Abstract
Myocardial infarction causes significant mortality and morbidity. Timely diagnosis allows clinicians to risk stratify their patients and select appropriate treatment. Biomarkers have been used to assist with timely diagnosis, while an increasing number of novel markers have been identified to predict outcome following an acute myocardial infarction or acute coronary syndrome. This may facilitate tailoring of appropriate therapy to high-risk patients. This review focuses on a variety of promising biomarkers which provide diagnostic and prognostic information.
Heart-type Fatty Acid Binding Protein and copeptin in combination with cardiac troponin help diagnose myocardial infarction or acute coronary syndrome in the early hours following symptoms. An elevated N-Terminal Pro-B-type Natriuretic Peptide has been well validated to predict death and heart failure following a myocardial infarction. Similarly other biomarkers such as Mid-regional pro-Atrial Natriuretic Peptide, ST2, C-Terminal pro-endothelin 1, Mid-regional pro-Adrenomedullin and copeptin all provide incremental information in predicting death and heart failure. Growth differentiation factor-15 and high-sensitivity C-reactive protein predict death following an acute coronary syndrome. Pregnancy associated plasma protein A levels following chest pain predicts risk of myocardial infarction and revascularisation. Some biomarkers such as myeloperoxidase and high-sensitivity C-reactive protein in an apparently healthy population predicts risk of coronary disease and allows clinicians to initiate early preventative treatment. In addition to biomarkers, various well-validated scoring systems based on clinical characteristics are available to help clinicians predict mortality risk, such as the Thrombolysis In Myocardial Infarction score and Global Registry of Acute Coronary Events score. A multimarker approach incorporating biomarkers and clinical scores will increase the prognostic accuracy. However, it is important to note that only troponin has been used to direct therapeutic intervention and none of the new prognostic biomarkers have been tested and proven to alter outcome of therapeutic intervention.
Novel biomarkers have improved prediction of outcome in acute myocardial infarction, but none have been demonstrated to alter the outcome of a particular therapy or management strategy. Randomised trials are urgently needed to address this translational gap before the use of novel biomarkers becomes common practice to facilitate tailored treatment following an acute coronary event.
Similar content being viewed by others
Introduction
Coronary artery disease (CAD) and its end result, myocardial infarction (MI) continue to be a significant cause of mortality and morbidity in the western world. Over the past 50 years, it has become clear that the cascade of thrombotic events following atherosclerotic plaque rupture causes occlusion of the coronary artery, interrupting blood supply and oxygen to myocardium thus resulting in infarction. Myocardial necrosis following infarction is followed by heart failure, myocardial rupture or arrhythmias. Early treatment of myocardial ischaemia to prevent necrosis with treatments such as fibrinolysis, coronary artery bypass grafting and percutaneous coronary intervention have improved outcome [1].
Over time it has become clear that in order for such treatments to be of maximal benefit, timely diagnosis is important. Here, biomarkers become important, to help us improve our diagnostic accuracy of the disease, as treatments are not without risk. Furthermore, biomarkers also provide prognostic information about the disease, which then aids clinicians in deciding how aggressively they need to treat the disease.
Definitions
Biomarkers are measurable and quantifiable biological parameters which serve as indices for health and physiology assessments [2]. This includes disease risk and diagnosis. The diagnosis of acute myocardial infarction (AMI) [3] can be made with the detection of a rise/fall of cardiac troponin (at least one value above the 99th percentile of the upper reference limit) and one of 1) symptoms of ischaemia, 2) electrocardiogram (ECG) changes of new ischaemia, 3) new pathological Q waves or 4) imaging evidence of new loss of viable myocardium.
Both the ECG and cardiac troponin are biomarkers, but the focus of this review will be on serum proteins/markers which have become increasingly important to improve our diagnosis of myocardial infarction, in some cases identifying people at risk of having an infarct and in others to predict long term prognosis following an actual event.
What makes a good biomarker?
A good biomarker is something that is easily measured and can be used as a surrogate marker for disease and its severity [4]. For instance, blood sugar can be used to diagnose diabetes [5] whilst glycosylated haemoglobin (HbA1c) monitors blood sugar control. Because cardiovascular disease continues to be a huge burden in most countries, it is important to identify high risk patients in order to prevent morbidity or mortality in later life. Medications and treatments also come at a cost and therefore simple and cheap tests have become increasingly necessary to decide how to target treatment. A good biomarker will diagnose or predict risk accurately (that is, high specificity and sensitivity), promptly provide affordable but meaningful results, and should provide this incrementally over existing markers or clinical characteristics.
Biomarkers in acute myocardial infarction
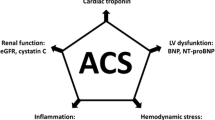
Some of these newer biomarkers and their relationship to various pathophysiological processes are depicted in Figure 1.
Diagnostic biomarkers
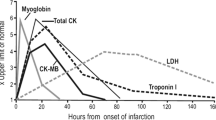
Two well known biomarkers in use for diagnosis of acute myocardial infarction are Creatine-Kinase-MB isoform and Cardiac Troponin. In 2000, Cardiac Troponin replaced CK-MB as the biomarker of choice for diagnosing a myocardial infarction [6]. Troponin is a protein released from myocytes when irreversible myocardial damage occurs. It is highly specific to cardiac tissue and accurately diagnoses myocardial infarction with a history of ischaemic pain or ECG changes reflecting ischaemia.
Cardiac troponin level is dependent on infarct size [7], thus giving clinicians an idea of the prognosis following an infarct. However, following reperfusion therapy, the actual troponin level can be misleading due to the washout phenomenon. Troponin levels peak at 12 hours, and stay elevated for 10 days or more. Whilst the use of Troponin for diagnosing AMI and risk stratification to aid decision making has revolutionised the management of patients presenting with chest pain, the 12-hour wait for the levels to peak remains the Achilles heel of this biomarker. Newer, more sensitive troponin assays [8] have been introduced to rectify this weakness. A positive Troponin is associated with increased risk of an adverse outcome at 30 days (HR 1.96, P = 0.003). In addition, the following two biomarkers may help facilitate early diagnosis of AMI, although neither has been compared with the newer high sensitivity troponin assays.
C-terminal-provasopressin (Copeptin)
Copeptin is the more stable surrogate of arginine vasopressin (AVP), with well-known effects on osmoregulation and cardiovascular homeostasis [9]. Post AMI, vasopressin is thought to (1) increase peripheral vasoconstrictor activity thus increasing afterload and ventricular stress [10]; (2) increase protein synthesis in myocytes leading to hypertrophy [11] and (3) vasoconstriction of coronary arteries. These effects are mediated via the V1 receptor, whilst effects on the V2 receptor mediate water retention in the renal tubules. These receptors are now targets for pharmacological therapy [12, 13]. Copeptin is released in stoichiometric proportion to vasopressin and is stable and easily assayed.
Copeptin can rule out MI earlier in addition to a negative Troponin T test [14]. At the time of presentation a copeptin level of < 14 pg/ml and a Trop T level of < 0.01 could rule out a myocardial infarction with an area under the curve (AUC) of receiver operating characteristic curve (ROC) of 0.97 (negative predictive value of 99.7%), thus obviating the need for monitoring and serial blood tests in a majority of patients. Copeptin is a good marker of neurohormonal stress, making it also useful in risk stratification in sepsis [15] and other diseases and hence is not specific to the cardiovascular system.
