Abstract
Background
Indoxyl sulfate (IS) is produced by action of the intestinal flora on tryptophan in protein diet, and it is normally excreted by the kidney. IS is a protein-bound uremic toxin, and it is difficult to be removed by conventional hemodialysis (HD) methods; so, it accumulates in HD patients and may contribute to major cardiovascular morbidity and mortality.
Aim
To study the effect of dietary synbiotic (prebiotic and probiotic) supplementation on IS level in prevalent HD patients.
Patients and methods
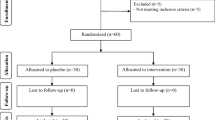
This single-blind, placebo-controlled trial was conducted on 80 prevalent HD patients (between January 2017 and March 2017) in Ain Shams University Hospital. Patients were divided into 2 groups: group 1 was given synbiotic (SYN) and group 2 was given placebo for 6 weeks. Blood levels of IS, CRP, creatinine, blood urea nitrogen (BUN), sodium, potassium, calcium, and phosphorus were measured at baseline and after 6 weeks.
Results
There was a significant reduction in serum IS level in groups 1 and 2 in comparison to their baselines (P value = 0.000 and 0.019 respectively); however, the change in IS level in group 1 after SYN supplementation (64% with IR 72.38–33.33) was more than that shown in group 2 (did not receive SYN) (18.47% with IR 26.75–26.75) with a highly significant P value, 0.000. Also, there were significant reductions in the levels of creatinine, BUN, phosphorus (P values < 0.001), and CRP (P values 0.002) in group 1 respectively with no similar changes noticed in group 2.
Conclusion
SYN supplementation in HD patients can reduce serum levels of IS and other uremic toxins like BUN and creatinine. Also, it may help to reduce serum phosphorus and CRP levels.
Similar content being viewed by others
Background
Indoxyl sulfate is a major protein-bound uremic toxin produced by the action of gut microbiota on tryptophan amino acid in dietary protein. Gut microbiota converts tryptophan to indole; then, it is conjugated with sulfate in the liver to form IS [1]. Serum IS levels are 10–20 times higher in HD patients compared to normal people due to its poor dialysis clearance as it is a protein-bound uremic toxins [2].
Elevated IS levels in chronic kidney disease (CKD) are associated with hazardous effects like progression of CKD [3] cardiovascular disease (CVD) and all-cause mortality in HD patients [4].
The gut microbiome consisted of more than 100 trillion bacterial cells which has a beneficial effect to their hosts [5]. In CKD patients, there is an alteration of the normal gut microbiome (dysbiosis) with an increase in aerobic and anaerobic bacteria and decreases in Prevotellaceae and Lactobacillaceae families [6]. Dysbiosis is associated with abnormal production of uremic toxins which can be corrected by supplementation of specific types of probiotics and prebiotics [7].
The prebiotics are non-digestible dietary fibers that escape digestion in intestine and promote growth of commensal bacteria in the colon [8], and oligosaccharides are considered to be the main source of prebiotics that have been identified [9]. Both Lactobacillus and Bifidobacteria can ferment prebiotics resulting in the production of the beneficial short chain fatty acids [10]. Prebiotic supplementation alone can decrease gut derived bacterial toxins as IS and p-cresyl sulfate (PCS) and can decrease the progression of CKD [11], as prebiotics can enhance the development and proliferation of beneficial gut microbiota either naturally present in the gut or supplied externally as probiotic [12].
Probiotic had been defined as “living microorganisms which when given in adequate amounts results in beneficial outcomes to the host” [13], and a meta-analysis by Jia et al. show that probiotic supplementation can decrease PCS in CKD patients [14].
Methods
Aim of the study
The aim of the study was to investigate the effects of SYN (co-administration of pre- and probiotics) as a potential treatment for reducing IS production in HD patients.
Study design
This study was a single-center, single-blind, placebo-controlled, randomized trial. Participants have undergone allocation into either SYN supplements (group 1) or placebo (group 2) for 6 weeks. Laboratory profile was performed prior to and after the end of the 6 weeks to assess the effect of the given SYN.
