Abstract
Background
Telmisartan, a potent angiotensin II type-1 receptor blocker as well as partial PPAR–gamma agonist, has emerged as a versatile therapeutic agent with diverse pharmacological actions beyond its primary indication for essential hypertension. This review explores the complex mechanisms of action of telmisartan and clarifies its effectiveness in an inflammation, cancer, metabolic, and CNS disorders.
Main body
Telmisartan inhibits many biochemical processes involved in the control of the cardiovascular system, such as vascular smooth muscle contraction, aldosterone production, and sympathetic tone modulation, by specifically targeting the angiotensin II type-1 receptor. Its distinct partial agonist action toward peroxisome proliferator-activated receptor gamma also imparts anti-inflammatory, antiproliferative, and antioxidant activities, making it a viable treatment for various diabetic patients who have atherosclerosis and myocardial infarction.
Conclusion
Telmisartan's diverse pharmacological actions, encompassing anti-inflammatory, neuroprotective, nephroprotective, anticancer, and anti-anxiety properties, position it as a promising treatment option for a broad spectrum of medical conditions.
Similar content being viewed by others
Background
Telmisartan, a nonpeptide antagonist directed at the angiotensin II type-1 (AT1) receptor, has surfaced as a versatile therapeutic option, demonstrating notable effectiveness and longevity in managing essential hypertension [1]. Its unique mode of action entails the targeted and enduring inhibition of the AT1 receptor's reactivity to angiotensin II, while preserving the functionality of other receptor systems implicated in cardiovascular control [2]. This selectivity not only forms the basis of its powerful antihypertensive action but also lays the groundwork for its diverse pharmacological characteristics [3].
In addition to its role as an angiotensin receptor blocker (ARB), telmisartan exhibits partial agonist activity toward the peroxisome proliferator-activated receptor gamma (PPAR-γ) [4]. This unique dual activity confers a myriad of additional benefits, including antioxidative, anti-inflammatory, and antiproliferative effects, particularly in conditions such as atherosclerosis [5]. Such pleiotropic effects position telmisartan as a promising therapeutic option for diabetic patients with myocardial infarction, offering a comprehensive approach to cardiovascular management beyond blood pressure control [6].
Additionally, telmisartan's capacity to mitigate fibrotic alterations linked to diabetes-related cardiac fibrosis by activating endogenous PPARδ [4] and enhancing STAT3 [7, 8] expression highlights its potential in addressing diverse pathological pathways [9]. The activation of PPARδ not only maintains a balance in metabolism and inflammation [10] but also contributes to cardiac protection, thereby broadening telmisartan's therapeutic scope beyond hypertension [11].
Furthermore, telmisartan's influence extends beyond cardiovascular well-being, encompassing a range of advantageous effects on metabolic syndrome, neuroprotection [12], and nephroprotection. Its anti-inflammatory [13], antioxidative [14], antineoplastic [15], and nephroprotective [16] attributes further emphasize its potential as a valuable therapeutic agent across various diseases and disorders [17].
In this extensive review, we explore the multifaceted mechanisms of action of telmisartan, shedding light on its diverse pleiotropic effects and underscoring its potential as a fundamental component in the management of cardiovascular conditions and beyond. In this study, we examined over 323 research papers, with 199 of them being thoroughly included in our analysis. By meticulously analyzing the available literature, our objective is to offer valuable insights into the wide-ranging therapeutic capabilities of telmisartan, facilitating its exploration across diverse clinical scenarios.
Methodology
The scope of the review article on telmisartan will encompass its pharmacological properties, therapeutic indications, and emerging research trends for treatment of other than cardiovascular disorders. The objectives include evaluating the efficacy of telmisartan in the management of various CNS, cancer, diabetes, and its complications, exploring its potential mechanisms of action, and discussing its comparative effectiveness. For comprehensive coverage of literature on telmisartan, relevant databases such as PubMed, Web of Science, Scopus, and Google Scholar were utilized. A comprehensive search strategy was developed using keywords such as “telmisartan plus neurological disorders,” “telmisartan + metabolic syndrome,” “telmisartan and Diabetes,” Cancer and telmisartan, etc., and search filters will be employed to refine the search and capture relevant literature on telmisartan's pharmacology. Search results were screened based on relevance to the review's objectives. Titles and abstracts were reviewed to assess their suitability for inclusion in the review. Studies focusing on telmisartan's pharmacological properties were prioritized for further evaluation. Inclusion criteria include studies published in English, observational studies, meta-analyses investigating telmisartan's efficacy for other than cardiovascular disorders in preclinical studies. Exclusion criteria comprise clinical trials, case reports, and studies with insufficient data or irrelevant outcomes. During the study, we examined over 323 research papers, with 199 of them being thoroughly included in our analysis. Full-text articles of potentially relevant studies were retrieved through institutional subscriptions, interlibrary access, or direct contact with authors. Selected studies were critically evaluated for their quality and validity. Factors including study design, sample size, methodology, and risk of bias were considered in the assessment.
Relevant data from selected studies were extracted using a standardized data extraction form. Key information including study characteristics, type of animal models, intervention details, outcomes, and conclusions was systematically recorded for creation of review. Findings from the selected studies were synthesized to address the review's research questions and objectives. Synthesized information was organized and presented in a tabular form for different disorders and diseases.
The review process involved continuous literation, with ongoing refinement of search strategies and inclusion/exclusion criteria to ensure comprehensive coverage of literature and alignment with the review's objectives. Additionally, updates were made to incorporate new findings and emerging research trends on telmisartan's therapeutic applications beyond cardiovascular disorders. (Methodology section was included to provide clarity and reproducibility).
Neuroinflammation and telmisartan
Angiotensin receptor II induces inflammation and oxidative stress via ROS production through the NADPH oxidase complex [18, 19]. Toll-like receptors (TLRs) and PPARγ receptors in the CNS play vital roles in neuroinflammation [20]. Excessive RAS activation, especially through AT1 receptors, contributes to brain inflammation [21, 22]. IL-1β, generated by microglia, has diverse roles and is implicated in neurodegenerative disorders [23, 24].
Telmisartan induces PPARγ activation independently of AT1R, preventing NFκB-mediated inflammatory cascades [13]. PPARγ activation leads to a dose-dependent increase in SARM expression, a negative regulator of pro-inflammatory cytokines [25].
Telmisartan reduces LPS-induced inflammation in neuronal cells via SARM activation through TLR4 signaling independently of AT1R [13]. TLR4 activation triggers NFκB, AP1, and IRF3, with a focus on MyD88-mediated pro-inflammatory cytokine mechanisms [26].
