Abstract
Background
Acute myocardial infarction (AMI) is a leading cause of death worldwide. The first hours of acute myocardial infarction are correlated with the highest risk of death. Therefore, early diagnosis of the infarction seriously affects the efficacy of the treatment administered to the patient. Misdiagnosing patients with chest pain often leads to inappropriate admission of them as acute myocardial infarction patients. The physical examination of the patient, the electrocardiogram, and the assessment of cardiac biomarkers all play an important role in the early diagnosis of acute ischemia, along with the patient's medical history.
Main body
The present review highlights a number of different biomarkers that are released and elevated in blood during an acute myocardial infarction.
Conclusions
Analysis of cardiac biomarkers has become the first-line diagnostic tool used in the diagnosis of acute myocardial infarction. Novel markers of acute myocardial infarction, when added to routinely used markers, can provide added value not only in the earlier detection of acute myocardial infarction but also in monitoring the clinical progress of the disease, predicting its consequences, evaluating its prognosis, detecting recurrence, and managing its treatment. This leads to a lower mortality rate associated with acute myocardial infarction.
cMyC, IMA, S100, and MicroRNAs can serve as markers of early diagnosis of acute myocardial infarction, whereas myeloperoxidase, sCD40L, PAPPA, and TNF-α can be used to monitor the clinical progress of the disease. In addition, H-FABP, GDF-15, F2 isoprostanes, and ST2 can serve as predictors of AMI complications and mortality. Copeptin, ST2, and SIRT can be useful as prognostic markers of acute myocardial infarction.
Similar content being viewed by others
Background
The World Health Organization (WHO) has defined acute myocardial infarction (AMI) as having two of the three diagnostic criteria which include chest pain, Q-wave development in the ECG, and elevated plasma activity of a number of enzymes including creatine kinase (CK) CK-MB (myocardial band), aspartate transaminase (AST), and lactate dehydrogenase (LDH).
The lately proposed definitions of acute myocardial infarction have confirmed the importance of sensitive serological biomarkers in the diagnosis of the disease, as well as the use of cardiac troponins (cTn) as the gold standard [1]. Several databases for public health research including PubMed, Directory of Open Access Journals, and PLOS were searched for publications on the newly discovered and currently used diagnostic markers for acute myocardial infarction to highlight the importance of these biomarkers in the early diagnosis of acute myocardial infarction.
Main text
Pathophysiology of acute myocardial infarction
Coronary artery disease (CAD) is characterized by an atherosclerotic process taking place on the coronary vasculature. The first signs of atherosclerosis can be noticed in infants, followed by a regression in childhood, reappearance in puberty, and progression over time [2]. Asymptomatic disease can become symptomatic anytime with angina, exertional or at rest, myocardial infarction (MI), or sudden death. The incidence and prevalence of CAD are difficult to assess as different definitions are used and due to its detection only upon symptom appearance. The prevalence of angina increases with age and is higher in middle-aged females than males. It seems like mortality from CAD is decreasing; however, prevalence is not, undoubtedly as a result of better survival [2].
Stable CAD is generally characterized by episodes of reversible myocardial demand/supply mismatch, related to ischemia or hypoxia, which are usually inducible by exercise, emotion, or other types of stress and are commonly associated with transient chest discomfort [2]. Stable CAD can manifest itself due to plaque-related obstruction of coronary arteries, their spasms, microvascular dysfunction, or left ventricular dysfunction [2]. MI is defined as myocardial cell death due to prolonged ischemia. Clinically, the term MI is used when there is evidence of myocardial necrosis in a clinical setting, consistent with acute myocardial ischemia and with a rise in cardiac troponin [3]. The two types of MI on which clinical decision-making is based are ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI). In contrast to NSTEMI patients, patients with STEMI are referred to immediate reperfusion treatment strategies. Furthermore, MI is classified into 5 types depending
on its pathophysiology, clinics, and prognostics [3]:
-
Type I: Spontaneous MI:
Atherosclerotic plaque disturbance resulting in thrombus formation and decreased myocardial blood flow or distal platelet emboli with ensuing myocyte necrosis
-
Type II: MI secondary to an ischemic imbalance:
Imbalance between myocardial oxygen delivery and/or demand due to other conditions (not coronary artery disease), e.g., coronary endothelial dysfunction, coronary artery spasm,
coronary embolism, cardiac arrhythmias, heart failure, hypotension, hypertension, renal failure …etc.
-
Type III: Myocardial infarction resulting in mortality when biomarker values are unavailable
-
Type IVa: Myocardial infarction correlated with percutaneous coronary intervention (PCI)
-
Type IVb: Myocardial infarction correlated with stent thrombosis
-
Type V: Myocardial infarction correlated with coronary artery bypass grafting (CABG)
Atherosclerosis is a chronic, inflammatory, fibroproliferative disease of the intima of large and medium-sized arteries. It includes many pathophysiological mechanisms: retention and modification of lipoproteins, recruitment of monocytes and T-lymphocytes, and the accumulation of excessive fibrous tissue [4]. The development and progression of atherosclerosis depends on many risk factors, including male gender, hypertension, diabetes, smoking, elevated cholesterol, and genetic susceptibility [2].
In the outer walls of the vessel (branch points and vascular curvatures), the intima is thickened and the subendothelial proteoglycan-rich layer and a deeper musculoelastic layer with elastic fibers and smooth muscle cells (SMC) start to form [4].
