Abstract
Background
Diagnosis and management of pediatric cancer develop a major life event that might impact psychosocial functioning and quality of life (QoL) even long after the initial therapy has been completed. Treatment outcomes have been measured in terms of survival time, but they also significantly impact survivors’ quality of life. The pediatric differentiated thyroid carcinoma survivors’ QoL has rarely been evaluated. This study aims to translate and validate the Indonesian version of the ThYCA-QoL questionnaire.
Results
The median age of 105 eligible survivors was 20.88 years old. Eighty percent of the survivors were female, were married or in a relationship (86.7%), and had paid jobs or were full-time students (71.7%). The median follow-up time was 64.82 months. Cronbach-α co-efficient was > 0.70 for psychological, concentration, throat, and mouth problems. For sympathetic, neuromuscular, voice, and sensory, the scores were < 0.70, where a multi-trait scaling analysis showed that all item correlations were > 0.40. Validity was assessed using the Pearson correlation coefficient for y-QoL with r > 0.60 and p < 0.01.
Conclusions
The Indonesian ThYCA-QoL questionnaire is a reliable and valid tool to evaluate pediatric patients’ QoL after treatment. This simple assessment tool can be used to evaluate and manage pediatric thyroid cancer patients’ HRQoL.
Similar content being viewed by others
Introduction
Pediatric cancer diagnosis and treatment constitute a significant life event that can have an impact on psychosocial functioning and quality of life (QoL), even years after the first course of treatment is over. Despite its rarity, pediatric thyroid carcinoma (PTC) has an incredibly good prognosis [1]. Its rising incidence requires a greater focus on this disease’s long-term effects and treatment [2,3,4]. After the first therapy, which frequently entails a complete thyroidectomy followed by the infusion of radioactive iodine (131-I), a lifetime of follow-up is then started [5]. One-third of PTC survivors in Indonesia also experience post-operative problems, including permanent or transient hypoparathyroidism, which need further medical care [6]. Hypothyroidism as well as hypoparathyroidism have been explained as complications that would affect the QoL of PTC survivors [7]. Although there have been studies evaluating QoL in adolescent and adult survivors of thyroid cancer, there is a dearth of studies evaluating QoL in the pediatric population. When compared to healthy controls, QoL in adolescent and adult thyroid cancer survivors reported increased levels of fatigue, anxiety, and depression as well as lower health-related quality of life (HRQoL) [7, 8].
Thyroid cancer-specific symptoms may appear during therapy and follow-up in PTC survivors. Most long-term adult survivors with a median follow-up of 9.6 years had clinical manifestations unique to their condition. Compared to older survivors, individuals in their early twenties reported having more complaints related to thyroid cancer [7, 8].
In pediatric survivors, complaints specifically related to thyroid cancer have not yet been assessed. QoL in PTC survivors is independent of thyrotropin (TSH) level since the main management of PTC includes total thyroidectomy and TSH suppression [7, 8].
Varying outcomes have been seen in the numerous research that have investigated HRQol following thyroidectomy. It is interesting to note that while some thyroid cancer survivors have decreased HRQoL, others exhibit similar HRQoL to the general population [7]. The absence of HRQoL questionnaires created specifically for thyroid cancer patients is one of the limitations of such studies. Most of them use generic tools that were neither developed nor evaluated for this cancer group. English thyroid-specific tool THYCA-QoL was created in 2013 by Husson et al. [9, 10]. This tool has already been tried out in French [11], the Netherlands [12], the UK [13], and the Persian [14]. A validated Indonesian version of a quality-of-life survey is not yet available.
This result can be used to evaluate and manage PTC’s HRQoL to enhance the HRQoL of this demographic.
Materials and methods
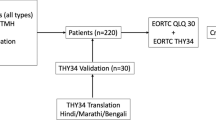
This study is a cross-sectional one and the participants were selected at the Thyroid Cancer Center Outpatient Clinic of Oncology, Head and Neck Surgery Department at the Hasan Sadikin General Hospital, Faculty of Medicine, Padjadjaran University, Bandung, West Java, Indonesia, from 2021 to 2022. Included subjects who had previously been diagnosed with thyroid cancer before the age of 18-year-old. A follow-up of less than 5 years, a diagnosis made after the age of eighteen, or a PTC diagnosis as a second malignant neoplasm (SMN) are all exclusion factors.