Ethical considerations
Ethical approval has been granted through the Human Research Ethics Committee, Ain Shams University, Egypt, on 17 March 2016; reference number of approval: 69/2016.
Target population
The trial recruited 80 prevalent HD patients undergoing regular HD at the Ain Shams University HD Unit for at least 6 months before the start of the study, aged ≥ 18 years, and able to provide informed consent.
Exclusion criteria
-
1.
Receiving/or have received radiation to the bowel or large bowel resection
-
2.
Consumed prebiotics or probiotics or antibiotic therapy within 1 month of study commencement
-
3.
Previously diagnosed with irritable bowel syndrome, Crohn’s disease, or ulcerative colitis
-
4.
Severely malnourished or severely immune-compromised patients
-
5.
Patients who had acute gastroenteritis during the study or in the last month before the study
-
6.
Antibiotic therapy in the last month before the study or during the study
-
7.
Any patient who has severe alteration of bowel habits during the study, e.g., diarrhea
Dietary counseling
All participants have undergone dietary counseling to establish baseline dietary fiber intakes. Patients were encouraged to maintain stable protein and fiber intakes, with specific attention to maintaining the same sources of these nutrients (i.e., animal vs. plant protein and soluble vs. insoluble fiber) throughout the study.
Prebiotic and probiotic intervention
For probiotic, Lactobacillus and Bifidobacteria genera were selected as they have a very limited ability to produce IS, and the probiotics in this study contained at least 2 billion colony-forming units (CFU) in the form of yoghurt prepared by the Dairy and Food Microbiology lab of the National Research Center, Egypt, including 5 bacterial strains:
-
1.
Lactobacillus rhamnosus
-
2.
Lactobacillus acidophilus
-
3.
Lactobacillus casei
-
4.
Bifidobacterium brevis
-
5.
Bifidobacterium longum
The prebiotic component included lactulose syrup by the dose of 15 g/day in the early morning period.
Supplement dosing and duration
For the study group, a daily dose of 15 g of prebiotics (lactulose) mixed with 10 g of the probiotic (each gram containing 1 × 109 colony-forming units (CFU)) was given to the study group for 6 weeks. The dosage of prebiotics and probiotics is based on previous successful trials [15].
Both prebiotic and probiotic were mixed together in one can, and patients were given 7 cans weekly at the beginning of the dialysis week for each patient and were instructed to take only one can daily, and this was repeated for 6 weeks at the beginning of the dialysis week.
For the placebo group, they received only ordinary yogurt cans prepared by the Dairy and Food Microbiology lab of the National Research Center, and the starters for this yoghurt were Streptococcus thermophilus and Lactobacillus bulgaricus which are not probiotics (without the 5 strains of bacteria and was not mixed with any prebiotic).
All patients were asked to keep the synbiotic (study group) or the ordinary yogurt (placebo group) in the refrigerator.
Randomization
Simple randomization method using tables of random numbers was used to allocate patients who are meeting the inclusion and exclusion criteria in either study group or placebo group.
Primary outcome
Serum IS
Venous blood was collected following an overnight fast at 2 time points throughout the study (i.e., before initiation of SYN and 6 weeks later) before the dialysis session. Samples were centrifuged at 3000 rpm for 10 min before being stored at − 80 °C. Samples were sent for analysis of serum concentration of IS using ELISA.
Participants were provided with a standard evening meal preceding their overnight fast before each blood collection.
Secondary outcomes
The following parameters were assessed at baseline and after 6 weeks before the dialysis session: blood hemoglobin (Hb), serum creatinine, BUN, sodium, potassium, calcium, phosphorus, and CRP.
Safety and adherence
No serious adverse events were reported to the ethics committee, whether deemed to be supplement related or not. All participants showed adherence to supplements.
Statistical analysis
Data recorded were analyzed by the Statistical Package for Social Sciences, version 20.0 (SPSS Inc., Chicago, IL, USA). Normally distributed quantitative values were presented as mean ± standard deviation (SD), while non-normally distributed quantitative values were expressed as median and interquartile range (IR). Qualitative values were presented as frequency and percentage. When comparing between two means, independent samples t-test of significance was used. Independent Kruskal-Wallis test and independent sample Mann-Whitney test were used for comparing non-parametric quantitative variables between groups. Chi-square (χ2) test of significance was used to compare proportions between qualitative parameters. The confidence interval was set to 95%, and the margin of error accepted was set to 5%. So, the P value was considered significant if P value ≤ 0.05 and highly significant with P value ≤ 0.001, while P value > 0.05 was considered insignificant.