Apo B-containing lipoproteins (e.g., LDL) accumulate in the proteoglycan-rich layer. Fat droplets are then oxidized by free radicals to act as proinflammatory mediators that induce the synthesis of endothelial adhesion molecules and chemokines which, in turn, stimulate the recruitment of macrophages [5]. Macrophages phagocyte oxidized LDL particles, giving them the appearance of foam cells. Foam cells assemble in the thickened intima and form yellow fatty streaks [4].
When lipoproteins are further accumulated in fatty streaks, lipid pools begin to form under the layer of foam cells with no observed disruption of vascular intimal structure.
The progression of intermediate lesions to more advanced Lesions forms an irreversible turning point in the process of atherosclerosis, e.g., fibroatheromas. A necrotic, lipid-rich core is formed by the degradation of the extracellular matrix, death of local SMCs, and apoptosis of foam cells promoting lipid accumulation. Serum lipoproteins are thought to be a major lipid source in advanced lesions. On the intravascular side, fibroatheromas are covered with a fibrous cap, consisting mostly of collagen-rich fibrotic tissue. Additionally, fibroatheromas consist of microscopic intra- and extracellular calcifications, mediated by osteogenic SMCs. Calcification volumes vary and can occupy most of the plaque [4]. Complicated plaques are a different type of lesions. They are defined by the presence of an occlusive or nonocclusive thrombus. A thrombus can complicate intermediate or advanced lesions and is a direct consequence of plaque rupture or plaque erosion (no microscopic evidence of rupture) [4].
With aging, plaques can progress and grow to become so large that the coronary artery lumen is reduced beyond the critical point at which blood flow to cardiomyocytes is not sufficient, exposing them to an anaerobic environment and causing ischemia to set in. With the onset of ischemia, stable angina pectoris develops. On the contrary, certain plaques do not reduce lumen/flow significantly and therefore do not cause symptoms. The severity of flow obstruction depends on plaque size, vasospasms, and arterial remodeling [6]. Non-stenotic plaque is clinically silent and cannot be seen on angiography. Therefore, it can only be managed by conservative therapy [7]. Stenotic plaques cause angina pectoris and are managed not only with drug therapy, but often also require revascularization therapy.
Flow through coronary arteries is limited when there is more than an 80% reduction in the luminal area or more than a 50% reduction in diameter as seen on coronary angiography. In lower grade stenosis, only flow reserve is decreased. In stable CAD, plaques progress gradually in with the above-mentioned process of atherosclerosis, sometimes complicated by plaque ruptures that are, in the vast majority, clinically silent, causing non-obstructive thrombus formation. Many complicated plaques have several plaque rupture sites. The non-obstructive thrombus is partly removed by natural lysis; the remaining part is then covered with natural heparinoids to neutralize the thrombogenicity of the exposed collagen. After 36 h, SMCs migrate to this area and cover the surface with newly produced connective tissue, restoring plaque integrity. With the restoration of the fibrous cap (thickening), the affected plaque increases in size and contracts by healing SMCs (negative remodeling) [4, 7, 8]. In most cases, MI and unstable angina are caused by the formation of a thrombus on a ruptured or eroded plaque with or without simultaneous vasospasm [8]. The thrombus causing unstable angina and NSTEMI is usually nonocclusive and dynamic, whereas, on the other hand, an occlusive and persistent thrombus is found in STEMI patients. Plaque rupture is the major cause of thrombosis in coronary vasculature and is more frequent in men [4].
When plaque ruptures, the fibrous cap tears and exposes the highly thrombogenic lipid core to blood. The lipid core contains a tissue factor, collagens, and lipid microcrystals, all of which accelerate thrombosis, which starts in the plaque itself and extends intra-luminally. When there is no sign of fibrous cap rupture, term plaque erosion is used. In plaque erosion, subendothelial tissue, such as the basal membrane, is exposed to blood, and the thrombus that forms is therefore adherent to the plaque surface. The underlying mechanism of both processes is an enhanced vascular inflammatory activity within the plaque itself. Metalloproteinase secretion by macrophages within the plaque is highly activated by inflammatory cytokines causing the degradation of the connective tissue matrix including collagen. Also, the activated macrophages cause endothelial and SMC cell death by apoptosis. Apoptosis of endothelial tissue with lysis of adhesion proteins is the basis of endothelial erosion. Apoptosis of SMCs with fibrous cap lysis leads to endothelial rupture [4, 8].
The development of a thrombus and its extent are highly variable and depend on local flow disturbances, blood coagulability, and thrombogenicity of the exposed tissue [4].
The thrombus may progress rapidly or can develop over the course of days, with intermittent flow disturbances due to dynamic processes: thrombosis, and thrombolysis with or without vasospasms. A thrombus can form predominantly intra-luminally or inside the plaque. A large occlusive thrombus can form on a small ruptured plaque or it can expand the plaque from the inside and cause its complete disassembly [8]. Only 14% of occlusions leading to MI develop on high grade stenoses (more than 70% in diameter) and more than two thirds of MI occur on non-occlusive lesions (<50% in diameter) [2]. Therefore, the severity of stenosis on angiography is not a determining factor of an individual’s risk for the development of MI, whereas the number of rupture-susceptible plaques is a crucial risk factor [2].
Risk factors
Atherosclerosis risk factors are also the most common risk factors for acute myocardial infarction. These factors include aging, obesity, smoking, hypertension, diabetes, and hypercholesterolemia, which consists of an increased low-density lipoprotein (LDL) and a decreased HDL. Unusually strenuous muscular exercise and emotional stress are also risk factors [9].