At the outpatient clinic, everyone was required to independently fill out the written questionnaires. Medical records were used to gather data on the demographics as well as clinical characteristics (such as diagnostic cancer stage, type of pathology, and type of therapy received) of each subject. The Helsinki Declaration was followed in the conduct of this investigation, and it was approved by the Institutional Ethical Review Boards at Hasan Sadikin General Hospital. Each participant signed an informed consent in a written form before enrolling in this study.
The survey items were based on the original version of the ThyCA-QoL, which consists of 24 items of thyroid cancer-specific questionnaire developed based on the EORTC guidelines [8, 9]. First, it was translated from English to Indonesian by a qualified professional translator fluent in both languages. A group of specialists examined the translated questionnaire after which small adjustments were made to adapt and improve the translation. Afterward, the translated Indonesian questionnaire was translated back to English to verify the questionnaire’s original meaning, it was translated by a second certified professional translator. Finally, the Indonesian ThyCA-QoL questionnaire was evaluated on respondents.
The responder is then asked (verbally by the interviewer or via an open-ended question) to explain what they believed each questionnaire item and their related response signified. Using this method, the researcher may make sure that the translated items have the same meaning as the original items and that the translated questionnaire is clear. We used respondent-to-item ratios of 1:3 since the pediatric thyroid cancer patient population was quite small.
The questionnaire consists of seven multi-item scales namely neuromuscular, voice, concentration, sympathetic, throat and mouth, psychological and sensory problems, and six single items relating to problems with scars, feeling chilly, tingling at hands/feet, gained weight, headaches, and interest in sex. Additionally, the questionnaire has a deadline: each item has a week to be completed, except for sexuality, which was four weeks. The Likert scale, which ranges from 1 (not at all) to 4 (very much), was used to evaluate the intensity of the complaints. More complaints correspond to symptoms with higher scores.
The Indonesian version of the EORTC QLQ-C30 (version 3.0) questionnaire includes thirty items and measures five functional scales (physical, role, emotional, cognitive, and social functioning), global health status (GHS), financial difficulties, and eight symptom scales (fatigue, nausea, and vomiting, pain, dyspnea, insomnia, appetite loss, constipation, diarrhea). The evaluation time frame is the previous week of the questionnaire reception. All items are scored on a Likert four-point response ranging from 1, “not at all” to 4, “very much”, except for the global health status scale including 2 items scored on a seven-point modified linear analog scale ranging from 1, “very poor” to 7, “excellent” [15]. The EORTC scoring manual procedure provides a score from 0 to 100 for each dimension.
Primary and secondary outcome
The primary outcome of this study is a translated and validated Indonesian version of the thyroid cancer-specific quality of life (ThyCA-QoL) questionnaire. The secondary outcome is to obtain data on demographic and clinical characteristics and ThyCA-QoL questionnaire results of pediatric thyroid cancer patients at the Hasan Sadikin General Hospital from 2021 to 2022.
Inclusion and exclusion criteria
Included subjects who had previously been diagnosed with thyroid cancer before the age of 18-year-old who visited the outpatient clinic of the thyroid cancer center, Oncology, Head and Neck Surgery Department at the Hasan Sadikin General Hospital/Faculty of Medicine, Padjadjaran University, Bandung, West Java, Indonesia, from 2021 to 2022. Exclusion criteria include a follow-up < 5 years, diagnosis after the age of 18 years old, or having been diagnosed with PTC as a second malignant neoplasm (SMN).
Statistical analysis
Descriptive methods were used for the purpose of evaluating demographic and clinical data. The items in the questionnaire were each translated and evaluated for validity. The validity of the questionnaire was assessed between the EORTC QLQ-C30 and the Indonesian ThyCA-QoL questionnaire. Criterion validity was also assessed using a correlation matrix between Indonesian ThyCA-QoL scale summary scores and the QLQ-C30 scale scores. The correlation between the Indonesian ThyCA-QoL and the QLQ-C30 scores assessing the same HRQoL domain was expected to be greater or equal than 0.4, in absolute value. Conversely, the correlation between each ThyCA-QoL scale score and other scales of the QLQ-C30 assessing other HRQoL domains was expected to be lower than 0.4. By identifying the Cronbach coefficient, which gauges internal consistency, the reliability was evaluated.