Results
This study was conducted on 80 prevalent HD patients recruited from the Ain Shams University Hospital which were divided into 2 groups: group 1 received SYN for 6 weeks, and group 2 received placebo.
Both groups were comparable as regards age, sex, duration of HD, and etiology of renal failure with non-significant P value 0.11, 1.0, 0.06, and 0.70, respectively.
At baseline, there was no significant difference between both groups as regards IS level and other laboratory tests (Table 1).
After 6 weeks, there was a highly significant reduction of IS in the SYN group and significant reduction in the placebo group (Table 2), P value 0.00 and 0.019 respectively, but the change in serum IS was more evident in the synbiotic group compared to the placebo group (Table 3) with a highly significant P value of 0.00.
There was a significant reduction in serum levels of creatinine, BUN, phosphorus, and CRP after 6 weeks of SYN supplementation, P value < 0.001, < 0.001, < 0.001, and 0.002 respectively, with no similar changes in the other group (Table 2).
Discussion
In CKD patients, there is alteration in the normal intestinal flora (dysbiosis) with predominance of harmful bacteria which results in increased production of gut uremic toxins with increased permeability of intestinal barrier to these products as endotoxins, amines, and phenols [16].
The aim of this study was to establish the effect of SYN administration on serum IS levels in HD patients.
Participants were provided with either SYN therapy or placebo for 6 weeks. All patients completed the study, and no drop-out was observed, and follow up samples were obtained after the 6-week trial period.
The primary outcome was serum IS level; the secondary outcomes included Hb, creatinine, BUN, sodium, potassium, calcium, phosphorus, and CRP levels.
IS which is a gut derived, protein-bound uremic toxin and difficult to be cleared with HD has been associated with increased CVD and total mortality in HD patients [17].
Although there was significant reduction in serum IS level in groups 1 and 2 in comparison to their baselines (P value = 0.000 and 0.019 respectively), the change in IS level in group 1 (64% with IR 72.38–33.33) was more than that shown in group 2 (18.47% with IR 26.75–26.75) with a highly significant P value 0.000 which is attributed to the supplementation of SYN to group 1.
In the study group, reduction in IS level could be explained by the effect of synbiotic given to the patient, while in the placebo group, reduction of IS may be secondary to the usage of yoghurt as placebo which may contain some beneficial bacteria like Lactobacillus, and this shows that using even ordinary yoghurt may decrease IS level in HD patients.
In agreement with our study is the study done by Takayama et al. in which oral administration of SYN for 5 weeks (Bifidobacterium longum with 0.11 g of oligosaccharides) resulted in the decrease in serum levels of IS in HD patients [18]. Also, a study by Taki et al. showed significant reduction in IS level in HD patients after oral administration of Bifidobacterium longum in a gastro-resistant seamless capsule [19]. Similar results were established by Ogawa et al. where significant reduction in serum IS level were noticed in HD patients after oral administration of synbiotic (Bifidobacterium longum together with oligosaccharides) with no similar finding in the control group [20].
On the other hand, the double-blind placebo-controlled crossover SYNERGY study (Synbiotics Easing Renal Failure by Improving Gut Microbiology) conducted on 37 CKD pre-dialysis patients shown that there was no significant difference in serum IS levels after SYN supplementation for 6 weeks between both groups. However, this non-agreement with our study may be attributed to the difference in the study population (CKD patients were included, not hemodialysis patients as in our study) [21].
Some of the secondary outcomes varied significantly before and after the trial (creatinine, BUN, phosphorus, CRP), while others did not show such variation (potassium, sodium, calcium).