Heart-Type Fatty Acid Binding Protein (H-FABP)
H-FABP is a low molecular weight protein involved in myocardial fatty-acid metabolism [16]. It is also found in small quantities in brain, kidney and skeletal tissue and levels can go up in acute ischaemic strokes and intense exercise. It is rapidly released early in myocardial infarction and necrosis into the cytosol. H-FABP has been shown in mouse studies to be an early marker of ischaemia [17] (before morphological evidence of myocardial necrosis) and can therefore help with diagnosis of MI earlier [17–19]. However, studies attempting to use H-FABP alone for early diagnosis of AMI have produced disappointing results. One review of six studies found that the pooled positive predictive value to be 65.8% and pooled negative predictive value to be 82.0% [20]. Other more recent studies demonstrated that H-FABP levels were clearly associated with the composite end point of death, myocardial infarction and heart failure at 10 months [21, 22]. When levels of H-FABP were measured post-ACS and divided into quartiles, the top quartile was associated with all-cause mortality 6.59 times higher than the lowest quartile, after adjusting for hsCRP and Troponin. In fact, when added to Troponin for risk stratification, a negative troponin and H-FABP level < 5.8 mcg/L was associated with zero mortality at six months; a negative Troponin but H-FABP level > 5.8 mcg/L was associated with a 4.93-fold increase in risk of death and 7.93-fold increase in risk if Troponin was positive and H-FABP > 5.8 mcg/L.
Prognostic biomarkers
Before broaching the subject of biomarkers it is important to note that as a result of various randomized control trials and registry studies, various risk factors have been identified and entered into scoring systems that allow a clinician to risk stratify disease [23]. Popular tools include the TIMI score [24], derived from the Thrombolysis in Myocardial Infarction study, and the PURSUIT score [25] (from Platelet glycoprotein IIb/IIIa in unstable angina: Receptor sUppression using Integrillin Therapy). The GRACE score is another particularly robust clinical tool [26], which uses clinical indicators to calculate risk, (from the Global Registry of Acute Coronary Events study), utilizing weighted information about renal dysfunction, haemodynamic status, age, Killip Class, cardiovascular history, and history of a cardiac arrest, as well as elevated cardiac enzymes and type of ECG changes. On its own this score has an excellent c-statistic of 0.84 for predicting in-hospital death.
Newly introduced biomarkers should complement and have incremental prognostic value over and above these simple risk scores. It is therefore no surprise that biomarkers providing prognostic information following an acute coronary syndrome reflect the various physiological pathways described in the GRACE score (for example, haemodynamic status vs. biomechanical stress and neurohumoral pathways). Currently, the only accepted biomarker affecting a change in management of a patient with an acute coronary syndrome is the cardiac troponin.
1) Biomarkers of biomechanical stress
BNP/NTproBNP
One of the best known biomarkers of biomechanical stress is the B-type Natriuretic Peptide (BNP). Secreted by the ventricles in response to cardiomyocytes under tension [27], BNP binds and activates receptors causing reduction in systemic vascular resistance, central venous pressure and natriuresis. BNP has been studied extensively and provides prognostic information following an MI [28–30]. This biomarker has a short half-life but is released with the N-terminal portion of the pro-BNP peptide (NTproBNP), a peptide much more stable in serum and can be measured easily [31]. The understanding of its biochemistry is far from complete, in particular post-translational metabolism of the peptides, which may affect accurate determination of the levels of active BNP [32].
NTproBNP/BNP provides incremental information on cardiovascular death at one year in the older population above and beyond GRACE score [33]. On its own, it is at least as good as the GRACE score when predicting in-hospital mortality following AMI [34]; it also improves the accuracy of the prognosis when added to the GRACE score. In Non ST-elevation acute coronary syndromes (NSTEACS), this biomarker predicts in-hospital and 180 day death or heart failure [35]. Studies are summarised in Table 1[28–30, 34–39].
The TACTICS-TIMI 18 study [35] randomized 1,676 patients to conservative vs. early invasive therapy. Patients' BNP was measured within 24 hours and compared. This study found that the six-month mortality if BNP was below versus above cut-off of 80 pg/ml was 1.4% versus 8.4%, and risk of mortality or congestive heart failure below versus above cut-off was 3.6% vs. 16.3%. However, like another study [36], it did not identify patients who would benefit from early invasive revascularisation.
Mid-Regional pro-Atrial Natriuretic Peptide (MRproANP)
Like BNP, ANP has similar neurohormonal effects and has a similar secretory profile post AMI. Prior studies have attempted to accurately measure levels of ANP and N-ANP, with limited success [30, 40]. N-ANP has been demonstrated to be associated with late mortality following AMI [41]. Such early N-ANP assays were often affected by interferences and instability of analyte. Because of disappointing results, ANP was thought to provide limited prognostic information. However, the discovery of the novel MRproANP fragment [42], a substantially more stable peptide compared to N-ANP and ANP [43] due to the assay epitopes being located internally on the proANP molecule (and hence stability to exoprotease activity), has led to the finding that MRproANP is at least as good at predicting death and heart failure as NTproBNP [44]. When MRproANP levels were divided into quartiles, the top quartile was associated with a hazard ratio (HR) of 3.87 (vs. NTproBNP HR 3.25) predicting death at follow-up. Both biomarkers had similar AUC of ROC (0.83). MRproANP is emerging therefore to be an important predictor of adverse events following an AMI.
Growth Differentiation Factor-15(GDF-15)
GDF-15 is a member of the Transforming Growth Factor Beta cytokine superfamily. It is not normally expressed in the heart, but under episodes of stress (for example, ischaemia and reperfusion) its levels go up in a variety of tissues, including cardiomyocytes. It has an antihypertrophic effect, demonstrated in knockout mice which develop early cardiac hypertrophic growth following pressure overload [45]. GDF-15 provides prognostic information following an MI or ACS. One study found that increasing tertiles of GDF-15 levels in patients presenting with NSTEACS was associated with an increasing risk (1.5%, 5% and 14.1% respectively) of death at one year (AUC of ROC 0.757) [46]. Studies are summarised in Table 2[46–49].
These findings were validated in ST-elevation MI (STEMIs) [48] and prospectively validated in an unselected group of patients with AMI, where GDF-15 was found to be independently predictive of adverse events (death and heart failure) [49]. One study (FRISC-II) which randomized patients to conservative and early invasive strategy in patients with NSTEMI found GDF-15 to predict death or recurrent MI in the conservative group but not in the invasive group [47] suggesting that GDF-15 improves patients selection for early invasive strategy. It also directly compared the use of Troponin T vs the use of GDF-15 to select patients for early invasive therapy. Troponin-positive patients but with a GDF-15 level < 1,200 ng/L had no mortality benefit from early invasive therapy.
However, GDF-15 is not specific for cardiovascular disorders and has been found to be elevated in a variety of malignancies (prostate, colon, glial).
ST2
ST2 is an IL1-receptor-like protein which was found to be elevated in serum of hearts under mechanical stress [50]. ST2 predicts cardiovascular death following ACS [51]. ST2 turned out to be the target for an Interleukin called IL-33 which seems to have a cardioprotective role, and only appears when myocytes are under biomechanical stress [52]. In mouse studies, IL-33 was found to markedly antagonize angiotensin-II and phenylephrine-induced cardiomyocyte hypertrophy. It is thought that ST2/IL33 interaction also reduces atheroma burden [53]. Post AMI though, it correlates somewhat with NTproBNP [54], and both these biomarkers predict death after MI (at six months) or heart failure. Investigations into the use of IL33/ST2 pathway activation as a therapeutic target are still ongoing [55]. ST2 is also elevated in acute asthma [56] and autoimmune disease [57]. The specificity of ST2 to myocardial tissue stretch will need to be determined before it can be used at the bedside [58].