Diagnosis
The diagnosis of myocardial infarction is built on clinical and laboratory findings that include the electrocardiogram ECG and cardiac biomarkers of myocardium cell injury. Cardiac biochemical markers are signals released by the affected myocardium when myocardial damage occurs [9]. The most common causes of infarction are acute coronary syndromes, which include myocardial infarction and unstable angina, as well as other conditions that affect the heart muscle, including heart surgery, trauma, myocarditis, and others. In these cases, cardiac biomarkers may be detected and measured in blood samples. Cardiac biomarkers have a role in the diagnosis, risk assessment, and care of patients with chest pain. The primary ECG may be undiagnosing. And that despite the increasing awareness of doctors and diagnostic tools, the rate of undiagnosed myocardial infarction still ranges between 1.5 and 2%. Guidelines for the diagnosis of myocardial infarction have lately been updated, and the results of cardiac marker measurement have been incorporated into the clinical definition of myocardial infarction [9].
Creatine kinase-MB (CK-MB), cardiac troponins T and I, myoglobin, homocysteine, and C-reactive protein have been utilized to evaluate suspected acute myocardial infarction. Also, CK-MB and cardiac troponins T and I can be used in the detection and care of high-risk patients [9]. The diagnosis of myocardial infarction at the beginning of the nineties of the last century was based mainly on the increase in serum CK-MB activity. Although the sensitivity and specificity of myocardial injury have increased with the discovery of troponins, CK-MB remains the gold standard for diagnosis. Acute myocardial infarction is now defined as an elevation of some serum biochemical markers and a decrease in others concomitant with symptoms of ischemic injury, new pathological Q-waves in the ECG, ischemic changes in the ECG, and both histological changes and changes related to intervention in coronary artery consistent with acute myocardial infarction [9].
Biomarkers for acute myocardial infarction
Acute myocardial infarction is defined as the death of cardiomyocytes caused by prolonged ischemia. Myocardial cell death occurs at least 6 h after myocardial ischemia has occurre d[1]. Rapid identification of acute myocardial infarction is necessary to start effective treatment and improve prognosis. Newer recommendations confirm the importance of the evaluation of early cardiac biomarkers along with ECG in the diagnosis, as ECG alone is often insufficient for the diagnosis of acute myocardial infarction. AST was the first cardiac biomarker to be used [1]. This enzyme is insensitive to cardiac tissue, as it is found in skeletal muscle, liver, brain, and kidneys, and therefore it is no longer used in the diagnosis of acute myocardial infarction.
Then, the activity of total creatine kinase enzyme (CK) in plasma was evaluated to diagnose acute myocardial infarction, as it is a good indicator of skeletal muscle injury. Years later, LDH was used to diagnose acute myocardial infarction. In 1979, the World Health Organization recommended the adoption of the three previously mentioned tests together for the diagnosis of heart infarction [1]. The development of immunoassays in the 1980s revolutionized the field of cardiac biomarker evaluation [10].
Inflammatory markers
C-reactive protein is an acute phase protein that is secreted by hepatocytes in response to an inflammatory stimulus. C-reactive protein stimulates the expression of adhesion molecules and stimulates inflammatory cells.
Studies have also shown that this protein is elevated in patients with unstable angina. However, C-reactive protein cannot be used as a diagnostic marker due to its low sensitivity and specificity. C-reactive protein is a good prognostic indicator, as significantly higher plasma concentrations are associated with a bad prognosis [1]. Pentraxin-3, which belongs to the PTX family of proteins, is produced by vascular endothelial cells, vascular smooth muscle cells, macrophages, and neutrophils in response to an inflammatory stimulus [1].
Elevated plasma PTX-3 concentration has been suggested as a prognostic biomarker in patients with unstable angina, myocardial infarction, and heart failure [11]. PTX-3 is more sensitive to vasculitis than CRP, and it can also be used to predict the occurrence of atherosclerosis [12]. Interleukin-6 (IL-6) is another indicator of early atherosclerosis, as it plays a key role in the recruitment and activation of inflammatory cells in response to ischemia and subsequent reperfusion of injured myocardium. IL-6 also activates the hepatic production of C-reactive protein. Elevated serum concentrations of C-reactive protein and interleukin-6 are associated with the development of atherosclerosis as well as type 2 diabetes in insulin-resistant individuals [1]. Galectin-3 (Gal-3) is a member of the inflammatory mediator family and is related to the extent of myocarditis and fibrosis, which in turn negatively correlates with the ventricular ejection fraction [13]. Gal-3 is believed to be involved in plaque formation, instability, and rupture [14]. Gal-3 is associated with left ventricular dilation and is a contributing factor in predicting outcomes and guiding monitoring of patients with acute and chronic heart failure [15,16,17].
The serum concentration of Interleukin-37 (IL-37) is significantly elevated in acute coronary syndrome and an elevated basal concentration of this parameter is associated with poor outcome. Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an inflammatory biomarker unsuitable for use as a therapeutic target, while its plasma concentration is associated with predicting the risk of cardiovascular outcomes in patients with stable coronary heart diseases (CHD) [18].
Markers of plaque destabilization
Myeloperoxidase is a metalloproteinase that is produced by polymorphonuclear leukocytes and macrophages. Myeloperoxidase stimulates the initiation of the production of reactive oxygen species important for the development of atheroma and plaque rupture.
Therefore, its elevated serum activity is a marker of Plaque Destabilization, and it can also be used as a predictive marker for the development of future cardiovascular events [19,20,21]. Pregnancy-associated plasma protein A (PAPPA) is also a metalloproteinase that plays an active role during atherosclerotic plaque rupture. This enzyme is produced mainly by the placenta syncytiotrophoblasts, as well as by fibroblasts, vascular endothelial cells, and vascular smooth muscle cells. PAPPA has been associated with plaque development and instability in atherosclerosis [1].