In this study, there was significant reduction in serum creatinine level in synbiotic group (P < 0.001) with no similar changes in group 2 (P value = 0.084). Also, there was a significant reduction of BUN in group 1 (P value < 0.001) after 6 weeks of SYN supplementation with non-significant finding in group 2 (P value 0.137) which goes with the Alatriste et al. study in which thirty CKD patients received a probiotic (Lactobacillus casei Shirota: (LcS)), and there was a more than 10% decrease in the serum urea concentrations in patients with stage 3 and 4 CKD who received the prebiotic [22]. Another study by Ranganathan et al. showed a significant reduction of BUN in CKD patients after probiotic supplementation for 6 months (P < 0.05) [23].
Unlike a randomized double-blind controlled trial performed by Guida et al. with 30 patients, CKD stages 3 to 4 were randomized to receive either SYN or placebo for 4 weeks, and no significant reduction in serum creatinine or BUN occurred during the study in both groups [20].
As regards serum phosphorus level, there was a highly significant reduction in phosphorus level in group 1 after 6 weeks of SYN supplementation (P value < 0.001) and insignificant difference in group 2 (P value 0.468).
The reduction of serum phosphorus level may be due to the effect of Bifidobacteria and Lactobacillus bacteria in the probiotic which are potent phosphorus-accumulating organism. However, this finding needs further work up to be confirmed [24].
Ogawa et al. showed similar finding as SYN supplementation for 4 weeks in HD patients had led to a significant reduction in serum phosphorus level with no significant changes in the control group [20].
CRP was included in our study, as a marker of chronic inflammatory state, and there was no significant difference between both groups at baseline (P value = 0.31), but after 6 weeks of the trial, there was a significant reduction in CRP level in group 1 compared to baseline with a highly significant P value (P value = 0.002), and the difference in group 2 was comparable with a non-significant P value (P = 0.157).
The reduction of CRP level may be due to the highly significant reduction of serum IS in the SYN group as IS is a pre-inflammatory uremic toxin [25].
This agrees with Natarajan et al. in which there was significant reduction in CRP level after 2 months of probiotic supplementation [26]. Another study by Viramontes-Hörner showed that after 2 months of SYN supplementation, there was significant reduction in CRP level compared to nonsignificant changes in the control group [27].
As regards the mean hemoglobin levels (Hb), there was no statistically significant difference between both groups before the intervention. The change in Hb before and after the trial was found to be non-statistically significant in both groups (P values 0.249 and 0.735 respectively).
The same applies to the results of potassium (K), sodium (Na), and calcium (Ca).
Conclusions
In prevalent HD patients, SYN supplementation can decrease the level of serum IS which is a protein-bound gut-derived toxin, and it is difficult to be removed by different dialysis methods. SYN supplementation to HD patients also reduced serum creatinine, BUN, phosphorus, and CRP levels; however, this needs more studies to be confirmed.
Limitation of the study
The limitations in this study included adherence of outpatient dialysis participants to same dietary habits during the study period which is doubtful despite dietary counseling and using ordinary yoghurt as placebo which may contain probiotics like Lactobacillus bacteria.
Recommendation for future studies
Future studies should have larger sample size and longer follow-up duration and should focus on the benefits of reducing IS level like CVD risk factor biomarkers, e.g., lipid profile, and it is better to use capsules containing either the synbiotic or placebo instead of ordinary yoghurt which may contain probiotics. Also, further studies are needed to show the effect of SYN supplementation on levels of creatinine, BUN, phosphorus, potassium, and its ability to delay dialysis in chronic kidney patients.
Availability of data and materials
The datasets used and/or analyzed in this manuscript are available from the corresponding author upon reasonable request.