ET1/CTproET1
Endothelin-1 or the more stable C-Terminal portion of pro-Endothelin-1(CTproET1) has also been found to be predictive of death or heart failure following an AMI [59]. ET1 is a potent vasoconstrictor peptide found originally in vascular endothelial cells but has subsequently been isolated in pulmonary, renal and smooth muscle cells [60]. It activates ETA and ETB receptors; ETA receptors are located predominantly on smooth muscle tissue of blood vessels, mediating vasoconstriction and sodium retention, whereas ETB receptors are located predominantly on endothelial cells mediating nitric oxide release, natriuresis and diuresis [61]. Endothelin appears to be detrimental post-MI, extending the infarct [62] and reducing coronary blood flow [63]. It is also grossly elevated following cardiogenic shock [64]. ET-1 is very unstable and measuring its levels can be problematic due to binding with receptors and other proteins. However CTproET1 is a stable by-product of the release of the precursor which indirectly measures activity of the endothelial system. ET1 is increased in proportion to the severity of the disease post AMI [65, 66]. Likewise CTproET1 is also elevated post-MI, and levels above the median predict death or heart failure (HR 5.71, P= 0.002). This variable is independent of age, Killip class and past medical history. Plasma concentration of CTproET-1 peaks at Day 2 [59].
2) Biomarkers of neurohormonal pathway activation
Mid-Regional-pro-Adrenomedullin (MRproADM)
Adrenomedullin was first identified in human phaeochromocytoma cells [67]. It is highly expressed in endothelial cells [68]. Adrenomedullin mediates an increase in cAMP with resultant vasodilatation and hypotension [69]. Its other roles have not been well defined, but some have suggested a cardioprotective role at the time of the insult. The activity of adrenomedullin in the cardiovascular system is similar to that of BNP; that is, increase of nitric oxide production causing vasodilatation, natriuresis and diuresis [70–72]. Like BNP it is released in proportion to the severity of heart failure [73, 74], and is inversely related to the left ventricular ejection fraction (LVEF)[75, 76]. Adrenomedullin (ADM) is difficult to measure in plasma as it is partially complexed with complement [77]; in addition it is also rapidly cleared from the circulation. Indirect quantification of this peptide can be made by measuring the mid-regional fragment of the proAdrenomedullin peptide, which is more stable and secreted in equimolar concentrations as ADM. Initial studies with ADM have produced conflicting results as to the prognostic value of the peptide [40]. However, a recent study using the more stable MRproADM has shown that post AMI, increased MRproADM was associated with death, heart failure or both at one year, over and above information gained from NTproBNP alone [78]. Combining the two markers increased the AUC of the ROC from 0.77 and 0.79 to 0.84. MRproADM is very similar to NTproBNP, it is higher in females than males, and is increased with age.
Copeptin
The same study group also investigated Copeptin as a prognostic biomarker. They found that Copeptin predicted mortality or heart failure at 60 days post AMI [79]. In addition, the relationship of copeptin to LV dysfunction persisted for a prolonged period after the acute event [80]. Copeptin provided complementary prognostic information to NTproBNP, increasing the AUC of the ROC from 0.76 and 0.79 to 0.84.
Figure 1 is a summary of the Kaplan-Meier event free survival curves for some of the above mentioned biomarkers (namely NTproBNP, MRproANP, MRproADM, CTproET, copeptin, GDF-15 and ST2) in a prospectively collected cohort of AMI patients (derived from the Leicester Acute Myocardial Infarction Peptide (LAMP) study, illustrating the events associated with biomarker quartiles over about seven years. All of these biomarkers significantly predict major adverse events following AMI (Figure 2).
3) Biomarkers of plaque instability and inflammation
HsCRP (High-sensitivity C-reactive Protein)
Acute coronary syndromes are caused by vulnerable plaques. It is thought that one of the driving forces causing atheromatous plaques to rupture or erode, causing a cascade of events leading to coronary artery occlusion, is inflammation in the plaques. An elevated C-reactive protein measured in seemingly healthy adults was associated with increased cardiovascular risk [81, 82]. CRP itself mediates atherothrombosis [83–87]. This is supported by a fairly large body of evidence. Newer, higher sensitivity assays of CRP that detect lower levels of CRP (<5 mg/L) risk stratify patients into low, intermediate and high risk, with intermediate and high risk individuals benefiting from aggressive therapy [88]. While the benefits of HsCRP testing in a primary setting to screen for ischaemic heart disease is very clear, its use post-ACS or -MI is less clear. CRP is elevated post-acute coronary syndrome almost exclusively in the setting of myocardial necrosis indicating the level of myocardial inflammation.
One study found that CRP measurements (taken between 12 and 24 hours post event) predicted occurrence of heart failure (HR = 2.6, P = 0.04) and death (HR = 2.7, P = 0.02) post-MI [89]. Elevated peak CRP in the early phase of MI was related to early mechanical complications, including cardiac rupture [90], ventricular aneurysm and thrombus formation. CRP levels post-MI peak at two to four days, then take 8 to 12 weeks to subside to baseline levels. Interestingly, CRP levels post acute MI do not predict re-infarction. Additional acute coronary events can only be predicted after CRP levels have receded to baseline levels (after about 12 weeks).
One of the difficulties with CRP is that it is non-specific in the presence of other inflammatory conditions (rheumatoid arthritis, malignancy, vasculitis). A new assay for Human Pentraxin 3 is now available. Human Pentraxin 3 is an isoform which is secreted exclusively in vascular endothelium and therefore may be more specific to the vascular plaque inflammatory activity [91]. It remains to be seen if this biomarker can provide incremental information.
Myeloperoxidase (MPO)
Leucocytes play a central role in atherosclerotic plaque rupture [92–96]. Myeloperoxidase in leucocytes may activate metalloproteinases and inactivate plasminogen activator inhibitor. Leucocytes also consume nitric oxide catalytically, causing vasoconstriction and endothelial dysfunction. Myeloperoxidase has been found in atheromatous plaques [96]. Patients with chronic angina have circulating neutrophils with large quantities of MPO, which decrease substantially post-ACS [97].
One trial showed that post-acute coronary syndrome MPO levels higher than median predicted future death and MI at one year [98]. It also found that after an AMI, MPO levels peak early, then decrease over time and do not correlate with Troponin or the neutrophil count. It is not affected by fibrinolytic therapy but it is unclear if it is affected by Primary Intervention. MPO levels do not predict heart failure. MPO levels higher than the median, though, predict death or MI after one year [99, 100], whether or not NTproBNP level is below or above median. The risk is much higher if both MPO and NTproBNP levels are above the median.
Two population studies show that MPO (and CRP) in healthy individuals are both associated with future development of CAD [101, 102].
Collectively the current evidence supports the need for further studies into the actual role of MPO, and whether elevated MPO levels in the serum directly correlates with MPO released from circulating neutrophils.
Pregnancy associated Plasma Protein A (PaPPA)
PaPPA is a proatherosclerotic metalloproteinase [103] which is highly expressed in unstable plaques and their extracellular matrices [104]. It is not expressed in stable plaques. Circulating PaPPA has been found to be much higher in unstable angina and AMI, correlating also with insulin-like growth factor and CRP, but not with Troponin. Interestingly, PaPPA > 2.9 mIU/L predicts a 4.6-fold increase in risk of cardiovascular death, MI or revascularisation even without a raised Troponin [105]. Its mode of action of cleaving insulin-like growth factor 1 (IGF-1) was found to counteract endothelial dysfunction by binding to high affinity binding sites in the endothelium which then triggers nitric oxide release. PaPPA has been isolated in other damaged tissue promoting repair, giving it an inflammation repressor role [106].