Telmisartan reduces IL-1β-induced COX-2 expression, PGE2 release, and ROS production. Telmisartan mitigates IL-1β-induced upregulation of IL-1R1 receptor and NOX-4 mRNA expression [27]. Telmisartan attenuates hydrogen peroxide-induced COX-2 gene expression and reduces JNK and c-Jun activation. Telmisartan's neuroprotective effects are independent of PPARγ activation, as confirmed in primary rat cortical neurons [28, 29].
In conclusion, telmisartan demonstrates a multifaceted approach to neuroprotection by modulating specific pathways associated with inflammation and oxidative stress. These findings highlight its potential therapeutic role in neurodegenerative diseases.
Ocular inflammation and telmisartan
Endotoxin-induced uveitis (EIU) serves as an animal model for acute ocular inflammation induced by lipopolysaccharide (LPS) [30, 31]. Severe vision impairment complications include retinal vasculitis, retinal detachment, and glaucoma. Angiotensin II, a key renin-angiotensin system effector, interacts with AT1 and AT2 receptor [32, 33]. Recent studies reveal diverse biological roles of angiotensin II, including modulation of angiogenesis, vascular remodeling, and inflammation [34]. Angiotensin II enhances vascular permeability [35], induces chemokines and adhesion molecules, and influences inflammatory cell proliferation and differentiation. AT1R blockade, including telmisartan, effectively attenuates these inflammatory processes [36,37,38]. Upregulation of AT1R is associated with ocular inflammation in EIU [39]. Telmisartan effectively attenuates inflammatory parameters, including ICAM-1-mediated leukocyte adhesion and infiltration in EIU eyes. LPS stimulation leads to the upregulation of inflammatory mediators contributing to EIU development. ICAM-1 plays a pivotal role in leukocyte adhesion; its upregulation is inhibited by telmisartan. Telmisartan suppresses retinal ICAM-1 upregulation and mitigates various EIU-induced cytokines [40, 41]. The anti-inflammatory effects are associated with downregulation of NF-κB-induced molecules [42]. Insights into the RAS highlight its involvement in various inflammatory conditions, such as atherosclerosis, cerebral infarction, and pancreatitis [36]. Telmisartan substantially reduces anterior-chamber cell infiltration but shows limited impact on protein leakage [43].
Anti-inflammatory effects of telmisartan in Experimental Autoimmune Uveitis (EIU), a model for studying ocular inflammation. Telmisartan is noted for its ability to target important inflammatory mediators such as ICAM-1 (intercellular adhesion molecule-1), various cytokines, and molecules induced by NF-κB (nuclear factor-kappa B), a key transcription factor involved in inflammation. The reference to ICAM-1 suggests that telmisartan may inhibit the adhesion of immune cells to vascular endothelial cells, thereby reducing inflammation. Additionally, by modulating cytokines, which are signaling molecules involved in the immune response, telmisartan likely attenuates the inflammatory cascade in EIU. NF-κB-induced molecules further emphasize the drug's ability to interfere with transcriptional processes that promote inflammation.
Diabetes-induced vascular inflammation and telmisartan
Individuals with diabetes experience exposure of the endothelium to uncontrolled high glucose levels [44]. Endothelial dysfunction is the fundamental pathophysiology in diabetic macrovascular complications [45, 46]. Hyperglycemia triggers inflammatory responses crucial in the development of diabetic macrovascular diseases, including atherosclerotic coronary artery and cerebrovascular diseases [39]. Adhesion of inflammatory leukocytes to the vascular endothelium is a pivotal step in atherosclerosis development [47]. Adhesion molecules like VCAM-1, intercellular adhesion molecule-1, and endothelial-leukocyte adhesion molecule-1 play a role in leukocyte adhesion, leading to vascular inflammation [48]. Angiotensin II type-1 receptor blockers (ARBs), including telmisartan, are prescribed for hypertensive patients with diabetes mellitus [49]. Early reduction of inflammatory leukocyte homing and attachment to the endothelium is considered an effective therapeutic strategy. Telmisartan, an ARB, protects against vascular inflammation induced by hyperglycemia. Reports suggest that telmisartan reduces vascular inflammation by inhibiting the expression of IKKβ in endothelial cells [1]. Telmisartan induces GSK3β-Ser9 phosphorylation in endothelial cells. GSK3β-Ser9 phosphorylation decreases hyperglycemia-induced NFκB p65-Ser536 phosphorylation, VCAM-1 expression, and adhesion of THP-1 monocytes. GSK3β-S9A, a constitutively active mutant of GSK3β, restores the inhibition of NFκB p65-Ser536 phosphorylation, VCAM-1 expression, and THP-1 monocyte adhesion by telmisartan [1].
Telmisartan inhibits IKKβ expression in a GSK3β-Ser9 phosphorylation-dependent manner. Among various ARBs, only telmisartan demonstrates an increase in GSK3β-Ser9 phosphorylation. Telmisartan treatment mitigates HFD-induced upregulation of NFκB p65-Ser536 phosphorylation, VCAM-1 expression, and IKKβ expression in aortic tissues. Telmisartan alleviates hyperglycemia-exacerbated vascular inflammation by inducing GSK3β-Ser9 phosphorylation, inhibiting IKKβ expression, NFκB p65-Ser536 phosphorylation, and VCAM-1 expression in a PPARγ-independent manner. Telmisartan operates within diabetes by engaging with crucial signaling molecules implicated in inflammation and vascular irregularities, including GSK3β, IKKβ, NF-κB, and VCAM-1. Through its influence on these pathways, telmisartan can alleviate the inflammatory reactions linked to diabetes and its accompanying complications, offering promising therapeutic possibilities for individuals with diabetes.
Chronic inflammation
The renin–angiotensin system produces angiotensin II, activating the AT1 receptor, leading to oxidative stress and inflammation [50]. Telmisartan, an AT1 receptor antagonist, also acts as a partial agonist on peroxisome proliferator-activated receptor-γ (PPAR-γ), providing antioxidative and anti-inflammatory effects [51]. Recent studies highlight telmisartan's additional PPAR-γ partial agonist activity, impacting metabolic and inflammatory pathways, improving left ventricular functions, and showing benefits in post-infarct ventricular remodeling [51, 52]. Telmisartan's dose–response relationship in animal models of chronic inflammation suggests antiproliferative and anti-arthritic activities, inhibiting inflammatory reactions [53]. Tissue injury triggers pro-inflammatory cytokine release, but telmisartan's PPAR-γ activation decreases hypertrophic prostanoid production, potentially modulating inflammation [54, 55]. Telmisartan's antioxidant and anti-inflammatory effects involve preventing nuclear factor-κB (NF-κB) signaling pathway activation [56].