Ethics
This study received approval from Hasan Sadikin General Hospital’s Ethics Committee number 0516070802 on June 15th, 2022. Then, written informed consent forms (Supplementary file 1) were obtained from each patient. The 1964 Helsinki Declaration and any updates thereto, as well as any relevant ethical standards, were followed in all procedures conducted in research involving human participants. These practices also adhere to institutional, governmental, and/or ethical research committee rules.
Results
Demographic and clinical characteristics of pediatric thyroid cancer
Based on the inclusion and exclusion criteria, 105 survivors were qualified for this long-term QoL study and agreed to participate. The demographic data along with clinical features are presented in Table 1. At the time of examination, the survivors’ ages ranged from 17 to 25, with a 20.88-year-old median age. Most survivors (80%) were female, married or in a relationship (86.7%), employed full-time, or enrolled in school full-time (71,7%). Table 1 explains the tumor, therapy, and follow-up characteristics of the survivors. The median follow-up time was 64.82 months (ranging from 60.00 to 85.00 months after diagnosis). Each survivor had a complete thyroidectomy, with subsequent administration of radioiodine (131-I) and levothyroxine suppression.
Reliability and validity
The multi-trait scaling analysis was used to evaluate the reliability of the multi-item scales by estimating the Cronbach-α co-efficient. The degree of convergence between different items used to represent the idea is shown by the Cronbach coefficient. In this study, we found the Cronbach-α co-efficient > 0.70 for psychological, concentration, throat, and mouth problems. Even in the other items such as sympathetic, neuromuscular, voice, and sensory, the scores were < 0.70, where a multi-trait scaling analysis showed that all item correlations were > 0.40. Validity was assessed using the Pearson correlation coefficient for ThyCA-QoL with r > 0.60 and p < 0.01 as shown in Tables 2 and 3. The 24-item questionnaire that resulted was given the name Indonesian ThyCA-QoL questionnaire. (Supplementary file 2)
Overall correlation coefficients between each ThyCA-QoL scale and its matching QLQ-C30 scale were all less than 0.4, indicating acceptable criterion validity (Table 3). The ThyCA-QoL and QLQ-C30 scales may or may not correlate since a high score on the functional scale of the QLQ-C30 suggests a high degree of functioning, whereas a high score on the symptomatic scale shows an elevated level of health-related quality of life impairment. All ThyCA-QoL scales and all subscales of the EORTC QLQ-C30 exhibited statistically significant relationships, except for one item (sexual interest). When compared to the “emotional functioning” and “cognitive functioning” measures on the EORTC QLQ C-30, the “psychological” and “concentration” scales on the ThyCA-QoL questionnaire showed strong correlations (correlation coefficients: r > 0.60; p < 0.01). “Neuromuscular,” “concentration,” and “psychological”, “physical functioning,” “role functioning,” “fatigue,” “nausea/vomiting,” “pain,” “dyspnea,” and “sleep/insomnia” scores were all moderately linked with the EORTC QLC C-30s.
Discussion
Our research demonstrated that the four multi-item scales’ internal consistency was sufficient for reliability. Internal consistency is important to measure how closely the items in the questionnaire subscale are correlated. We found low-reliability scores for neuromuscular, voice, and sensory, which reflect limited variability of those items. In terms of the items’ convergence and discriminant validity, our study confirmed using multi-trait scaling analysis that there were no scaling errors. Hence, the Indonesian ThyCA-QoL questionnaire can be used as a tool to assess the QoL of pediatric thyroid cancer survivors in Indonesia.
Even though this study supports the reliability and validity of the Indonesian version of the ThyCA-QoL questionnaire, several limitations ought to be noted. Despite the sub-optimum results of the reliability test on the neuromuscular, voice, and sensory subscales, those values are quite close to the cutoff point of < 0.70. However, we also found in some of the references that the value of Cronbach-α co-efficient > 0.60 is also acceptable. Moreover, the population of this study may not represent all thyroid cancer survivors as the majority of participants were diagnosed with stage I. Nevertheless, it is our understanding that the population of this study can represent the majority of well-differentiated pediatric thyroid cancer patients which in the AJCC 8th TNM System is classified mostly at stage I. Further study to reduce bias in the study sample is needed to affirm the reliability and validity of the Indonesian ThyCa-QoL questionnaire.