Like CRP, PaPPA is expressed when there is a heavy burden of unstable atheromatous plaque, including in carotid arteries [107]. It also predicts risk of cardiovascular death [105]. Unlike CRP, it does not predict heart failure. Instead, it predicts future MI and revascularisation. Evidence for the use of this biomarker clinically remains scarce and whilst promising, more studies and standardized assays will be needed to improve its clinical utility.
4) Other Novel biomarkers
MMP9, MMP2, TIMP1
The structural integrity of myocardial Extracellular Matrix (ECM) is dependent on endogenous zinc-dependent endopeptidases known as matrix metalloproteinases (MMP). These enzymes are regulated by tissue inhibitors of metalloproteinases (TIMPs). MMPs may degrade myocardial ECM leading to the development of LV dilatation and heart failure and their inhibition in experimental models of AMI has been associated with reduced LV dilatation and wall stress. Although NTproBNP, TIMP1 and MMP 9 were associated with cardiovascular death, heart failure or both, they were not associated with re-infarction [108]. MMP2 is also elevated post MI [109] and is associated with poor prognosis [110]. MMP3 peaks at 72 hours and plateau levels are associated with increase in LV volume and a lower ejection fraction at follow up [111].
Stability of biomarkers
Many of the new biomarkers introduced have enhanced stability in vitro, so that preanalytical contributions to variation are minimised to some extent. For example, the midregional epitopes assayed in MRproANP and MRproADM are less susceptible to exoprotease action, and prohormone fragment assays tend to be more stable than assays for the actual hormone (for example, NTproBNP, CTproET1, copeptin).
Future directions
Although there are large numbers of emerging novel biomarkers, our understanding of the roles and biochemistry of these various peptides in the disease process is still fairly limited. It is difficult to draw specific conclusions from the current body of evidence regarding the mechanisms through which a biomarker could affect the prognosis. Many of the studies use death or major adverse cardiovascular events as endpoints because they are easy to measure, but either of these endpoints could be a culmination of a variety of pathophysiological processes. As such, currently available biomarkers have not been able to add much to helping us tailor our treatment (over and above Troponin). Randomised trials based on the use of biomarkers to alter therapy would be very informative. Although there is evidence that combining biomarkers may increase the accuracy of the tests, the best combinations for diagnosis or prognosis need to be defined. Some analogies can be drawn from heart failure studies; NTproBNP has been used as a biomarker for diagnosis of heart failure [112]. It does not provide clinicians with information about the aetiology nor which specific treatments to initiate. It does however guide therapy [113], as a surrogate marker of disease severity.
There is some evidence that a multi-marker strategy can improve diagnosis, risk stratification and prognostication of patients [114]. Of the above biomarkers, the ones most likely to be adopted into bedside practice in the near future are NTproBNP, MRproANP,MRproADM, copeptin and GDF-15. The theoretical benefit of a multi-marker approach would be tailored therapy as each biomarker would measure a separate disease sub-process. A multi-marker panel of tests could then be used to create an algorithm to aid clinical-decision making. We are, however, a long way from this eventual goal.
Abbreviations
- ADM:
-
Adrenomedullin
- AMI:
-
Acute Myocardial Infarction
- ANP:
-
Atrial natriuretic peptide
- AVP:
-
Arginine vasopressin
- BNP:
-
B-type natriuretic peptide
- CAD:
-
Coronary artery disease
- CTproET1:
-
C-terminal pro-endothelin 1
- CKMB:
-
Creatine kinase MB isoform
- ECG:
-
Electrocardiogram
- ECM:
-
Extracellular matrix
- ET1:
-
Endothelin-1
- ETA:
-
Endothelin receptor type A
- ETB:
-
Endothelin receptor type B
- GDF-15:
-
Growth differentiation factor 15
- GRACE:
-
Global registry of acute coronary events study
- H-FABP:
-
Heart type fatty acid binding protein
- HsCRP:
-
High sensitivity C-reactive protein
- IGF-1:
-
Insulin-like growth factor 1
- IL-33:
-
Interleukin 33
- LV:
-
Left ventricle
- LVEF:
-
Left ventricular ejection fraction
- MI:
-
Myocardial infarction
- MMP:
-
Matrix metalloproteinase
- MPO:
-
Myeloperoxidase
- MRproANP:
-
Mid regional pro-atrial natriuretic peptide
- MRproADM:
-
Mid regional proadrenomedullin
- N-ANP:
-
N-terminal pro-atrial natriuretic peptide
- NSTEACS:
-
Non-ST elevation acute coronary syndromes
- NTproBNP:
-
N-terminal pro-B type natriuretic peptide
- PaPPA:
-
Pregnancy associated Plasma Protein A
- ROC AUC:
-
Area under the receiver operating characteristic curve
- TIMI:
-
Thrombolysis in Myocardial infarction study
- TIMP:
-
Tissue inhibitors of metalloproteinases
- Trop:
-
Troponin
References
Tunstall-Pedoe H, Vanuzzo D, Hobbs M, Mahonen M, Cepaitis Z, Kuulasmaa K, Keil U: Estimation of contribution of changes in coronary care to improving survival, event rates, and coronary heart disease mortality across the WHO MONICA Project populations. Lancet. 2000, 355: 688-700. 10.1016/S0140-6736(99)11181-4.
Biological Markers - MeSH Result. [http://www.ncbi.nlm.nih.gov/mesh/68015415?ordinalpos=1&itool=EntrezSystem2.PEntrez.Mesh.Mesh_ResultsPanel.Mesh_RVDocSum]
Thygesen K, Alpert JS, White HD: Universal definition of myocardial infarction. J Am Coll Cardiol. 2007, 50: 2173-2195. 10.1016/j.jacc.2007.09.011.
Vasan RS: Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006, 113: 2335-2362. 10.1161/CIRCULATIONAHA.104.482570.
WHO Expert Committee on Diabetes Mellitus: Second report. World Health Organ Tech Rep Ser. 1980, 646: 1-80.
Alpert JS, Thygesen K, Antman E, Bassand JP: Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000, 36: 959-969. 10.1016/S0735-1097(00)00804-4.
Antman EM, Tanasijevic MJ, Thompson B, Schactman M, McCabe CH, Cannon CP, Fischer GA, Fung AY, Thompson C, Wybenga D, Braunwald E: Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. N Engl J Med. 1996, 335: 1342-1349. 10.1056/NEJM199610313351802.
Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, Bickel C, Baldus S, Warnholtz A, Frohlich M, Sinning CR, Eleftheriadis MS, Wild PS, Schnabel RB, Lubos E, Jachmann N, Genth-Zotz S, Post F, Nicaud V, Tiret L, Lackner KJ, Münzel TF, Blankenberg S: Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009, 361: 868-877. 10.1056/NEJMoa0903515.
Itoi K, Jiang YQ, Iwasaki Y, Watson SJ: Regulatory mechanisms of corticotropin-releasing hormone and vasopressin gene expression in the hypothalamus. J Neuroendocrinol. 2004, 16: 348-355. 10.1111/j.0953-8194.2004.01172.x.
Goldsmith SR: Vasopressin as Vasopressor. American Journal of Medicine. 1987, 82: 1213-1219. 10.1016/0002-9343(87)90228-2.
Fukuzawa J, Haneda T, Kikuchi K: Arginine vasopressin increases the rate of protein synthesis in isolated perfused adult rat heart via the V1 receptor. Mol Cell Biochem. 1999, 195: 93-98. 10.1023/A:1006980517557.