Telmisartan exhibits pleiotropic effects, including anti-inflammatory, antioxidative, and antiproliferative actions in atherosclerosis and myocardial infarction.
It also demonstrates a protective effect against gastric mucosal lesions induced by stress and indomethacin [56, 57].
Telmisartan functions as a partial agonist at PPAR-γ, inducing catalase gene expression and inhibiting NF-κB. These actions collectively combat oxidative stress and downregulated a majority of pro-inflammatory responses [51, 58].
The anti-inflammatory impact of telmisartan is attributed to its PPAR-γ agonist activity. The modulation of PPAR-γ expression observed during various inflammatory disorders provides a robust foundation for utilizing potent PPAR-γ ligands, like telmisartan, to attenuate or modulate the progression of inflammation. This finding underscores the potential of PPAR-γ as a therapeutic target in inflammatory conditions, given its altered expression in several inflammatory disorders [59].
This sequence outlines the key mechanisms of action of telmisartan, emphasizing its dual role as an AT1 receptor antagonist and a partial agonist at PPAR-γ, contributing to its multifaceted effects on inflammation, oxidative stress, and related pathways.
Ulcerative colitis and telmisartan
There is a global rise in inflammatory bowel diseases (IBDs), particularly UC. Anti-TNF antibodies are commonly used for UC treatment. Telmisartan is a promising therapeutic candidate with anti-inflammatory properties. Telmisartan suppresses TNF-α-induced activation of nuclear factor-kB (NF-κB) in vascular endothelial cells [60]. Varying doses of telmisartan lead to decreased tissue levels of TNF-α and increased anti-inflammatory activity. Telmisartan's benefits extend to modulating colonic inflammation, oxidative stress, and apoptosis in inflammatory bowel disease [61].
Telmisartan administration mitigates pathological changes induced by arachidonic acid (AA)-induced colitis model, including oxidative stress, alterations in colonic weight, ulceration, tissue necrosis, and inflammatory infiltrate [62].
Telmisartan treatment accelerates the shift from the acute to the chronic phase of inflammation in UC [63]. Effectively it reduces neutrophil infiltration, as indicated by diminished myeloperoxidase (MPO) levels [64]. Telmisartan suppresses the expression of TNF-α and intervenes in the AA-induced colitis model by reducing malondialdehyde (MDA) levels, showcasing its protective role against oxidative cellular injury. Telmisartan increases levels of interleukin-10 (IL-10), known for its anti-inflammatory properties [63].
Telmisartan offers diverse anti-inflammatory effects in treating ulcerative colitis. By inhibiting NF-κB activation triggered by TNF-α, it reduces tissue TNF-α levels and enhances overall anti-inflammatory activity. Telmisartan also combats oxidative stress and apoptosis, common in colonic inflammation, and shields against AA-induced colitis. It expedites the shift from acute to chronic inflammation, curtails neutrophil infiltration, and dampens TNF-α expression. Furthermore, it lowers malondialdehyde levels, indicating defense against oxidative cellular harm, and boosts IL-10 levels, reinforcing its anti-inflammatory prowess. These combined actions highlight telmisartan's promise as a therapeutic option for ulcerative colitis.
Crohn’s disease and telmisartan
Crohn’s disease (CD) patients are often underweight, and there's a significant increase in the ratio of intra-abdominal adipose tissue to total abdominal fat in these individuals [65]. Mesenteric fat serves as a crucial indicator of intestinal inflammation in CD [66].
MAT in CD patients exhibits notable inflammatory infiltrate and altered adipocyte morphology compared to healthy subjects [67]. MAT comprises various cell types, including adipocytes, preadipocytes, macrophages, endothelial cells, fibroblasts, and leukocytes. Telmisartan administration has a positive impact on mesenteric adipocytes in a mouse model of spontaneous colitis, restoring morphological changes and increasing adipocyte diameter. Mesenteric adipocytes contribute to C-reactive protein production in CD [68]. Leptin and adiponectin, hormones produced by adipose tissue, play roles in IBD pathogenesis [69].
Telmisartan treatment significantly alters the production of leptin and adiponectin in MAT, potentially influencing inflammatory processes. The neurotensin/miR-155 signaling pathway, involved in adipose inflammation and adipocyte differentiation, is modulated by telmisartan [70, 71].
Telmisartan treatment inhibits this pathway in MAT, suggesting a potential therapeutic contribution to attenuate MAT alteration and gut inflammation in CD. The renin-angiotensin system plays a role in the pathophysiology of colitis. Angiotensin receptor antagonists, including telmisartan, demonstrate effectiveness in preventing experimental colitis. Telmisartan exhibits a multifaceted impact on visceral adipose tissues, reducing leptin expression, increasing adiponectin levels, and attenuating MAT inflammatory parameters [72]. Telmisartan administration has a beneficial effect in an animal model of spontaneous colitis, reducing MAT inflammation, cytokine production, and ameliorating mesenteric adipose tissue alterations[73].
In conclusion, Telmisartan shows promise as a therapeutic option for managing inflammatory bowel diseases, particularly Crohn's disease, by influencing various aspects of adipose tissue composition, hormone regulation, and inflammatory pathways. The diverse actions of Telmisartan suggest its potential as a comprehensive approach to address the complex mechanisms associated with CD (Table 1 and Fig. 1).
Telmisartan in CNS disorders
Depression, characterized by anhedonia and persistent sadness, poses significant threats to life and cognitive functions [74, 75]. Stress plays a crucial role in depression development and associated memory issues. Ethical concerns limit direct research on depression causes and treatments in affected individuals. Chronic stress contributes to oxidative stress, leading to the generation of reactive oxygen species and compromised central nervous system functioning [76].
Chronic unpredictable mild stress (CUMS) rat model, introduced by Willner, simulates daily stressors and is instrumental in exploring depression origins and testing antidepressant interventions [77]. Telmisartan, a commonly used angiotensin receptor blocker (ARB), easily crosses the blood–brain barrier, inducing central AT1 receptor blockade [78].
Telmisartan holds promise as a potential oral antidepressant, possessing neuroprotective properties and mitigating cognitive impairments induced by chronic stress in rats [79].
