(3):634–641
Funding
Is not applicable.
Author information
Authors and Affiliations
Contributions
Maha Assem was responsible for the writing of the manuscript and doing ultrasound for the participants. Yasmine Fathy was responsible for the pathology. Dina Esam collects the data. Shrook revised the scientific material
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study satisfied the requirements of the Revised Helsinki Declaration biomedical ethics, approved by the Research Ethics Committee, Faculty of Medicine, Cairo University.
Informed consent was obtained from all participants before inclusion.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hussein, M.A., Elesawy, Y.F., Ghoweba, D.E.A.AR. et al. Correlation of ultrasound features in the TIRADS scoring system with cytological findings in the FNAC of thyroid nodules and their association with the metabolic status. Egypt J Intern Med 36, 29 (2024). https://doi.org/10.1186/s43162-024-00290-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-024-00290-z