The soluble cluster of differentiation 40 ligand (sCD40L), which belongs to the TNF-α family of proteins, is highly expressed on the surface of platelets located in the intraluminal thrombus. Activation of the inflammatory and coagulation pathways during thrombus formation leads to the release of CD40L into circulation, which is an indicator of plaque rupture and the subsequent development of myocardial infarction [1].
TNF-α is a pleiotropic cytokine produced by endothelial cells, vascular smooth muscle cells, and macrophages. The serum TNF-α concentration is significantly elevated in advanced heart failure. The role of this protein in atherosclerosis lies in the production of tissue inhibitors of metalloproteinases by fibroblasts. Therefore, the production of excess amounts of metalloproteinases leads to the rupture of the atherosclerotic plaque [1]. TNF-α also activates IL-6 production by vascular smooth muscle cells, confirming the role of TNF-α in regulating the inflammatory cascade. Therefore, elevated TNF-α concentrations may be an indicator of a nonfatal recurrent myocardial infarction or a fatal cardiovascular event [1].
Myocardial necrosis markers
Traditional markers
Troponins of three types C, T, and I are complexes consisting of three protein subunits, which are located on the thin filaments of cardiac and skeletal muscle fibers. Troponin C is the binding component of calcium, while troponin T is the binding component of tropomyosin, and troponin I is the inhibitory component. Troponin C isoforms in cardiac and skeletal muscle are completely identical, so it is not highly specific for cardiomyopathy.
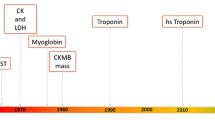
While the isoforms of troponin T and troponin I differ in cardiac and skeletal muscle, they are highly specific for cardiac tissue necrosis. Troponin T is mainly found in its bound form with contractile components in cardiomyocytes and is also found free in the cytoplasm, and therefore, it shows a double release of the free component first and then the bound component later [1]. Troponin I is highly myocardial specific, as it has not been isolated from skeletal muscle, making it a typical marker for myocardial injury. Troponins are released into circulation about 6–8 h after myocardial injury, peak plasma concentrations after 12–24 h, and plasma concentrations remain elevated for 7–10 days. The only drawback of troponins is their delayed clearance, so it is difficult to use them to identify recurrent myocardial infarction [1]. Myoglobin is a small cytoplasmic oxygen-binding protein found both in cardiac and skeletal muscle [1]. Both CK and CK-MB were mentioned as cardiac biomarkers in 1979. Creatine kinase (CK) is mainly found in cardiac and skeletal muscle, and it has three isoenzymes: MM, MB, and BB. Where CK-MM represents the musculoskeletal portion of the total CK, while CK-MB is that portion of the total enzyme assembly found in the heart muscle, and CK-BB is the brain portion of the total enzyme CK. The activity of total creatine kinase in serum has been evaluated in myocardial infarctions. Serum CK-MB activity rises 4–9 h after the onset of chest pain, peaks after approximately 24 h, and returns to baseline values within 48–72 h [1]. Measurement of CK-MB activity in serum is superior to measurement of troponins only in its early clearance, which helps detect re-infarction. Serum CK-MB activity and serum troponin concentration are both evaluated for the diagnosis of myocardial infarction. Figure 1 shows the routine pattern of cardiac markers associated with myocardial infarction [1].
Routine model of cardiac markers associated with myocardial infarction [22]
Novel markers
-
Cardiac myosin-binding protein C (cMyC)
Cardiac myosin-binding protein C (cMyC) is one of the three isoforms of myosin-binding protein C that is expressed in cardiac tissue, while the other two isoforms are expressed in skeletal muscle [18]. This protein appears in the circulation earlier than highly sensitive cardiac troponin hs-cTn after necrosis of cardiomyocytes [23]. While cMyC has a higher efficacy than hs-cTn in excluding or confirming patients, the two markers have similar diagnosis accuracy [24]. A study has shown that cMyC has similar discriminatory power as hs-cTn and that it can perform better than hs-cTn early after the occurrence of symptoms [18].
-
Heart fatty acid-binding protein (H-FABP)
It is a small, low molecular weight cytosolic protein found in the myocardial tissue that is responsible for transporting fatty acids from the plasma membrane to the β-oxidation sites in both mitochondria and the peroxisome, as well as transporting these acids to the sites of lipid synthesis in the endoplasmic reticulum. This protein is found mainly in the cardiac muscle, and it is also found to a lesser extent in the brain, kidneys, and skeletal muscles. Cardiac fatty acid-binding protein is released early and severely after cardiac muscle cell membrane rupture, as it shows a rise in its plasma concentration within 30 min of the occurrence of myocardial injury, and this concentration reaches its peak after 6–8 h, to return to its baseline value after 24 h approximately. The cardiac fatty acid-binding protein can also be used as a predictive marker of mortality after acute coronary syndrome [25, 26].
-
Ischemia-modified albumin (IMA)
The concentration of this protein in the blood increases significantly in the case of ischemia, and therefore it helps in the diagnosis of acute ischemia before the development of myocardial necrosis. The ischemia-modified albumin can be measured by the binding of cobalt to the damaged amino terminus of the albumin. An increase in the concentration of this protein occurs immediately after ischemia and returns to its baseline values within 6–12 h, so it can may be useful for the early detection of ischemia [27, 28].