Gheorghiade M, Gattis WA, O'Connor CM, Adams KF, Elkayam U, Barbagelata A, Ghali JK, Benza RL, McGrew FA, Klapholz M, Ouyang J, Orlandi C, Acute and Chronic Therapeutic Impact of a Vasopressin Antagonist in Congestive Heart Failure (ACTIV in CHF) Investigators: Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: a randomized controlled trial. JAMA. 2004, 291: 1963-1971. 10.1001/jama.291.16.1963.
Abraham WT, Shamshirsaz AA, McFann K, Oren RM, Schrier RW: Aquaretic effect of lixivaptan, an oral, non-peptide, selective V2 receptor vasopressin antagonist, in New York Heart Association functional class II and III chronic heart failure patients. J Am Coll Cardiol. 2006, 47: 1615-1621. 10.1016/j.jacc.2005.11.071.
Reichlin T, Hochholzer W, Stelzig C, Laule K, Freidank H, Morgenthaler NG, Bergmann A, Potocki M, Noveanu M, Breidthardt T, Christ A, Boldanova T, Merki R, Schaub N, Bingisser R, Christ M, Mueller C: Incremental value of copeptin for rapid rule out of acute myocardial infarction. J Am Coll Cardiol. 2009, 54: 60-68. 10.1016/j.jacc.2009.01.076.
Jochberger S, Morgenthaler NG, Mayr VD, Luckner G, Wenzel V, Ulmer H, Schwarz S, Hasibeder WR, Friesenecker BE, Dunser MW: Copeptin and arginine vasopressin concentrations in critically ill patients. J Clin Endocrinol Metab. 2006, 91: 4381-4386. 10.1210/jc.2005-2830.
Glatz JF, Luiken JJ, van Nieuwenhoven FA, Van der Vusse GJ: Molecular mechanism of cellular uptake and intracellular translocation of fatty acids. Prostaglandins Leukot Essent Fatty Acids. 1997, 57: 3-9. 10.1016/S0952-3278(97)90485-3.
Glatz JF, van Bilsen M, Paulussen RJ, Veerkamp JH, van der Vusse GJ, Reneman RS: Release of fatty acid-binding protein from isolated rat heart subjected to ischemia and reperfusion or to the calcium paradox. Biochim Biophys Acta. 1988, 961: 148-152.
Chan CP, Sanderson JE, Glatz JF, Cheng WS, Hempel A, Renneberg R: A superior early myocardial infarction marker. Human heart-type fatty acid-binding protein. Z Kardiol. 2004, 93: 388-397. 10.1007/s00392-004-0080-6.
Chen L, Guo X, Yang F: Role of heart-type fatty acid binding protein in early detection of acute myocardial infarction in comparison with cTnI, CK-MB and myoglobin. J Huazhong Univ Sci Technolog Med Sci. 2004, 24: 449-451. 459
Body R: Towards evidence based emergency medicine: Best BETs from the Manchester Royal Infirmary. Bet 2. Heart Fatty Acid binding protein for rapid diagnosis of acute myocardial infarction in the emergency department. Emerg Med J. 2009, 26: 519-522. 10.1136/emj.2009.076182.
O'Donoghue M, de Lemos JA, Morrow DA, Murphy SA, Buros JL, Cannon CP, Sabatine MS: Prognostic utility of heart-type fatty acid binding protein in patients with acute coronary syndromes. Circulation. 2006, 114: 550-557. 10.1161/CIRCULATIONAHA.106.641936.
Kilcullen N, Viswanathan K, Das R, Morrell C, Farrin A, Barth JH, Hall AS: Heart-type fatty acid-binding protein predicts long-term mortality after acute coronary syndrome and identifies high-risk patients across the range of troponin values. J Am Coll Cardiol. 2007, 50: 2061-2067. 10.1016/j.jacc.2007.08.021.
de Araujo Goncalves P, Ferreira J, Aguiar C, Seabra-Gomes R: TIMI, PURSUIT, and GRACE risk scores: sustained prognostic value and interaction with revascularization in NSTE-ACS. Eur Heart J. 2005, 26: 865-872. 10.1093/eurheartj/ehi187.
Scirica BM, Cannon CP, Antman EM, Murphy SA, Morrow DA, Sabatine MS, McCabe CH, Gibson CM, Braunwald E: Validation of the thrombolysis in myocardial infarction (TIMI) risk score for unstable angina pectoris and non-ST-elevation myocardial infarction in the TIMI III registry. Am J Cardiol. 2002, 90: 303-305. 10.1016/S0002-9149(02)02468-2.
Boersma E, Pieper KS, Steyerberg EW, Wilcox RG, Chang WC, Lee KL, Akkerhuis KM, Harrington RA, Deckers JW, Armstrong PW, Lincoff AM, Califf RM, Topol EJ, Simoons ML: Predictors of outcome in patients with acute coronary syndromes without persistent ST-segment elevation. Results from an international trial of 9461 patients. The PURSUIT Investigators. Circulation. 2000, 101: 2557-2567.
Granger CB, Goldberg RJ, Dabbous O, Pieper KS, Eagle KA, Cannon CP, Van De Werf F, Avezum A, Goodman SG, Flather MD, Fox KA: Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003, 163: 2345-2353. 10.1001/archinte.163.19.2345.
Levin ER, Gardner DG, Samson WK: Natriuretic peptides. N Engl J Med. 1998, 339: 321-328. 10.1056/NEJM199807303390507.
de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E: The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001, 345: 1014-1021. 10.1056/NEJMoa011053.
Arakawa N, Nakamura M, Aoki H, Hiramori K: Plasma brain natriuretic peptide concentrations predict survival after acute myocardial infarction. J Am Coll Cardiol. 1996, 27: 1656-1661. 10.1016/0735-1097(96)00067-8.
Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K: Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation. 1996, 93: 1963-1969.
Mueller T, Gegenhuber A, Dieplinger B, Poelz W, Haltmayer M: Long-term stability of endogenous B-type natriuretic peptide (BNP) and amino terminal proBNP (NT-proBNP) in frozen plasma samples. Clin Chem Lab Med. 2004, 42: 942-944. 10.1515/CCLM.2004.153.
Luckenbill KN, Christenson RH, Jaffe AS, Mair J, Ordonez-Llanos J, Pagani F, Tate J, Wu AH, Ler R, Apple FS: Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem. 2008, 54: 619-621. 10.1373/clinchem.2007.097998.
Lorgis L, Zeller M, Dentan G, Sicard P, Buffet P, L'Huillier I, Beer JC, Vincent-Martin M, Makki H, Gambert P, Cottin Y: Prognostic value of N-terminal pro-brain natriuretic peptide in elderly people with acute myocardial infarction: prospective observational study. BMJ. 2009, 338: b1605-10.1136/bmj.b1605.
Khan SQ, Narayan H, Ng KH, Dhillon OS, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL: N-terminal pro-B-type natriuretic peptide complements the GRACE risk score in predicting early and late mortality following acute coronary syndrome. Clin Sci (Lond). 2009, 117: 31-39. 10.1042/CS20080419.
Morrow DA, de Lemos JA, Sabatine MS, Murphy SA, Demopoulos LA, DiBattiste PM, McCabe CH, Gibson CM, Cannon CP, Braunwald E: Evaluation of B-type natriuretic peptide for risk assessment in unstable angina/non-ST-elevation myocardial infarction: B-type natriuretic peptide and prognosis in TACTICS-TIMI 18. J Am Coll Cardiol. 2003, 41: 1264-1272. 10.1016/S0735-1097(03)00168-2.