Abbreviations
- BUN:
-
Blood urea nitrogen
- CRP:
-
C reactive protein
- CVD:
-
Cardiovascular disease
- IR:
-
Interquartile range
- IS:
-
Indoxyl sulfate
- HD:
-
Hemodialysis
- Hb:
-
Hemoglobin
- PCS:
-
p-Cresyl sulfate
- SD:
-
Standard deviation
- SYN:
-
Synbiotic
References
Hyšpler R, Tichá A, Šafránek R, Moučka P, Nývltová Z, Štochlová K, Dusilová-Sulková S, Zadák Z (2018) Indoxyl sulfate elimination in renal replacement therapy: influence of citrate- versus acetate-buffering component during bicarbonate dialysis. Dis Markers 2018:3985861. https://doi.org/10.1155/2018/3985861
Yoshikawa D, Ishii H, Suzuki S, Takeshita K, Kumagai S, Hayashi M, Niwa T, Izawa H, Murohara T (2014) Plasma indoxyl sulfate and estimated glomerular filtration rate. Circ J 78(10):2477–2482. https://doi.org/10.1253/circj.cj-14-0401
Tumur Z, Shimizu H, Enomoto A, Miyazaki H, Niwa T (2010) Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am J Nephrol 31(5):435–441. https://doi.org/10.1159/000299798
Abe T, Onoda M, Matsuura T, Sugimura J, Obara W, Sasaki N, Kato T, Tatsumi K, Maruyama T (2021) Evaluation of a new measurement method of indoxyl sulfate in hemodialysis patients. Ther Apher Dial 25(1):44–49. https://doi.org/10.1111/1744-9987.13500
Chen YY, Chen DQ, Chen L, Liu JR, Vaziri ND, Guo Y, Zhao YY (2019) Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med 17(1):5. https://doi.org/10.1186/s12967-018-1756-4
Ramezani A, Raj DS (2014) The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol 25(4):657–670. https://doi.org/10.1681/ASN.2013080905
Meijers B, Evenepoel P, Anders HJ (2019) Intestinal microbiome and fitness in kidney disease. Nat Rev Nephrol 15(9):531–545. https://doi.org/10.1038/s41581-019-0172-1
Hutkins RW, Krumbeck JA, Bindels LB, Cani PD, Fahey G Jr, Goh YJ, Hamaker B, Martens EC, Mills DA, Rastal RA, Vaughan E, Sanders ME (2016) Prebiotics: why definitions matter. Curr Opin Biotechnol 37:1–7. https://doi.org/10.1016/j.copbio.2015.09.001
Pokusaeva K, Fitzgerald GF, van Sinderen D (2011) Carbohydrate metabolism in Bifidobacteria. Genes Nutr 6(3):285–306. https://doi.org/10.1007/s12263-010-0206-6
Slavin J (2013) Fiber and prebiotics: mechanisms and health benefits. Nutrients 5(4):1417–1435. https://doi.org/10.3390/nu5041417
Li L, Xiong Q, Zhao J, Lin X, He S, Wu N, Yao Y, Liang W, Zuo X, Ying C (2020) Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: a randomized crossover study. Am J Clin Nutr 111(5):1087–1099. https://doi.org/10.1093/ajcn/nqz337
Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, Scott K, Stanton C, Swanson KS, Cani PD, Verbeke K, Reid G (2017) Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol 14(8):491–502. https://doi.org/10.1038/nrgastro.2017.75
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S, Calder PC, Sanders ME (2014) Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514. https://doi.org/10.1038/nrgastro.2014.66
Jia L, Jia Q, Yang J, Jia R, Zhang H (2018) Efficacy of probiotics supplementation on chronic kidney disease: a systematic review and meta-analysis. Kidney Blood Press Res 43(5):1623–1635. https://doi.org/10.1159/000494677
Rossi M, Klein K, Johnson DW, Campbell KL (2012) Pre-, pro-, and synbiotics: do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol 2012:673631. https://doi.org/10.1155/2012/673631
CigarranGuldris S, González Parra E, Cases Amenós A (2017) Gut microbiota in chronic kidney disease. Microbiota intestinal en la enfermedad renal crónica. Nefrologia: publicacionoficial de la Sociedad Espanola. Nefrologia 37(1):9–19. https://doi.org/10.1016/j.nefro.2016.05.008
Lin CJ, Wu V, Wu PC, Wu CJ (2015) Meta-Analysis of the associations of p-cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One 10(7):e0132589. https://doi.org/10.1371/journal.pone.0132589
Takyama F, Taki K, Niwa T (2003) Bifidobacterium in gastro-resistant seamless capsule reduces serum levels of indoxyl sulfate in patients on hemodialysis. Am J Kidney Dis 41(3 Suppl 1):S142–S145. https://doi.org/10.1053/ajkd.2003.50104