Chronic stress results in decreased locomotor activity, sucrose preference, and impaired novel object recognition [80]. Telmisartan, especially at 1 mg/kg/day, significantly improves the impaired ability of novel object recognition, suggesting a potential antidepressant effect. AT1 receptor blockers, including telmisartan, gain attention for potential antidepressant effects [81].
The renin-angiotensin system (RAS) plays a crucial role in the body's response to stress [82].
Resting-state functional magnetic resonance imaging (r-fMRI), measuring intrinsic neural activity, is valuable in neuropsychiatric disorder research [83]. Telmisartan's impact on depression explored using ALFF and ReHo methods in a rat model was the first study of its kind. Telmisartan's effects on stress-induced alterations in brain regions were explored using ALFF and ReHo methods. Telmisartan at 1 mg/kg showed potential in reversing or attenuating stress-induced alterations in brain regions. Telmisartan demonstrated potential in decreasing hypercoordination of neural activity, particularly in the thalamus. Increased ReHo in limbic system regions and altered connectivity in various cerebral regions suggested neuroimaging markers for depression [81].
Telmisartan alleviated depressive behaviors induced by unpredictable chronic mild stress in BALB/c mice. Telmisartan's antidepressant effects were linked to its impact on serotonin transporter expression through PPARδ activation [84, 85].
Telmisartan's neuroprotective and antidepressant properties were associated with its impact on oxidative stress and pro-inflammatory mediators [86].
Its partial PPARδ agonistic property was considered crucial for reducing cytokine levels and improving cognitive decline [87].
Depression is a significant contributor to morbidity and mortality, requiring a multifaceted approach for accurate diagnosis and treatment [88, 89].
Telmisartan's dual action as an AT1 receptor blocker and PPAR-gamma agonist provides neuroprotection against various brain disorders, including depression [17, 90].
Telmisartan shows promise as a depression treatment through multiple mechanisms. It protects neurons, enhances cognition, and influences the body's stress response by modulating the renin-angiotensin system. Telmisartan also restores neural activity in stressed brain regions, impacting serotonin transporter expression via PPARδ activation. Its anti-inflammatory and antioxidant properties, partially through PPARδ agonism, alleviate depressive symptoms. Acting as both an AT1 receptor blocker and PPAR-gamma agonist, telmisartan offers comprehensive neuroprotection against depression.
Telmisartan in epilepsy
Epilepsies involve sudden, abnormal, and excessive neuronal activity in the brain, affecting 5–10% of the population. Long-term therapy challenges and medication side effects contribute to issues with compliance. Epilepsy may be associated with comorbid conditions like hypertension, diabetes, and renal disorders. Research suggests that drugs addressing these disorders, including ACE inhibitors and AT II receptor antagonists, may have a role in preventing seizures. The brain's RAS influences various functions, including regulating cerebral blood flow, stress, depression, seizures, and memory consolidation. Angiotensin II, a RAS component, acts as a neurotransmitter in the central nervous system, influencing the release of other neurotransmitters [91]. Angiotensin II inhibits GABAergic synaptic transmission by activating presynaptic AT1 receptors. Drugs like ACE inhibitors and AT1 receptor antagonists, including telmisartan, have the potential to enhance GABAergic transmission, beneficial in preventing seizures. Telmisartan, an AT1 receptor antagonist, improves the anticonvulsant effects of medications like valproate, lamotrigine, and topiramate in mouse models.
Telmisartan's unique properties, including potential depression-like effects, contribute to its anticonvulsant properties. Telmisartan exhibits neuroprotective effects by reducing local angiotensin II expression, blocking AT1 receptors, and promoting the relative upregulation of AT2 receptor function. In a rat model, ACE and AT1 receptors were upregulated in the brain after repetitive seizures. Telmisartan, especially at a 10 mg/kg dose, significantly decreases hind limb extension duration in the maximal electroshock (MES) model. Telmisartan exhibits substantial seizure inhibition and protection in the pentylenetetrazol (PTZ) test, suggesting dose-dependent antiepileptic activity. Telmisartan's slow dissociation from receptors, penetration of the blood–brain barrier, and increased potency at brain AT1 receptors contribute to its effectiveness. Telmisartan modulates the renin-angiotensin system, affecting glutamate/GABA release, decreasing glutamate levels, increasing GABA levels, and facilitating seizure prevention. AT1 receptor blockers, including telmisartan, contribute to decreased glutamate levels, increased GABA levels, and potential seizure prevention [92].
Telmisartan's higher lipophilicity and potency at brain AT1 receptors make it effective in modulating these neurological functions [93]. Angiotensin affects ion channels, including voltage-dependent potassium and calcium currents, influencing neuronal excitability and seizures [94].
Stress-induced changes in cortical BZ1 receptor expression are regulated by AT1 receptor activity. AT1 receptor antagonists may protect against seizures induced by inflammatory cytokines in chronic inflammatory disorders [95]. Seizure generation involves the activation of inflammatory cytokines, and AT1 receptor antagonists can be considered for epilepsy treatment [96].
Telmisartan, with its AT1 receptor antagonistic properties, demonstrates promising antiepileptic effects. Understanding its mechanisms involving GABAnergic transmission, modulation of RAS, and impact on ion channels provides insights for potential therapeutic applications in epilepsy treatment. Together, these mechanisms underscore telmisartan's potential as an antiepileptic agent, providing neuroprotection, seizure management, modulation of neurotransmitter balance, and regulation of inflammatory pathways.
Telmisartan traumatic brain injury and cerebral edema
Cerebral edema is a serious complication of TBI, leading to elevated intracranial pressure and unfavorable clinical outcomes [97, 98].
The RAS is implicated in neuroinflammation and neurodegenerative disorders [99, 100].
Angiotensin receptor blockers (ARBs), especially telmisartan, effectively inhibit angiotensin II, offering anti-inflammatory and neuroprotective effects [101, 102]. Telmisartan, with its high lipid solubility, effectively penetrates brain tissue. Recognized for neuroprotective effects through angiotensin II receptor type-1 (AT1R) blockade [103].
Scientists investigated the anti-edemic effect of telmisartan through single oral gavage administration. Reduction in cerebral edema observed at 12 and 24 h post-TBI. The anti-edemic effect of telmisartan was not strictly dose-dependent [104]. Telmisartan demonstrated sustained inhibitory effects on AT1R, presenting an extended window for pharmacological intervention. IL-1β, a pro-inflammatory cytokine elevated in TBI, is implicated in cerebral edema [105, 106].