Jernberg T, Lindahl B, Siegbahn A, Andren B, Frostfeldt G, Lagerqvist B, Stridsberg M, Venge P, Wallentin L: N-terminal pro-brain natriuretic peptide in relation to inflammation, myocardial necrosis, and the effect of an invasive strategy in unstable coronary artery disease. J Am Coll Cardiol. 2003, 42: 1909-1916. 10.1016/j.jacc.2003.07.015.
Omland T, Persson A, Ng L, O'Brien R, Karlsson T, Herlitz J, Hartford M, Caidahl K: N-terminal pro-B-type natriuretic peptide and long-term mortality in acute coronary syndromes. Circulation. 2002, 106: 2913-2918. 10.1161/01.CIR.0000041661.63285.AE.
Bazzino O, Fuselli JJ, Botto F, Perez De Arenaza D, Bahit C, Dadone J: Relative value of N-terminal probrain natriuretic peptide, TIMI risk score, ACC/AHA prognostic classification and other risk markers in patients with non-ST-elevation acute coronary syndromes. Eur Heart J. 2004, 25: 859-866. 10.1016/j.ehj.2004.03.004.
Khan SQ, Quinn P, Davies JE, Ng LL: N-terminal pro-B-type natriuretic peptide is better than TIMI risk score at predicting death after acute myocardial infarction. Heart. 2008, 94: 40-43. 10.1136/hrt.2006.108985.
Richards AM, Nicholls MG, Yandle TG, Frampton C, Espiner EA, Turner JG, Buttimore RC, Lainchbury JG, Elliott JM, Ikram H, Crozier IG, Smyth DW: Plasma N-terminal pro-brain natriuretic peptide and adrenomedullin: new neurohormonal predictors of left ventricular function and prognosis after myocardial infarction. Circulation. 1998, 97: 1921-1929.
Squire IB, O'Brien RJ, Demme B, Davies JE, Ng LL: N-terminal pro-atrial natriuretic peptide (N-ANP) and N-terminal pro-B-type natriuretic peptide (N-BNP) in the prediction of death and heart failure in unselected patients following acute myocardial infarction. Clin Sci (Lond). 2004, 107: 309-316. 10.1042/CS20040087.
Morgenthaler NG, Struck J, Thomas B, Bergmann A: Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004, 50: 234-236. 10.1373/clinchem.2003.021204.
Ala-Kopsala M, Magga J, Peuhkurinen K, Leipala J, Ruskoaho H, Leppaluoto J, Vuolteenaho O: Molecular heterogeneity has a major impact on the measurement of circulating N-terminal fragments of A- and B-type natriuretic peptides. Clin Chem. 2004, 50: 1576-1588. 10.1373/clinchem.2004.032490.
Khan SQ, Dhillon O, Kelly D, Squire IB, Struck J, Quinn P, Morgenthaler NG, Bergmann A, Davies JE, Ng LL: Plasma N-terminal B-Type natriuretic peptide as an indicator of long-term survival after acute myocardial infarction: comparison with plasma midregional pro-atrial natriuretic peptide: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2008, 51: 1857-1864. 10.1016/j.jacc.2008.01.041.
Wang J, Xu N, Feng X, Hou N, Zhang J, Cheng X, Chen Y, Zhang Y, Yang X: Targeted disruption of Smad4 in cardiomyocytes results in cardiac hypertrophy and heart failure. Circ Res. 2005, 97: 821-828. 10.1161/01.RES.0000185833.42544.06.
Wollert KC, Kempf T, Peter T, Olofsson S, James S, Johnston N, Lindahl B, Horn-Wichmann R, Brabant G, Simoons ML, Armstrong PW, Califf RM, Drexler H, Wallentin L: Prognostic value of growth-differentiation factor-15 in patients with non-ST-elevation acute coronary syndrome. Circulation. 2007, 115: 962-971. 10.1161/CIRCULATIONAHA.106.650846.
Wollert KC, Kempf T, Lagerqvist B, Lindahl B, Olofsson S, Allhoff T, Peter T, Siegbahn A, Venge P, Drexler H, Wallentin L: Growth differentiation factor 15 for risk stratification and selection of an invasive treatment strategy in non ST-elevation acute coronary syndrome. Circulation. 2007, 116: 1540-1548. 10.1161/CIRCULATIONAHA.107.697714.
Kempf T, Bjorklund E, Olofsson S, Lindahl B, Allhoff T, Peter T, Tongers J, Wollert KC, Wallentin L: Growth-differentiation factor-15 improves risk stratification in ST-segment elevation myocardial infarction. Eur Heart J. 2007, 28: 2858-2865. 10.1093/eurheartj/ehm465.
Khan SQ, Ng K, Dhillon O, Kelly D, Quinn P, Squire IB, Davies JE, Ng LL: Growth differentiation factor-15 as a prognostic marker in patients with acute myocardial infarction. Eur Heart J. 2009, 30: 1057-1065. 10.1093/eurheartj/ehn600.
Weinberg EO, Shimpo M, De Keulenaer GW, MacGillivray C, Tominaga S, Solomon SD, Rouleau JL, Lee RT: Expression and regulation of ST2, an interleukin-1 receptor family member, in cardiomyocytes and myocardial infarction. Circulation. 2002, 106: 2961-2966. 10.1161/01.CIR.0000038705.69871.D9.
Shimpo M, Morrow DA, Weinberg EO, Sabatine MS, Murphy SA, Antman EM, Lee RT: Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004, 109: 2186-2190. 10.1161/01.CIR.0000127958.21003.5A.
Sanada S, Hakuno D, Higgins LJ, Schreiter ER, McKenzie AN, Lee RT: IL-33 and ST2 comprise a critical biomechanically induced and cardioprotective signaling system. J Clin Invest. 2007, 117: 1538-1549. 10.1172/JCI30634.
Zhang SH, Reddick RL, Piedrahita JA, Maeda N: Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science. 1992, 258: 468-471. 10.1126/science.1411543.
Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, Rifai N, Cannon CP, Gerszten RE, Lee RT: Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008, 117: 1936-1944. 10.1161/CIRCULATIONAHA.107.728022.
Seki K, Sanada S, Kudinova AY, Steinhauser ML, Handa V, Gannon J, Lee RT: Interleukin-33 prevents apoptosis and improves survival after experimental myocardial infarction through ST2 signaling. Circ Heart Fail. 2009, 2: 684-691. 10.1161/CIRCHEARTFAILURE.109.873240.
Oshikawa K, Kuroiwa K, Tago K, Iwahana H, Yanagisawa K, Ohno S, Tominaga SI, Sugiyama Y: Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001, 164: 277-281.
Kuroiwa K, Arai T, Okazaki H, Minota S, Tominaga S: Identification of human ST2 protein in the sera of patients with autoimmune diseases. Biochem Biophys Res Commun. 2001, 284: 1104-1108. 10.1006/bbrc.2001.5090.
Diez J: Serum soluble ST2 as a biochemical marker of acute heart failure: future areas of research. J Am Coll Cardiol. 2008, 52: 1466-1467. 10.1016/j.jacc.2008.07.045.
Khan SQ, Dhillon O, Struck J, Quinn P, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL: C-terminal pro-endothelin-1 offers additional prognostic information in patients after acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) Study. Am Heart J. 2007, 154: 736-742. 10.1016/j.ahj.2007.06.016.
Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988, 332: 411-415. 10.1038/332411a0.
Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, Masaki T: The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci USA. 1989, 86: 2863-2867. 10.1073/pnas.86.8.2863.
Haynes WG, Strachan FE, Webb DJ: Endothelin ETA and ETB receptors cause vasoconstriction of human resistance and capacitance vessels in vivo. Circulation. 1995, 92: 357-363.