-
Growth-differentiation factor-15 (GDF-15)
This protein belongs to the transforming growth factor-β family of cytokines that are mainly expressed in the placenta, although it may be expressed in various tissues under abnormal conditions. Elevated plasma GDF-15 concentrations during cardiac ischemia suggest the diagnosis of acute coronary syndrome (ACS). It can be used as a predictor of mortality after ACS rather than as a diagnostic marker for ACS due to its low specificity [1].
-
Copeptin
It is the carboxy-terminal portion of the precursor of Vasopressin that is secreted along with Vasopressin itself.
Since Copeptin is released from the pituitary in large quantities early in acute myocardial infarction (AMI), its plasma concentration in an AMI patient rises significantly within minutes of the injury. Copeptin can be used as a diagnostic and prognostic marker for cardiomyopathy [1].
-
F2 isoprostanes
These compounds are metabolites of arachidonic acid. F2 isoprostanes are secreted by many cells including monocytes during atherosclerosis. Studies have shown the presence of high concentrations of these compounds in the urine of patients with unstable angina. F2 isoprostanes can be used as markers to predict complications in nonfatal myocardial infarction, as well as to predict the progression of heart failure and death [1].
-
Salivary biomarkers associated with myocardial necrosis
Saliva provides an easy, simple, and non-invasive detection method for many systemic diseases. Saliva contains components of serum, gingival fluid, and oral mucosal leaching, making it an important diagnostic tool. Salivary AMI biomarkers include myoglobin, C-reactive protein, myeloperoxidase, troponin C, and CK-MB. The above-mentioned markers, when used with ECG, have shown a positive association in patients compared with healthy controls [29]. The concentration of salivary myoglobin was significantly increased within 48 h of the occurrence of chest pain in acute myocardial infarction, and it was also positively correlated with the concentration of that protein in serum [1]. Miller et al. showed that salivary concentrations of a number of markers including CRP, TNF-α, matrix metalloproteinase-9 (MMP-9), and MPO were significantly higher in patients with myocardial infarction and were positively correlated with the concentrations of those proteins in serum. Studies have shown a significant increase in the concentration of salivary intracellular adhesion molecule-1 (ICAM-1) in patients with acute myocardial infarction, while other studies have shown a significant decrease in the concentration of sCD40L in the saliva of these patients [30]. Foley et al. demonstrated the association of the salivary concentrations of troponin I and C-reactive protein with their serum concentrations in patients with myocardial injury. Therefore, these studies suggest that saliva can be used as a substitute for serum in the diagnosis of myocardial infarction [31].
-
Other biomarkers
Suppression of tumorigenicity 2 (ST2)
Suppression of tumorigenicity 2 (ST2) belongs to the IL-1 receptor family and possesses two isoforms, the transmembrane ST2L isoform and the soluble isoform sST2 [18]. The soluble form showed a high expression in patients with acute myocardial infarction, accompanied by an increase in GDF-15 and HFABP, and a decrease in Fetuin-A [32]. Elevated ST2 concentrations are predictive of adverse cardiac events in patients with acute coronary syndrome [33]. Serum ST2 concentration is positively correlated with IL-33 and BNP concentrations, and each of these biomarkers is an independent predictor of adverse cardiac events in patients with myocardial infarction undergoing percutaneous coronary intervention (PCI).
Research in animal models has confirmed that IL-33 inhibits cardiomyocyte apoptosis and improves cardiac function after myocardial infarction [34]. Also, modulation of IL-33/ST2 signaling may have a beneficial effect against negative post-infarction remodeling of the heart [34]. The soluble form of ST2 also has a useful role in the diagnosis of heart failure, especially in patients with elevated IL-33 concentrations [35]. Also, this form is significantly associated with cardiac death and readmission for exacerbated heart failure [36, 37].
Sirtuin proteins (SIRT)
The Sirtuin family of proteins (SIRT) includes 7 proteins (SIRT1–SIRT7) that have been known to be stress modulators and epigenetic-related enzymes involved in the cellular events controlling aging-related disorders and cardiovascular disease [38]. Compared to other myocardial biomarkers, the concentration of the soluble isoform of suppression of tumorigenicity 2 (ST2) is less affected by renal function and other conditions. Baseline ST2 concentrations are strong predictors in heart failure at chronic and acute stages, independently from BNP levels [39]. SIRT1 is the most distinguished of these for its protective roles in inflammation, vascular aging, heart disease, and atherosclerotic plaque development. Whole-genome expression analysis showed that SIRT1 transcription levels were decreased in peripheral blood monocytes isolated from patients with acute myocardial infarction, unstable angina, or other acute coronary syndromes. The researchers also showed that SIRT1 mRNA expression was negatively correlated with IL-6 gene expression.
The SIRT1 mRNA expression was significantly decreased in heart failure [18].
S100 proteins
They are calcium-binding low molecular weight proteins. There are at least 30 types of these proteins. The S100 protein consists of two isomers, a and b, where the protein can consist of two subunits of the same type (aa or bb) or of two different types (ab) [40]. The heart and skeletal muscle have been shown to contain large amounts of S100A, while most of the S100B is found in the brain. S100 proteins contribute to the regulation of protein phosphorylation, cell growth and differentiation, transcription factors, calcium transport and storage (calcium homeostasis), enzymatic activities and the inflammatory response. S100 isoforms have formerly been demonstrated to play key roles in the immune system as damage-associated molecular patterns (DAMPs), antimicrobial peptides, pro-inflammation stimulators, chemo-attractants, and metal scavengers during an innate immune response. Thus, they are critical in treating autoimmune diseases [40]. Also, many of these proteins are beneficial biomarkers for certain types of cance r[41]. S100 proteins are also biomarkers of inflammatory diseases. Serum S100A0 is a useful marker for the diagnosis of acute myocardial infarction, and is also better than CK-MB in distinguishing acute myocardial infarction from angina pectoris. S100A1 is abundant in the heart, particularly in ventricular myocytes [41]. Therefore, it can be used in the post-mortem diagnosis of early-stage acute myocardial infarction [42].