Telmisartan has shown efficacy in mitigating IL-1β-induced inflammatory responses in various brain injury models. Telmisartan improved neurological function, reduced lesion volume, and exhibited neuroprotective effects in the TBI model. Investigation into telmisartan's impact on the pro-inflammatory cytokine IL-1β. Telmisartan was found to inhibit the assembly and activation of the NLRP3 inflammasome [105, 107]. This inhibition provides a mechanism for telmisartan's anti-edemic role in TBI. The study confirmed the role of NLRP3 inflammasome-regulated IL-1β in traumatic cerebral edema. Telmisartan demonstrated potential in sustaining BBB integrity, reducing edema, and improving neurological function through NLRP3 inflammasome modulation [108].
Telmisartan, an angiotensin receptor blocker, offers a versatile approach to managing brain injury and cerebral edema. By inhibiting angiotensin II activity, it provides anti-inflammatory and neuroprotective benefits. Its lipid solubility enables direct brain tissue access for targeted action, primarily inhibiting AT1R to reduce inflammation and enhance neurological function. Telmisartan also disrupts NLRP3 inflammasome activation, reducing IL-1β-induced inflammation. Its sustained AT1R inhibition diminishes cerebral edema post-TBI, while also maintaining blood–brain barrier integrity. Telmisartan shows promise as a therapeutic agent for brain injury and cerebral edema management.
Anxiolytic effect of telmisartan
Anxiety disorders, affecting 7–30% of the world's population, represent a significant mental health challenge, contributing to economic burdens and health concerns [109]. Neurotransmitters like serotonin and GABA are implicated in anxiety pathophysiology, with selective serotonin reuptake inhibitors (SSRIs) recommended as first-line drugs. Emerging evidence suggests the involvement of the brain renin-angiotensin system in anxiety states, where angiotensin modulates neurotransmitter release [110]. Angiotensin receptor blockers (ARBs), used for cardiovascular conditions, have been associated with increased anxiety prevalence, particularly due to the blockade of AT1 > AT2 receptors in the brain [111]. Telmisartan, crossing the blood–brain barrier, exhibits significant anti-anxiety effects, possibly through AT1 receptor blockade in circumventricular organs and potential cerebral AT receptor blockade. The mechanism of telmisartan's anti-anxiety effects involves hypothesized upregulation of angiotensin levels and receptors in the brain during anxiety, influencing neurotransmitters like noradrenaline and serotonin [112]. Telmisartan's additional activities via PPAR-gamma and NADPH oxidase may contribute to its role in oxidative stress management, providing added benefits. [113].
In summary, telmisartan, through its action on the RAS and neurotransmitter modulation, emerges as a promising agent in managing anxiety, offering potential avenues for novel treatments targeting neurogenesis, plasticity, and cell survival (Table 2).
Telmisartan in cancer
Endometrial cancer
Endometrial cancers are prevalent malignant tumors affecting the female genital tract, with a rising incidence [114, 115]. Despite the increasing incidence, effective agents for advanced and recurrent cases are lacking [115].
Peroxisome proliferator-activated receptor gamma and its ligands are known to induce apoptosis in various cancer types, including endometrial cancer [116, 117].
PPAR-gamma belongs to a nuclear hormone receptor family linked to endometrial carcinoma risk factors like obesity, excess estrogen, type II diabetes, and hypertension [118, 119].
Telmisartan emerges as a therapeutic option, inducing DNA damage and apoptosis in endometrial cancer cells. Telmisartan, acting as a partial agonist of PPAR-gamma, activates the receptor independently through AT1R interaction [51].
Telmisartan induces DNA double-strand breaks (DSBs) before triggering apoptosis in endometrial cancer cells. Among ARBs, only telmisartan inhibits cell viability in endometrial cancer, and this effect is diminished by a PPAR-gamma inhibitor (GW9662) [120].
PPAR-gamma immunoreactivity is detected in endometrial carcinoma tissue, and PPAR-gamma ligands show antiproliferative activity [116].
In vivo experiments using a nude mouse model demonstrate apoptosis within the tumor area in mice treated with telmisartan. Telmisartan's antitumor activity without major side effects suggests potential effectiveness in individuals with minimal residual disease post-surgery, chemotherapy, or radiotherapy [120].
In summary, telmisartan exhibits promise in endometrial cancer treatment through its involvement in PPAR-gamma mediated pathways, highlighting its potential as a therapeutic agent for individuals with minimal residual disease.
Telmisartan in colon cancer
Telmisartan, an angiotensin II receptor blocker (ARB) for hypertension, has been found to activate PPARγ in colon cancer cells, suppressing malignancy through differentiation and apoptosis [121].
Telmisartan effectively reduces cell viability, inhibits proliferation, and induces apoptosis in selected colon cancer cell lines [15].
The addition of GW9662, a PPARγ blocker, did not hinder telmisartan's inhibition of cell proliferation and viability, suggesting a PPARγ-independent pathway. Combined use of telmisartan and GW9662 intensified the reduction in cell viability and antiproliferative effects, particularly in SW-480 and SW-620 cells. Telmisartan demonstrated an apoptotic effect similar to pioglitazone, and this effect was not hindered by GW9662, indicating PPARγ-independent apoptosis induction [122]. Ligand-dependent activation of PPARγ resulted in the downregulation of PPARγ mRNA and upregulation of target genes, including CSTA. Telmisartan, in a dose-dependent manner, affected relative PPARγ mRNA expression and upregulated CSTA [123].
Addition of GW9662 led to significant downregulation in relative PPARγ mRNA expression, suggesting a PPARγ-independent pathway. Telmisartan's effects were superior to pioglitazone in certain cells, especially in the presence of the PPARγ blocker GW9662 [122].
Telmisartan's apoptotic effect was comparable to the full PPARγ agonist pioglitazone, and this action appeared to be independent of PPARγ [124, 125].
Examination of gene expression shed light on the influence of GW9662 on PPARγ and CSTA mRNA expression, providing valuable insights into the molecular mechanisms underlying telmisartan's effects on colon cancer cells [123].
In summary, telmisartan exhibits notable effects on colon cancer cells through a PPARγ-independent pathway, with enhanced efficacy in combination with GW9662. The study provides crucial molecular insights into telmisartan's actions on colon cancer cells, emphasizing its potential as a therapeutic agent.
Hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the primary liver malignancy and a significant cause of cancer-related deaths globally [126, 127]. Telmisartan's impact on proliferation was assessed in various HCC cells, including HLF, HLE, HepG2, HuH-7, and PLC/PRF/5. Telmisartan effectively inhibited the proliferation of poorly differentiated HCC cells (HLF, HLE, and HepG2) while showing reduced sensitivity in well-differentiated HCC cells (HuH-7 and PLC/PRF/5) [128].