Kurihara H, Yamaoki K, Nagai R, Yoshizumi M, Takaku F, Satoh H, Inui J, Yazaki Y: Endothelin: a potent vasoconstrictor associated with coronary vasospasm. Life Sci. 1989, 44: 1937-1943. 10.1016/0024-3205(89)90406-2.
Cernacek P, Stewart DJ: Immunoreactive endothelin in human plasma: marked elevations in patients in cardiogenic shock. Biochem Biophys Res Commun. 1989, 161: 562-567. 10.1016/0006-291X(89)92636-3.
Stewart DJ, Kubac G, Costello KB, Cernacek P: Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol. 1991, 18: 38-43. 10.1016/S0735-1097(10)80214-1.
Tomoda H: Plasma endothelin-1 in acute myocardial infarction with heart failure. Am Heart J. 1993, 125: 667-672. 10.1016/0002-8703(93)90155-3.
Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, Eto T: Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993, 192: 553-560. 10.1006/bbrc.1993.1451.
Sugo S, Minamino N, Kangawa K, Miyamoto K, Kitamura K, Sakata J, Eto T, Matsuo H: Endothelial cells actively synthesize and secrete adrenomedullin. Biochem Biophys Res Commun. 1994, 201: 1160-1166. 10.1006/bbrc.1994.1827.
Takahashi K, Satoh F, Hara E, Sone M, Murakami O, Kayama T, Yoshimoto T, Shibahara S: Production and secretion of adrenomedullin from glial cell tumors and its effects on cAMP production. Peptides. 1997, 18: 1117-1124. 10.1016/S0196-9781(97)00186-1.
Lainchbury JG, Cooper GJ, Coy DH, Jiang NY, Lewis LK, Yandle TG, Richards AM, Nicholls MG: Adrenomedullin: a hypotensive hormone in man. Clin Sci (Lond). 1997, 92: 467-472.
Parkes DG, May CN: ACTH-suppressive and vasodilator actions of adrenomedullin in conscious sheep. J Neuroendocrinol. 1995, 7: 923-929. 10.1111/j.1365-2826.1995.tb00737.x.
Vari RC, Adkins SD, Samson WK: Renal effects of adrenomedullin in the rat. Proc Soc Exp Biol Med. 1996, 211: 178-183.
Jougasaki M, Rodeheffer RJ, Redfield MM, Yamamoto K, Wei CM, McKinley LJ, Burnett JC: Cardiac secretion of adrenomedullin in human heart failure. J Clin Invest. 1996, 97: 2370-2376. 10.1172/JCI118680.
Kato J, Kobayashi K, Etoh T, Tanaka M, Kitamura K, Imamura T, Koiwaya Y, Kangawa K, Eto T: Plasma adrenomedullin concentration in patients with heart failure. J Clin Endocrinol Metab. 1996, 81: 180-183. 10.1210/jc.81.1.180.
Jougasaki M, Wei CM, McKinley LJ, Burnett JC: Elevation of circulating and ventricular adrenomedullin in human congestive heart failure. Circulation. 1995, 92: 286-289.
Nakamura M, Yoshida H, Makita S, Arakawa N, Niinuma H, Hiramori K: Potent and long-lasting vasodilatory effects of adrenomedullin in humans. Comparisons between normal subjects and patients with chronic heart failure. Circulation. 1997, 95: 1214-1221.
Parkes DG, May CN: Direct cardiac and vascular actions of adrenomedullin in conscious sheep. Br J Pharmacol. 1997, 120: 1179-1185. 10.1038/sj.bjp.0701034.
Khan SQ, O'Brien RJ, Struck J, Quinn P, Morgenthaler N, Squire I, Davies J, Bergmann A, Ng LL: Prognostic value of midregional pro-adrenomedullin in patients with acute myocardial infarction: the LAMP (Leicester Acute Myocardial Infarction Peptide) study. J Am Coll Cardiol. 2007, 49: 1525-1532. 10.1016/j.jacc.2006.12.038.
Khan SQ, Dhillon OS, O'Brien RJ, Struck J, Quinn PA, Morgenthaler NG, Squire IB, Davies JE, Bergmann A, Ng LL: C-terminal provasopressin (copeptin) as a novel and prognostic marker in acute myocardial infarction: Leicester Acute Myocardial Infarction Peptide (LAMP) study. Circulation. 2007, 115: 2103-2110. 10.1161/CIRCULATIONAHA.106.685503.
Kelly D, Squire IB, Khan SQ, Quinn P, Struck J, Morgenthaler NG, Davies JE, Ng LL: C-terminal provasopressin (copeptin) is associated with left ventricular dysfunction, remodeling, and clinical heart failure in survivors of myocardial infarction. J Card Fail. 2008, 14: 739-745. 10.1016/j.cardfail.2008.07.231.
Ridker PM: Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003, 107: 363-369. 10.1161/01.CIR.0000053730.47739.3C.
Shishehbor MH, Bhatt DL, Topol EJ: Using C-reactive protein to assess cardiovascular disease risk. Cleve Clin J Med. 2003, 70: 634-640. 10.3949/ccjm.70.7.634.
Pasceri V, Willerson JT, Yeh ET: Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000, 102: 2165-2168.
Nakajima T, Schulte S, Warrington KJ, Kopecky SL, Frye RL, Goronzy JJ, Weyand CM: T-cell-mediated lysis of endothelial cells in acute coronary syndromes. Circulation. 2002, 105: 570-575. 10.1161/hc0502.103348.
Nakagomi A, Freedman SB, Geczy CL: Interferon-gamma and lipopolysaccharide potentiate monocyte tissue factor induction by C-reactive protein: relationship with age, sex, and hormone replacement treatment. Circulation. 2000, 101: 1785-1791.
Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, Dhillon B, Weisel RD, Li RK, Mickle DA, Stewart DJ: A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002, 106: 913-919. 10.1161/01.CIR.0000029802.88087.5E.
Devaraj S, Xu DY, Jialal I: C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003, 107: 398-404. 10.1161/01.CIR.0000052617.91920.FD.
Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E: C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005, 352: 20-28. 10.1056/NEJMoa042378.
Suleiman M, Khatib R, Agmon Y, Mahamid R, Boulos M, Kapeliovich M, Levy Y, Beyar R, Markiewicz W, Hammerman H, Aronson D: Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J Am Coll Cardiol. 2006, 47: 962-968. 10.1016/j.jacc.2005.10.055.
Anzai T, Yoshikawa T, Shiraki H, Asakura Y, Akaishi M, Mitamura H, Ogawa S: C-reactive protein as a predictor of infarct expansion and cardiac rupture after a first Q-wave acute myocardial infarction. Circulation. 1997, 96: 778-784.
Matsui S, Ishii J, Kitagawa F, Kuno A, Hattori K, Ishikawa M, Okumura M, Kan S, Nakano T, Naruse H, Tanaka I, Nomura M, Hishida H, Ozaki Y: Pentraxin 3 in unstable angina and non-ST-segment elevation myocardial infarction. Atherosclerosis. 2010, 210: 220-5. 10.1016/j.atherosclerosis.2009.10.033.
de Servi S, Mazzone A, Ricevuti G, Mazzucchelli I, Fossati G, Angoli L, Valentini P, Boschetti E, Specchia G: Expression of neutrophil and monocyte CD11B/CD18 adhesion molecules at different sites of the coronary tree in unstable angina pectoris. Am J Cardiol. 1996, 78: 564-568. 10.1016/S0002-9149(96)00367-0.