The research conducted in Iran was superior to this one. According to research conducted in Iran, most scales met the criteria for reliability analysis, with the exception of the weariness, pain, and nausea and vomiting scales (Cronbach coefficient values of 0.65, 0.69, and 0.66, respectively) [14].
All multi-item subscales had item own subscale correlations above 0.40, which demonstrated convergent validity. With the exception of item 4 on the physical functioning scale, all analyses successfully tested item discriminant validity. The results of the group-based analysis reveal substantial variations in QLQ-C30 functioning and symptom scores, with higher-grade patients having worse outcomes (p < 0.05).
Our research could be related to the French study. The internal structure of that study was comparable to the original questionnaire, with Cronbach coefficients ranging from 0.53 to 0.88 for the voice aspect [11].
From our perspective limitations of this study include a limited number of subjects, which may affect the result of this study.
At present, there is a lack of guidance on how to address measurement issues that could affect the construct validity of replication research. The results suggest that it is typical for the scales used in initial studies to lack evidence of validity. There are four measurement issues that replication researchers are likely to encounter: the absence of essential measurement data, the absence of evidence of validity, measurement discrepancies, and the lack of translation.
Furthermore, every region has certain characteristics of the language used by the native residents, which is also the limitation of this study as it was only done in one region: Bandung, West Java, Indonesia. Studies in other regions of Indonesia would add insights and benefits to this translated questionnaire.
Conclusion
The Indonesian ThyCA-QoL questionnaire proved to be a reliable and valid tool to assess pediatric patients’ quality of life after treatment. This tool is a simple assessment that can easily be used by physicians to appropriately evaluate and fulfill pediatric thyroid cancer patients’ HRQoL unmet needs.
Availability of data and materials
The data presented in this study are openly available in Zenodo at https://doi.org/10.5281/zenodo.7793583
Abbreviations
- QoL:
-
Quality of life
- PTC:
-
Pediatric thyroid carcinoma
- HRQoL:
-
Health-related quality of life
- TSH:
-
Thyrotropin
- ThYCA-QoL:
-
Thyroid cancer-specific quality of life
- SMN:
-
Second malignant neoplasm
References
Nies M, Dekker BL, Sulkers E, Huizinga GA, Klein Hesselink MS, Maurice-Stam H, et al. Psychosocial development in survivors of childhood differentiated thyroid carcinoma: a cross-sectional study. Eur J Endocrinol. 2018;178(3):215–23. https://doi.org/10.1530/EJE-17-0741.
Goldfarb M, Casillas J. Thyroid cancer–specific quality of life and health-related quality of life in young adult thyroid cancer survivors. Thyroid. 2016;26(7):923–32. https://doi.org/10.1089/thy.2015.0589.
Husson O, Nieuwlaat W-A, Oranje WA, Haak HR, van de Poll-Franse LV, Mols F. Fatigue among short- and long-term thyroid cancer survivors: results from the population-based PROFILES registry. Thyroid. 2013;23(10):1247–55. https://doi.org/10.1089/thy.2013.0015.
Aschebrook-Kilfoy B, James B, Nagar S, Kaplan S, Seng V, Ahsan H, et al. Risk factors for decreased quality of life in thyroid cancer survivors: Initial findings from the north American thyroid cancer survivorship study. Thyroid. 2015;25(12):1313–21. https://doi.org/10.1089/thy.2015.0098.
Vergamini LB, Frazier AL, Abrantes FL, Ribeiro KB, Rodriguez-Galindo C. Increase in the incidence of differentiated thyroid carcinoma in children, adolescents, and young adults: a population-based study. J Pediatr. 2014;164(6):1481–5. https://doi.org/10.1016/j.jpeds.2014.01.059.