Telmisartan induced G0/G1 cell cycle arrest in HLF cells by impeding the G0-to-G1 transition [129].
The observed cell cycle arrest was accompanied by a significant reduction in the levels of cyclin D1, cyclin E, and other cell cycle-related proteins [130].
Telmisartan increased the activity of the AMP-activated protein kinase (AMPK) pathway and concurrently inhibited the mammalian target of rapamycin (mTOR) pathway [131, 132].
Telmisartan contributed to apoptosis in HLF cells, as indicated by an increase in caspase-cleaved cytokeratin 18 (cCK18) levels and a reduction in the phosphorylation of ErbB3. The study identified 163 differentially expressed miRNAs in response to telmisartan, emphasizing its inhibitory effects, particularly in poorly differentiated HCC cells. Telmisartan's mechanisms involve cell cycle regulation, apoptosis induction, and modulation of key signaling pathways like AMPK/mTOR [14].
Telmisartan exhibits potent antiproliferative effects against hepatocellular carcinoma (HCC), especially in poorly differentiated cells. It arrests the cell cycle at G0/G1 phase, reduces cyclin D1 and cyclin E expression, and activates the AMPK pathway while inhibiting mTOR, hampering HCC proliferation. Telmisartan also induces apoptosis by increasing cCK18 levels and decreasing ErbB3 phosphorylation in HLF cells. Altered miRNA expression underscores its efficacy, particularly in poorly differentiated HCC. Overall, telmisartan regulates the cell cycle, induces apoptosis, and modulates key signaling pathways like AMPK/mTOR, making it a promising therapy for HCC.
Ovarian cancer and telmisartan
Ovarian cancer, ranking third in incidence among female reproductive system malignancies, poses a significant health challenge with epithelial ovarian cancer having the highest mortality rate among gynecological tumors [133]. Challenges at advanced stages highlight the critical need for early detection strategies [134].
Peroxisome proliferator-activated receptor gamma (PPARγ), a key player in various cancers, emerges as a potential avenue for cancer intervention [135]. Telmisartan, primarily an antihypertensive agent, gains attention for activating PPARγ. In HEY cells, telmisartan inhibits growth in a time- and dose-dependent manner, inducing apoptosis and reducing caspase-3 activity, consistent with outcomes in other cancer cells [136].
PPARγ's role takes center stage in regulating physiological processes, including ovarian tissue functions crucial for reproduction [137, 138]. Telmisartan's ability to elevate PPARγ expression reinforces its potential as a multifaceted therapeutic. Telmisartan's impact on MMP-9 expression, linked to cancer progression, further supports its potential in ovarian cancer treatment [139].
PPARγ agonists, including telmisartan, exhibit positive effects in contexts like renal fibrosis and early pregnancy chorionic villi, influencing trophoblast cell invasion [140]. Telmisartan's reduction in MMP-9 expression in HEY cells aligns with potential therapeutic effects observed in periodontitis and acute myocardial infarction [141, 142].
Telmisartan's mechanism of action in ovarian cancer encompasses PPARγ activation, suppressing cancer cell growth, promoting apoptosis, regulating MMP-9 expression, and offering potential multifaceted therapeutic effects (Fig. 2 and Table 3).
Telmisartan in diabetes
Telmisartan, recognized as an angiotensin II receptor antagonist specifically targeting the angiotensin II type-1 receptor, has become a widely utilized medication for the management of hypertension. In recent times, the focus on telmisartan's impact on peroxisome proliferator-activated receptors (PPARs) has expanded [10]. PPARs, belonging to the nuclear hormone receptor superfamily, are ligand-activated transcription factors. Telmisartan's distinctive feature lies in its reported partial PPARγ-agonistic effect, avoiding safety concerns associated with full PPARγ agonists [10].
Renowned for its partial PPARγ-agonistic effect, telmisartan exhibits capabilities in the regulation of glucose and lipid metabolism, improvement of insulin resistance, and potential effectiveness in addressing metabolic syndrome [10, 143].
Telmisartan's ability to lower glucose levels is linked to its capacity to inhibit reactive oxygen species and its agonistic influence on PPARγ [10]. The medication has demonstrated a reduction in oxidative stress, elevation of antioxidant levels, and enhancement of insulin sensitivity in individuals with diabetes [144]. The dual impact of telmisartan, involving partial activation of PPARγ and angiotensin type-1 receptor blockade, holds significance in preventing and managing type 2 diabetes mellitus (T2DM), metabolic syndrome, and atherosclerotic cardiovascular disease [10]. In hypertensive patients with T2DM, telmisartan exhibits favorable effects on insulin sensitivity, blood pressure, glucose and lipid metabolism, and endothelial function. Its cardioprotective attributes encompass the reduction of cardiac fibrosis and hypertrophy, prevention of adverse cardiac remodeling, and potential mitigation of diabetic cardiomyopathy [145]. Telmisartan's dual action on angiotensin type-1 receptor and PPARγ positions it as a valuable therapeutic option for hyperlipidemia, insulin resistance, hypertension, and stroke [146]. Additionally, the medication has displayed efficacy in improving the lipid profile, with notable reductions in triglycerides and increases in high-density lipoprotein cholesterol levels. Its potential hepatic partial PPARα agonist activity further contributes to its anti-dyslipidemic effects [147].
Telmisartan emerges as a promising therapeutic agent for T2DM, offering a comprehensive approach by addressing glucose regulation, lipid metabolism, insulin resistance, and providing cardiovascular and renal protection [10].
Telmisartan, known for its action as an angiotensin II receptor antagonist, also exhibits partial agonistic effects on PPARγ, distinct from full agonists. This feature enables telmisartan to regulate glucose and lipid metabolism and improve insulin sensitivity, making it valuable in managing type 2 diabetes mellitus (T2DM) and metabolic syndrome. By inhibiting reactive oxygen species and enhancing antioxidant levels through PPARγ activation, telmisartan reduces oxidative stress and enhances insulin sensitivity in diabetic individuals. Its dual action on PPARγ and angiotensin type-1 receptors provides a multifaceted approach to managing T2DM and metabolic syndrome.
Diabetic nephropathy
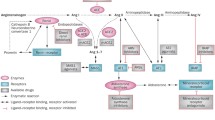
The renin-angiotensin system, vital for cardiovascular and renal functions, involves Angiotensin II (Ang II) binding to AT1 and AT2 receptors. Ang II, through AT1 receptor activation, induces vasoconstriction, sodium reabsorption, and various cellular processes in the kidney. Reactive oxygen species (ROS) [148] play a role in Ang II signaling, influencing critical events like transactivation of the epidermal growth factor receptor [149, 150].