Expression of S100A4 protects cardiomyocytes from ischemia [43]. Serum concentrations of S100B, S100A6, and S100P proteins were correlated with ischemic myocardial injury in acute coronary syndrome, and the expression of these proteins correlated with the size of the infarcted portion of the myocardium [41]. The S100B protein plays a crucial role in reducing cardiomyocyte hypertrophy and is an important therapeutic target for the treatment of heart diseases [41].
RNA molecules
-
1.
MicroRNAs
MicroRNAs (miRNAs/miRs) are small tissue-specific non-coding RNA molecules of 19-25 nucleotides that affect several biological processes including cell growth, proliferation, differentiation, and apoptosis [41]. MicroRNAs circulate in the plasma and have been evaluated as biological markers of cardiovascular disease [44].
Research has shown the presence of expression of microRNA-499 in the myocardium and skeletal muscle of mammals, and blood samples taken from patients showed a high concentration of microRNA-499 prior to the occurrence of acute myocardial infarction [41].
Circulating microRNA-499 has good sensitivity and specificity in differentiating acute myocardial infarction from other myocardial injuries, and is a biomarker for early diagnosis of acute myocardial infarction [45]. Zhang et al. showed that a decrease in microRNA-145 concentration is inversely associated with the risk of acute myocardial infarction. It is also thought that microRNA-145 in circulation is not only important for diagnosing myocardial infarction but may be valuable in predicting cardiac function and the risk of developing cardiac failure [46].
Jia and colleagues reported in 2015 that both miR-125b-5p and miR-30d-5p are valuable in the early diagnosis of acute myocardial infarction and that the latter may have a higher diagnostic value than cardiac troponin I (cTnI) [47]. MicroRNA-208b, microRNA-133a, miR-486, miR-150, and microRNA-21 may be novel biomarkers utilized in the diagnosis of acute myocardial infarction [41].
-
2.
Long non-coding RNAs
Zhong and colleagues indicated that differential expression of long non-coding RNA molecules may aid in understanding the molecular basis for the development of acute myocardial infarction and that these molecules may be utilized as biomarkers for non-invasive diagnosis [48]. Long non-coding RNAs are a group of RNA transcripts containing more than 200 nucleotides that are non-translatable but have the same effect as miRNAs [49]. Studies have reported that lncRNAs contribute to the regulation of heart failure pathogenesis and cardiovascular aging [41]. Previous studies showed that three long non-coding RNAs H19, MIAT, and MALAT1 are promising biomarkers for the diagnosis of acute myocardial infarction, as their concentrations were significantly increased in patients with acute myocardial infarction compared with healthy controls [50].
Conclusions
Analysis of cardiac biomarkers has become the first-line diagnostic tool used in the diagnosis of acute myocardial infarction. Novel markers of acute myocardial infarction, when added to routinely used markers, can provide added value not only in the earlier detection of acute myocardial infarction but also in monitoring the clinical progress of the disease, predicting its consequences, evaluating its prognosis, detecting recurrence, and managing its treatment. This leads to a lower mortality rate associated with acute myocardial infarction.
cMyC, IMA, S100, and MicroRNAs can serve as markers of early diagnosis of acute myocardial infarction, whereas myeloperoxidase, sCD40L, PAPPA, and TNF-α can be used to monitor the clinical progress of the disease. In addition, H-FABP, GDF-15, F2 isoprostanes, and ST2 can serve as predictors of AMI complications and mortality. Copeptin, ST2, and SIRT can be useful as prognostic markers of acute myocardial infarction.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author [H. K.], upon reasonable request.