Azhar Y, Achmad D, Lukman K, Hilmanto D. Pediatric Differentiated Thyroid Carcinoma Risk Factor for Analysis for Disease Free Survival. Indian J Med Pediatric Oncol. 2018;39(02):153–8. https://doi.org/10.4103/ijmpo.ijmpo_70_17.
de Oliveira Chachamovitz DS, dos Santos VP, Nogueira Cordeiro MF, de Castro CLN, Vaisman M, dos Santos TP, et al. Quality of life, muscle strength, and fatigue perception in patients on suppressive therapy with levothyroxine for differentiated thyroid carcinoma. Am J Clin Oncol. 2013;36(4):354–61. https://doi.org/10.1097/COC.0b013e318248d864.
Gamper E-M, Wintner LM, Rodrigues M, Buxbaum S, Nilica B, Singer S, et al. Persistent quality of life impairments in differentiated thyroid cancer patients: results from a monitoring programme. Eur J Nucl Med Mol Imaging. 2015;42(8):1179–88. https://doi.org/10.1007/s00259-015-3022-9.
Husson O, Haak HR, Mols F, Nieuwenhuijzen GA, Nieuwlaat W-A, Reemst PH, et al. Development of a disease-specific health-related quality of life questionnaire (THYCA-QoL) for thyroid cancer survivors. Acta Oncol. 2013;52(2):447–54. https://doi.org/10.3109/0284186X.2012.718445.
Husson O, Haak HR, Buffart LM, Nieuwlaat W-A, Oranje WA, Mols F, et al. Health-related quality of life and disease specific symptoms in long-term thyroid cancer survivors: a study from the population-based PROFILES registry. Acta Oncol. 2013;52(2):249–58. https://doi.org/10.3109/0284186X.2012.741326.
Scheller B, Santini J, Culie D, et al. Validation of the French version of the THYCA-QoL questionnaire for the health-related quality of life in thyroid cancer patients. Research Square. 2022; https://doi.org/10.21203/rs.3.rs-2237513/v1.
van Velsen EFS, Massolt ET, Heersema H, Kam BLR, van Ginhoven TM, Visser WE, et al. Longitudinal analysis of quality of life in patients treated for differentiated thyroid cancer. Eur J Endocrinol. 2019;181(6):671–9. https://doi.org/10.1530/EJE-19-0550.
McIntyre C, Jacques T, Palazzo F, Farnell K, Tolley N. Quality of life in differentiated thyroid cancer. Int J Surg. 2018;50:133–6. https://doi.org/10.1016/j.ijsu.2017.12.014.
Sanjari M, Esmaeeli M, Ahmadipour H. Thyroid Cancer-Specific Health-Related Quality of Life Questionnaire: Psychometric Properties of the Persian Version. Int J Prev Med. 2022;5(13):53. https://doi.org/10.4103/ijpvm.IJPVM_77_20.
Azhar Y, Achmad D, Rudiman R, Candrawinata VS. Validation of Thyroid Cancer-Specific Quality of Life Questionnaire in Indonesian as A Tool to Assess Quality of life among pediatric Thyroid Cancer patients. Zenodo. 2023; https://doi.org/10.5281/zenodo.7793583.
Acknowledgements
The authors would like to thank Nurvita Trianasari S. Si, MStat, staff of Business Management and Informatics, Faculty of Economy, Telkom University, Bandung, for providing statistical analysis.
Underlying data
All data underlying the results are available as part of the article and no additional source data are required.
Extended data
Zenodo: Validation of Thyroid Cancer-Specific Quality of Life Questionnaire in Indonesian as A Tool to Assess Quality of Life Among Pediatric Thyroid Cancer Patients, https://doi.org/10.5281/zenodo.7793583
This project contains the following extended data:
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Conception and design of the study, acquisition of data, analysis and interpretation of data, drafting the manuscript, revising the manuscript critically for important intellectual content, approval of the version of the manuscript to be published: Yohana Azhar, Dimyati Achmad, Reno Rudiman, Valeska Siulinda Candrawinata. All authors have read and agreed to the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Hasan Sadikin General Hospital (number 0516070802 on June 15th, 2022).
Consent for publication
Each participant signed written informed consent before enrolling in this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Azhar, Y., Achmad, D., Rudiman, R. et al. Validation of the Indonesian version of the thyroid cancer-specific quality of life as a tool to assess the quality of life among pediatric thyroid cancer patients. Ann Pediatr Surg 19, 44 (2023). https://doi.org/10.1186/s43159-023-00278-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43159-023-00278-4