In diabetic nephropathy (DN), PKC-α activation varies across renal structures, with implications for Na + -K + -ATPase inhibition and albumin uptake [151, 152]. Increased PKC-α expression correlates with TGF-β1 and VEGF levels, contributing to DN pathogenesis [153]. Telmisartan, an AT1R blocker, attenuates PKC-α and VEGF expression, suggesting nephroprotective effects via PKC-α in the RAS-PKC signaling cascade [154].
In vitro, telmisartan suppresses ROS generation induced by high glucose levels, protecting against cellular damage [155]. In diabetic mice, telmisartan reduces albuminuria, mesangial expansion, and inflammation-associated markers, demonstrating protective effects [156]. Telmisartan decreases oxidative stress markers (8-OHdG, Nox4), apoptosis (Bax), and improves kidney function and structure in diabetic rats. It regulates mitochondria-related pathways, inhibiting oxidative stress [157, 158].
Telmisartan's dual action as a PPAR-gamma agonist and AT1 receptor inhibitor contributes to renal protection It downregulates gene expression in the oxidative phosphorylation pathway, potentially inhibiting excessive mitochondrial ROS production [159, 160]. The upregulation of nephrin and podocin, crucial components of the slit diaphragm, signifies protective effects against diabetic nephropathy [161, 162].
Telmisartan exhibits multifaceted renoprotective effects, addressing oxidative stress, inflammation, and apoptosis in diabetic nephropathy. Its actions on PKC-α and various pathways provide valuable insights, suggesting its therapeutic potential in managing diabetic kidney disease (Fig. 3).
Telmisartan in diabetic nephropathy. TGF-b: Transforming growth factor beta, 8-OHdG: 8-hydroxydeoxyguanosine, (MCP)-1: monocyte chemoattractant protein, CD68: cluster differentiation, TNF-α: tumor necrosis factor alpha, PKC-α: protein kinase C alpha, Nox4: NADPH oxidase-4, NADPH: nicotinamide adenine dinucleotide phosphate
Diabetic neuropathy
Nerve healing is influenced by various factors, including the time between trauma and surgery, severity of trauma, type of damaged nerve, patient's age, and surgeon's experience [163]. Peripheral nerve regeneration is closely tied to apoptosis, and inhibiting the renin-angiotensin system (RAS) receptors, especially AT1 receptors, reduces apoptosis, inflammation, and oxidative stress [102]. Telmisartan, with its high affinity for AT1 receptors, demonstrates anti-inflammatory effects, supporting positive effects on nerve healing [102, 164].
The PPAR-γ ligand in telmisartan mediates its positive effects by inhibiting post-ischemic inflammation, neuronal degeneration, and apoptosis regeneration [165]. Telmisartan attenuates hemorrhage expansion, perihematomal edema formation, and neuropathic pain, attributed to its anti-inflammatory properties [102, 166]. It prevents nerve cells from injury by decreasing the apoptotic pathway, inhibiting caspase-3 activity, and reducing inflammatory cytokines [167, 168]. In conditions like chronic constriction injury (CCI), RAAS overactivation is associated with increased inflammatory mediators, oxidative stress, and pain-related markers [169, 170]. Telmisartan, by modulating RAAS components and downregulating signaling pathways like JAK2/STAT3 and P38-MAPK, [171] exerts beneficial effects in neuropathic pain modulation. ACE-Is are superior to ARBs in neuroprotective and antioxidant effects [172].
Telmisartan administration in diabetic rats prevents the progression of diabetic neuropathy (DN) and enhances thermal and mechanical analgesia [12]. Its anti-inflammatory and neuroprotective properties, [27] attributed to PPAR-γ activation, alleviate hyperglycemia-associated alterations and reduce pro-inflammatory biomarkers [12]. Telmisartan suppresses the production of inflammatory cytokines and inhibits thermal hyperalgesia development and progression. It attenuates nerve growth factor degeneration and behavioral abnormalities in diabetic animals [173].
In summary, telmisartan's multifaceted actions, involving anti-inflammatory, neuroprotective, and antioxidant effects, position it as a potential therapeutic candidate in nerve healing and diabetic neuropathy. Its modulation of RAAS components and downstream signaling pathways contributes to its beneficial effects on nerve regeneration and pain modulation (Fig. 4).
Diabetic retinopathy
The activation of the renin-angiotensin system, particularly through the angiotensin II type-1 receptor (AT1R), has been identified as a potential mechanism for damaging retinal neurons in individuals with diabetes [174]. TNFα, a key inflammatory factor, can disrupt the blood-retinal barrier (BRB), leading to leukocyte accumulation in the retina and promoting cell death [175]. Caspase, indicators of cell death, are activated early in the retina of individuals with diabetes. Telmisartan effectively reduces caspase-3 activity in the diabetic retina, providing protection against neuronal damage [174]. The AT1R blocker effect extends to modulating levels of brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), tyrosine hydroxylase (TH), glutathione (GSH), and caspase activity in the retina [174]. Telmisartan, by inhibiting AT1R activation, addresses imbalances in these factors contributing to neurodegeneration in diabetic retinopathy. Telmisartan's ability to inhibit AT1R, which is stimulated by angiotensin II, presents a promising approach for treating diabetic retinopathy (DR) [176]. Telmisartan demonstrates a multifaceted protective role in diabetic retinopathy (DR). By inhibiting the activation of the angiotensin II type-1 receptor (AT1R) [177], telmisartan intervenes in the renin-angiotensin system (RAS), a potential mechanism implicated in damaging retinal neurons in diabetes [178].