Dinerman JL, Mehta JL, Saldeen TG, Emerson S, Wallin R, Davda R, Davidson A: Increased neutrophil elastase release in unstable angina pectoris and acute myocardial infarction. J Am Coll Cardiol. 1990, 15: 1559-1563. 10.1016/0735-1097(90)92826-N.
Buffon A, Biasucci LM, Liuzzo G, D'Onofrio G, Crea F, Maseri A: Widespread coronary inflammation in unstable angina. N Engl J Med. 2002, 347: 5-12. 10.1056/NEJMoa012295.
Davies MJ, Thomas A: Thrombosis and acute coronary-artery lesions in sudden cardiac ischemic death. N Engl J Med. 1984, 310: 1137-1140.
Naruko T, Ueda M, Haze K, van der Wal AC, van der Loos CM, Itoh A, Komatsu R, Ikura Y, Ogami M, Shimada Y, Ehara S, Yoshiyama M, Takeuchi K, Yoshikawa J, Becker AE: Neutrophil infiltration of culprit lesions in acute coronary syndromes. Circulation. 2002, 106: 2894-2900. 10.1161/01.CIR.0000042674.89762.20.
Biasucci LM, D'Onofrio G, Liuzzo G, Zini G, Monaco C, Caligiuri G, Tommasi M, Rebuzzi AG, Maseri A: Intracellular neutrophil myeloperoxidase is reduced in unstable angina and acute myocardial infarction, but its reduction is not related to ischemia. J Am Coll Cardiol. 1996, 27: 611-616. 10.1016/0735-1097(95)00524-2.
Baldus S, Heeschen C, Meinertz T, Zeiher AM, Eiserich JP, Munzel T, Simoons ML, Hamm CW: Myeloperoxidase serum levels predict risk in patients with acute coronary syndromes. Circulation. 2003, 108: 1440-1445. 10.1161/01.CIR.0000090690.67322.51.
Khan SQ, Kelly D, Quinn P, Davies JE, Ng LL: Myeloperoxidase aids prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart. 2007, 93: 826-831. 10.1136/hrt.2006.091041.
Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, Winterbourn CC: Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007, 49: 1993-2000. 10.1016/j.jacc.2007.02.040.
Zhang R, Brennan ML, Fu X, Aviles RJ, Pearce GL, Penn MS, Topol EJ, Sprecher DL, Hazen SL: Association between myeloperoxidase levels and risk of coronary artery disease. JAMA. 2001, 286: 2136-2142. 10.1001/jama.286.17.2136.
Meuwese MC, Stroes ES, Hazen SL, van Miert JN, Kuivenhoven JA, Schaub RG, Wareham NJ, Luben R, Kastelein JJ, Khaw KT, Boekholdt SM: Serum myeloperoxidase levels are associated with the future risk of coronary artery disease in apparently healthy individuals: the EPIC-Norfolk Prospective Population Study. J Am Coll Cardiol. 2007, 50: 159-165. 10.1016/j.jacc.2007.03.033.
Laursen LS, Overgaard MT, Nielsen CG, Boldt HB, Hopmann KH, Conover CA, Sottrup-Jensen L, Giudice LC, Oxvig C: Substrate specificity of the metalloproteinase pregnancy-associated plasma protein-A (PAPP-A) assessed by mutagenesis and analysis of synthetic peptides: substrate residues distant from the scissile bond are critical for proteolysis. Biochem J. 2002, 367: 31-40. 10.1042/BJ20020831.
Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR, Virmani R, Oxvig C, Schwartz RS: Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001, 345: 1022-1029. 10.1056/NEJMoa003147.
Lund J, Qin QP, Ilva T, Pettersson K, Voipio-Pulkki LM, Porela P, Pulkki K: Circulating pregnancy-associated plasma protein a predicts outcome in patients with acute coronary syndrome but no troponin I elevation. Circulation. 2003, 108: 1924-1926. 10.1161/01.CIR.0000096054.18485.07.
Bayes-Genis A, Conover CA, Schwartz RS: The insulin-like growth factor axis: A review of atherosclerosis and restenosis. Circ Res. 2000, 86: 125-130.
Beaudeux JL, Burc L, Imbert-Bismut F, Giral P, Bernard M, Bruckert E, Chapman MJ: Serum plasma pregnancy-associated protein A: a potential marker of echogenic carotid atherosclerotic plaques in asymptomatic hyperlipidemic subjects at high cardiovascular risk. Arterioscler Thromb Vasc Biol. 2003, 23: e7-10. 10.1161/01.ATV.0000047448.76485.B8.
Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB: Plasma tissue inhibitor of metalloproteinase-1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008.
Kelly D, Cockerill G, Ng LL, Thompson M, Khan S, Samani NJ, Squire IB: Plasma matrix metalloproteinase-9 and left ventricular remodelling after acute myocardial infarction in man: a prospective cohort study. Eur Heart J. 2007, 28: 711-718. 10.1093/eurheartj/ehm003.
Dhillon OS, Khan SQ, Narayan HK, Ng KH, Mohammed N, Quinn PA, Squire IB, Davies JE, Ng LL: Matrix metalloproteinase-2 predicts mortality in patients with acute coronary syndrome. Clin Sci (Lond). 2009, 118: 249-57. 10.1042/CS20090226.
Kelly D, Khan S, Cockerill G, Ng LL, Thompson M, Samani NJ, Squire IB: Circulating stromelysin-1 (MMP-3): a novel predictor of LV dysfunction, remodelling and all-cause mortality after acute myocardial infarction. Eur J Heart Fail. 2008, 10: 133-139. 10.1016/j.ejheart.2007.12.009.
Steinhart B, Thorpe KE, Bayoumi AM, Moe G, Januzzi JL, Mazer CD: Improving the diagnosis of acute heart failure using a validated prediction model. J Am Coll Cardiol. 2009, 54: 1515-1521. 10.1016/j.jacc.2009.05.065.
Berger R, Moertl D, Peter S, Ahmadi R, Huelsmann M, Yamuti S, Wagner B, Pacher R: N-terminal pro-b-type natriuretic peptide-guided, intensive patient management in addition to multidisciplinary care in chronic heart failure: a 3-arm, prospective, randomized pilot study. J Am Coll Cardiol. 55: 645-653. 10.1016/j.jacc.2009.08.078.
Sabatine MS, Morrow DA, de Lemos JA, Gibson CM, Murphy SA, Rifai N, McCabe C, Antman EM, Cannon CP, Braunwald E: Multimarker approach to risk stratification in non-ST elevation acute coronary syndromes: simultaneous assessment of troponin I, C-reactive protein, and B-type natriuretic peptide. Circulation. 2002, 105: 1760-1763. 10.1161/01.CIR.0000015464.18023.0A.
Pre-publication history
The pre-publication history for this paper can be accessed here:http://www.biomedcentral.com/1741-7015/8/34/prepub
Acknowledgements
Prof. Ng is supported by the Leicester National Institute for Health Research Cardiovascular Biomedical Research Unit.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
Prof. Leong Ng has submitted patents on behalf of the University of Leicester on some of the biomarkers in this review, and has acted as a consultant to and received grants in aid from BRAHMS AG and Unipath PLC in the past. Dr. Daniel Chan has no competing interests to declare.
Authors' contributions
LN recruited the patients in the reported studies, performed the analyses and drafted the manuscript. DC participated in the analyses and drafting of the manuscript and figures.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under license to BioMed Central Ltd. This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Chan, D., Ng, L.L. Biomarkers in acute myocardial infarction. BMC Med 8, 34 (2010). https://doi.org/10.1186/1741-7015-8-34
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1741-7015-8-34