Abbreviations
- AMI:
-
Acute myocardial infarction
- WHO:
-
World Health Organization
- ECG:
-
Electrocardiogram
- CK:
-
Creatinine kinase
- AST:
-
Aspartate transaminase
- LDH:
-
Lactate dehydrogenase
- cTn:
-
Cardiac troponins
- H-FABP:
-
Heart fatty acid-binding protein
- hs-cTn:
-
Highly sensitive cardiac troponin
- BNP:
-
B-type natriuretic peptide
- IMA:
-
Ischemia-modified albumin
- GDF-15:
-
Growth-differentiation factor-15
- ST2:
-
Suppression of tumorigenicity 2
- SIRT:
-
Sirtuin proteins
- cyc-C:
-
Cystatin C protein
- lncRNAs:
-
Long non-coding RNAs
- PTX:
-
Pentraxin
- IL-6:
-
Interleukin-6
- IL-37:
-
Interleukin-37
- Gal-3:
-
Galectin-3
- LDL:
-
Low-density lipoprotein
- HDL:
-
High-density lipoprotein
- Lp-PLA2:
-
Lipoprotein-associated phospholipase A2
- TNF-α:
-
Tumor necrosis factor-α
- ACS:
-
Acute coronary syndrome
- MPO:
-
Myeloperoxidase
- MMP-9:
-
Matrix metalloproteinase-9
- ICAM-1:
-
Intercellular adhesion molecule-1
- MIAT:
-
Myocardial infarction associated transcript
- MALAT:
-
Metastasis-associated lung adenocarcinoma transcript 1
- DAMPs:
-
Damage-associated molecular patterns
- SMCs:
-
Smooth muscle cells
- STEMI:
-
ST-segment elevation myocardial infarction
- NSTEMI:
-
Non-ST-segment elevation myocardial infarction
References
Mythili S, Malathi N (2015) Diagnostic markers of acute myocardial infarction. Biomed Rep 3(6):743–748
Tibaut M, Mekis D, Petrovic D (2017) Pathophysiology of myocardial infarction and acute management strategies. Cardiovasc Hematol Agents Med Chem 14:150–159
van Beek DEC, van Zaane B, Buijsrogge MP, van Klei WA (2015) Implementation of the third universal definition of myocardial infarction after coronary artery bypass grafting: a survey study in Western Europe. J Am Heart Assoc 4:e001401
Bentzon JF, Falk E (2011) Acute Coronary Syndromes: A Companion to Braunwald’s. Heart Dis:42–52
Hansson GK (2005b) Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 352:1685–1695
Schoenhagen P, Ziada KM, Vince DG et al (2001) Arterial remodeling and coronary artery disease: the concept of “dilated” versus “obstructive” coronary atherosclerosis. J Am Coll Cardiol 38:297–306
Libby P, Theroux P (2005) Pathophysiology of coronary artery disease. Circulation 111:3481–3488
Davies MJ (2000) CORONARY DISEASE: The pathophysiology of acute coronary syndromes. Br Heart J 83:361–366
Rashid S, Malik A, Khurshid R, Faryal U, Qazi S (2018) The Diagnostic Value of Biochemical Cardiac Markers in Acute Myocardial Infarction. In: Myocardial Infarction. IntechOpen. Available via: https://doi.org/10.5772/intechopen.76150. Accessed 4 Feb 2022
Ahmad MI (2012) Biomarkers in Acute Myocardial Infarction. J Clin Exp Cardiolog [Internet] 03(11). Available from: http://dx.doi.org/10.4172/2155-9880.1000222
Suzuki S, Takeishi Y, Niizeki T, Koyama Y, Kitahara T, Sasaki T et al (2008) Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am Heart J 155(1):75–81
Knoflach M, Kiechl S, Mantovani A, Cuccovillo I, Bottazzi B, Xu Q et al (2012) Pentraxin-3 as a Marker of Advanced Atherosclerosis Results from the Bruneck, ARMY and ARFY Studies. PLoS One 7(2):e31474
Kang Q, Li X, Yang M, Fernando T, Wan Z (2018) Galectin-3 in patients with coronary heart disease and atrial fibrillation. Clin Chim Acta 478:166–170
Bivona G, Bellia C, Lo Sasso B, Agnello L, Scazzone C, Novo G et al (2016) Short-term Changes in Gal 3 Circulating Levels After Acute Myocardial Infarction. Arch Med Res 47(7):521–525
French B, Wang L, Ky B, Brandimarto J, Basuray A, Fang J et al (2016) Prognostic Value of Galectin-3 for Adverse Outcomes in Chronic Heart Failure. J Card Fail 22(4):256–262
van Vark L, Lesman-Leegte I, Baart S, Postmus D, Pinto Y, Orsel J et al (2017) Prognostic Value of Serial ST2 Measurements in Patients with Acute Heart Failure. J Am Coll Cardiol 70(19):2378–2388
Zivlas C, Triposkiadis F, Psarras S, Giamouzis G, Skoularigis I, Chrysanthopoulos S et al (2017) Left atrial volume index in patients with heart failure and severely impaired left ventricular systolic function: the role of established echocardiographic parameters, circulating cystatin C and galectin-3. Ther Adv Cardiovasc Dis 11(11):283–295
Wang X, Zhang F, Zhang C, Zheng L, Yang J (2020) The Biomarkers for Acute Myocardial Infarction and Heart Failure. BioMed Res Intern 2020:1–14
Khan S, Kelly D, Quinn P, Davies J, Ng L (2006) Myeloperoxidase aids prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart 93(7):826–831
Wang J, Zhang S, Jin Y, Qin G, Yu L, Zhang J (2007) Elevated levels of platelet, Monocyte aggregates and related circulating biomarkers in patients with acute coronary syndrome. Int J Cardiol 115:361–365
Cavusoglu E, Ruwende C, Eng C, Chopra V, Yanamadala S, Clark LT et al (2007) Usefulness of baseline plasma myeloperoxidase levels as an independent predictor of myocardial infarction at two years in patients presenting with acute coronary syndrome. Am J Cardiol 99:1364–1368
Index of /Common_New [Internet]. Tulipgroup.com. Available via: http://www.tulipgroup.com/Common_New/. Accessed 7 Feb 2022.