Taki K, Takayama F, Niwa T (2005) Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Renal Nutr 15(1):77–80. https://doi.org/10.1053/j.jrn.2004.09.028
Guida B, Germanò R, Trio R, Russo D, Memoli B, Grumetto L, Barbato F, Cataldi M (2014) Effect of short-term synbiotic treatment on plasma p-cresol levels in patients with chronic renal failure: a randomized clinical trial. Nutr Metab Cardiovasc Dis 24(9):1043–1049. https://doi.org/10.1016/j.numecd.2014.04.007
Rossi M, Johnson DW, Morrison M, Pascoe EM, Coombes JS, Forbes JM, Szeto CC, McWhinney BC, Ungerer JP, Campbell KL (2016) Synbiotics easing renal failure by improving gut microbiology (SYNERGY): a randomized trial. Clin J Am Soc Nephrol 11(2):223–231. https://doi.org/10.2215/CJN.05240515
Miranda Alatriste PV, Urbina Arronte R, Gómez Espinosa CO, Espinosa Cuevas M (2014) Effect of probiotics on human blood urea levels in patients with chronic renal failure. Nutr Hosp 29(3):582–590. https://doi.org/10.3305/nh.2014.29.3.7179
Ranganathan N, Ranganathan P, Friedman EA, Joseph A, Delano B, Goldfarb DS, Tam P, Rao AV, Anteyi E, Musso CG (2010) Pilot study of probiotic dietary supplementation for promoting healthy kidney function in patients with chronic kidney disease. Adv Ther 27(9):634–647. https://doi.org/10.1007/s12325-010-0059-9
Anand A, Sato M, Aoyagi H (2019) Screening of phosphate-accumulating probiotics for potential use in chronic kidney disorder. Food Sci Technol Res 25(1):89–96. https://doi.org/10.3136/fstr.25.89
Thongprayoon C, Kaewput W, Hatch ST, Bathini T, Sharma K, Wijarnpreecha K, Ungprasert P, D'Costa M, Mao MA, Cheungpasitporn W (2019) Effects of probiotics on inflammation and uremic toxins among patients on dialysis: a systematic review and meta-analysis. Dig Dis Sci 64(2):469–479. https://doi.org/10.1007/s10620-018-5243-9
Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, Mallappallil MC, Norin AJ, Friedman EA, Saggi SJ (2014) Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int 2014:568571. https://doi.org/10.1155/2014/568571
Viramontes-Hörner D, Márquez-Sandoval F, Martín-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodríguez A, Armendáriz-Borunda J, García-Bejarano H, Renoirte-López K, García-García G (2015) Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Renal Nutr 25(3):284–291. https://doi.org/10.1053/j.jrn.2014.09.008
Acknowledgements
Not applicable.
Funding
This study had no funding from any resource.
Author information
Authors and Affiliations
Contributions
MS: main idea, revised the manuscript; YS: edited and reviewed the manuscript, data analysis; ES: study design, follow-up of patients, and reviewed the manuscript; NS: study design, preparation of the symbiotic, data analysis; NN: collected clinical data, prepared the manuscript; MA: study design, literature research, data analysis, edited the manuscript, submitted the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was approved by the Research Ethics Committee of the Faculty of Medicine at Ain Shams University in Egypt on 17 March 2016, reference number of approval: 69/2016.
All patients included in this study gave written informed consent to participate in this research. If the patient was less than 16 years old or unconscious at the time of the study, written informed consent for their participation was given by their parent or legal guardian.
This paper has not been published or submitted for publication elsewhere.
Consent for publication
All patients included in this research gave written informed consent to publish the data contained within this study. If the patient was less than 16 years old, deceased, or unconscious when consent for publication was requested, written informed consent for the publication of this data was given by their parent or legal guardian.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hassan, M.S., Ahmed, Y.S., Sarhaan, E.I. et al. Effect of dietary synbiotic supplementation on serum indoxyl sulfate in prevalent hemodialysis patients. Egypt J Intern Med 34, 1 (2022). https://doi.org/10.1186/s43162-021-00096-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43162-021-00096-3