Kaier TE, Alaour B, Marber M (2019) Cardiac myosinbinding prot0065in C-from bench to improved diagnosis of acute myocardial infarction. Cardiovasc Drugs Ther 33(2):221–230
Kaier TE, Alaour B, Marber M (2018) Cardiac myosinbinding protein C: how a novel biomarker could transform chest pain triage. Biomark Med 12(8):823–826
Chan D, Ng LL (2010) Biomarkers in acute myocardial infarction. BMC Med 8(1):34
Haltern G, Peiniger S, Bufe A, Reiss G, Gülker H, Scheffold T (2010) Comparison of usefulness of heart-type fatty acid binding protein versus cardiac troponin T for diagnosis of acute myocardial infarction. Am J Cardiol 105(1):1–9
Mastella AK, Moresco RN, Da Silva DB, Becker AM, Duarte MM, Giovelli LL et al (2009) Evaluation of ischemia modified albumin in myocardial infarction and prostatic diseases. Biomed Pharmacother 63:762–766
Hjortshøj S, Kristensen SR, Ravkilde J (2010) Diagnostic value of ischemia-modified albumin in patients with suspected acute coronary syndrome. Am J Emerg Med 28(2):170–176
Malathi N, Mythili S, Vasanthi HR (2014) Salivary diagnostics: A brief review. ISRN Dent 2014:1–8
Miller CS, Foley JD, Bailey AL, Campell CL, Humphries RL, Christodoulides N et al (2010) Current developments in salivary diagnostics. Biomark Med 4(1):171–189
Foley JD III, Sneed JD, Steinhubl SR, Kolasa JR, Ebersole JL, Lin Y et al (2012) Salivary biomarkers associated with myocardial necrosis: results from an alcohol septal ablation model. Oral Surg Oral Med Oral Pathol Oral Radiol 114(5):616–623
Schernthaner C, Lichtenauer M, Wernly B, Paar V, Pistulli R, Rohm I et al (2017) Multibiomarker analysis in patients with acute myocardial infarction. Eur J Clin Invest 47(9):638–648
Jha D, Goenka L, Ramamoorthy T, Sharma M, Dhandapani VE, George M (2018) Prognostic role of soluble ST2 in acute coronary syndrome with diabetes. Eur J Clin Invest 48(9):e12994
Wang Y-P, Wang J-H, Wang X-L (2017) Roles of ST2, IL- 33 and BNP in predicting major adverse cardiovascular events in acute myocardial infarction after percutaneous coronary intervention. J Cell Mol Med 21(11):2677–2684
Luo NS, Zhang HF, Liu PM, Lin YQ, Huang TC, Yang Y et al (2017) Diagnostic value of combining serum soluble ST2 and interleukin-33 for heart failure patients with preserved left ventricular ejection fraction. Zhonghua Xin Xue Guan Bing Za Zhi 45(3):198–203
Bahuleyan CG, Alummoottil GK, Abdullakutty J (2018) Prognostic value of soluble ST2 biomarker in heart failure patients with reduced ejection fraction-a multicenter study. Indian Heart J 70(1):S79–S84
Mueller T, Gegenhuber A, Leitner I, Poelz W, Haltmayer M, Dieplinger B (2016) Diagnostic and prognostic accuracy of galectin-3 and soluble ST2 for acute heart failure. Clin Chim Acta 463:158–164
Onofrio N, Servillo L, Balestrieri ML (2018) SIRT1 and SIRT6 signaling pathways in cardiovascular disease protection. Antioxid Redox Sign 28(8):711–732
Perrone MA, Favresse J, D’Alessandro A, Albanese F, De Bruyne C, Ceccarelli S et al (2022) Soluble isoform of suppression of tumorigenicity 2 (ST2) biomarker in a large cohort of healthy pediatric population: Determination of reference intervals. J Clin Med 11(16):4693
Singh P, Ali SA (2022) Multifunctional role of S100 protein family in the immune system: An update. Cells 11(15):2274
Wu Y, Pan N, An Y, Xu M, Tan L, Zhang L (2020) Diagnostic and prognostic biomarkers for myocardial infarction. Front Cardiovasc Med 7:617277
Bi H, Yang Y, Huang J, Li Y, Ma C, Cong B (2013) Immunohistochemical detection of S100A1 in the postmortem diagnosis of acute myocardial infarction. Diagn Pathol 8:84. https://doi.org/10.1186/1746-1596-8-84
Doroudgar S, Quijada P, Konstandin M, Ilves K, Broughton K, Khalafalla FG et al (2016) S100A4 protects the myocardium against ischemic stress. J Mol Cell Cardiol 100:54–63
Wang C, Jing Q (2018) Non-coding RNAs as biomarkers for acute myocardial infarction. Acta Pharmacol Sin 39(7):1110–1119
Zhao J, Yu H, Yan P, Zhou X, Wang Y, Yao Y (2019) Circulating MicroRNA-499 as a diagnostic biomarker for acute myocardial infarction: A meta-analysis. Dis Markers 2019:6121696
Zhang W-Q, Xie B-Q (2017) A meta-analysis of the relations between blood microRNA-208b detection and acute myocardial infarction. Eur Rev Med Pharmacol Sci 21(4):848–854
Jia K, Shi P, Han X, Chen T, Tang H, Wang J (2016) Diagnostic value of miR-30d-5p and miR-125b-5p in acute myocardial infarction. Mol Med Rep 14(1):184–194
Martincorena I, Fowler JC, Wabik A, Lawson ARJ, Abascal F, Hall MWJ et al (2018) Somatic mutant clones colonize the human esophagus with age. Science 362(6417):911–917
Zhai H, Li X-M, Liu F, Chen B-D, Zheng H, Wang X-M et al (2017) Expression pattern of genome-scale long noncoding RNA following acute myocardial infarction in Chinese Uyghur patients. Oncotarget 8(19):31449–31464
Wang X-M, Li X-M, Song N, Zhai H, Gao X-M, Yang Y-N (2019) Long non-coding RNAs H19, MALAT1 and MIAT as potential novel biomarkers for diagnosis of acute myocardial infarction. Biomed Pharmacother 118(109208):109208
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
There are no contributing authors to this article. The corresponding author H.K. searched databases, collected data, planned, organized, and wrote the whole manuscript. The manuscript was read and approved by the corresponding author H.K.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khalil, H. Traditional and novel diagnostic biomarkers for acute myocardial infarction. Egypt J Intern Med 34, 87 (2022). https://doi.org/10.1186/s43162-022-00178-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-022-00178-w