The drug effectively reduces caspase-3 activity, providing protection against early cell death in the diabetic retina. Telmisartan's impact extends to the modulation of various factors crucial for neurodegeneration in diabetic retinopathy. It addresses imbalances in brain-derived neurotrophic factor (BDNF), ciliary neurotrophic factor (CNTF), tyrosine hydroxylase (TH), and glutathione (GSH), offering a promising approach for treating DR [179]. Its high affinity for AT1 receptor subtypes allows telmisartan to effectively block the RAS, leading to a sustained protective effect and slowing down the progression of diabetic retinopathy [180, 181]. The neuroprotective effects of telmisartan are further highlighted by its ability to elevate BDNF and GSH levels in both blood and the retina, responding to oxidative stress. By blocking AT1R activation induced by diabetes [182], telmisartan may deactivate NADPH oxidase, reducing oxidative stress and potentially increasing BDNF levels. The preservation of neurons, indicated by increased levels of TH, reinforces the protective impact of Telmisartan, particularly on dopaminergic amacrine cell functionality [183]. In addition, telmisartan effectively suppresses the elevated expression of VEGF-A, RAGE, and TNF-α in the retina, contributing to the mitigation of retinal complications [184]. Its specific binding to intraocular angiotensin receptor 1 (ATR 1) inhibits excessive activation of the Ang II-mediated RAS system, postponing the breakdown of the blood-retinal barrier (BRB) [184]. Telmisartan's actions encompass inhibition of AT1R, modulation of neuroprotective factors, reduction of oxidative stress, and suppression of pro-inflammatory mediators [185].
Telmisartan exerts a multifaceted protective effect against retinal damage in diabetic retinopathy by inhibiting AT1R, modulating neuroprotective factors, reducing oxidative stress, suppressing pro-inflammatory mediators, and preserving the blood-retinal barrier (Fig. 5).
Telmisartan and diabetic ulceration
Patients diagnosed with type 2 diabetes mellitus frequently experience a high incidence of acute gastric inflammation and ulcer disease, and this occurrence is notably linked to the duration of diabetes. Moreover, peptic ulcers associated with diabetes mellitus tend to be more severe, exhibiting a delayed healing rate and a higher propensity for complications, including gastrointestinal bleeding [186, 187].
The heightened vulnerability of gastric mucosa in diabetic animals to damage involves a multifaceted mechanism. This includes changes in gastric motility [188] compromised duodenal bicarbonate secretion, and diminished angiogenesis, along with dysfunction in capsaicin-sensitive neurons crucial for safeguarding the gastric mucosa [189]. Telmisartan, a medication utilized for various conditions, exhibits antioxidant and anti-inflammatory properties attributed to its inhibition of the nuclear factor-κB (NF-κB) signaling pathway. This pathway plays a pivotal role in the transcription of genes involved in oxidative stress and inflammation, including NADPH oxidase, tumor necrosis factor-α (TNF-α), and inducible nitric oxide synthase (iNOS). Angiotensin II, a known inducer of oxidative stress, activates NADPH oxidase, leading to the production of reactive oxygen species (ROS) like superoxide anion, hydrogen peroxide, and hydroxyl radicals [190]. Moreover, it triggers inflammatory pathways, promoting the synthesis of TNF-α, contributing to gastric mucosa damage. Telmisartan acts as a partial agonist for the nuclear peroxisome proliferator-activated receptor-γ (PPAR-γ). Activation of PPAR-γ stimulates the expression of the catalase gene and inhibits NF-κB activity, thereby mitigating oxidative stress and reducing pro-inflammatory reactions [191, 192]. Stimulation of PPAR-γ also enhances the production of leptin protein, known for its ability to reduce gastric acid secretion [193]. The drug's impact on gastric health extends to blocking the activation of caspase-3 in the stomach lining, preventing the cascade of events leading to cell apoptosis [194]. Pro-inflammatory cytokines, especially TNF-α, and oxygen-derived free radicals are triggers of cell death by activating caspases. Telmisartan's ability to inhibit apoptosis is likely due to its capacity to reduce reactive oxygen species production and inhibit the synthesis of TNF-α and excessive nitric oxide [102, 195].
In summary, Telmisartan's mechanism of action involves inhibiting NF-κB signaling, reducing oxidative stress, suppressing inflammatory responses, and preventing apoptosis, collectively contributing to its potential therapeutic role in protecting against gastric ulcer formation (Fig. 6 and Table 4).
Conclusion
Telmisartan demonstrates neuroprotective effects by targeting inflammation and oxidative stress pathways, potentially useful in neurodegenerative diseases. It also exhibits significant anti-inflammatory properties, particularly in mitigating acute ocular inflammation associated with endotoxin-induced uveitis (EIU). It shows promise in mitigating diabetes-induced vascular inflammation by reducing adhesion molecule expression and inflammatory leukocyte attachment. It also exhibits nephroprotective benefits by modulating PKC-α and VEGF expression. Telmisartan, alleviate neuropathic pain by modulating RAAS components and suppressing JAK2/STAT3 and P38-MAPK signaling pathways. Telmisartan, acting as an AT1 receptor antagonist and PPAR-γ partial agonist, displays antioxidative and anti-inflammatory effects, impacting metabolic and inflammatory pathways. It suppresses TNF-α-induced NF-κB activation, reducing neutrophil infiltration in ulcerative colitis.
Additionally, its ACE inhibitor and AT1 receptor antagonist properties may augment GABAergic transmission, potentially benefiting seizure prevention and reducing edema in traumatic brain injury. Telmisartan shows anti-anxiety effects by blocking AT1 receptors in the brain and may inhibit cerebral AT receptors. It also demonstrates potential as a therapy for endometrial and colon cancer by inducing DNA damage and apoptosis through PPAR-gamma activation. Telmisartan inhibits the proliferation of poorly differentiated hepatocellular carcinoma cells and reduces MMP-9 expression in ovarian cancer cells.
Limitations
Our findings are limited by our main focus on preclinical studies, which means we did not review the effects of telmisartan in clinical studies. Moreover, our attention was directed toward assessing telmisartan's impact on common diseases and disorders, rather than those that are rare.
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- ARB:
-
Angiotensin receptor blocker
- AT1:
-
Angiotensin II type-1
- CD:
-
Crohn’s disease
- EIU:
-
Endotoxin-induced uveitis
- GSK3β:
-
Glycogen synthase kinase-3 beta
- IBDs:
-
Inflammatory bowel diseases
- ICAM-1:
-
Intercellular adhesion molecule
- IFN-g:
-
Interferon gamma
- TNF:
-
Tumor necrosis factor
- IKKβ:
-
Inhibition if nuclear factor-kB (IkB) kinase beta
- IL:
-
Interleukin
- JNK:
-
Jun N-terminal kinase
- LPS:
-
Lipopolysaccharide
- MAT:
-
Medication-assisted treatment
- MCP:
-
Monocyte chemoattractant protein -1
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase levels
- PASI:
-
Psoriasis area and severity index
- PPAR-γ:
-
Peroxisome proliferator-activated receptor
- RANKL/RANK:
-
Signaling pathway
- SARM:
-
Selective androgen receptor modulator
- TLRs:
-
Toll-like receptors
- VCAM-1:
-
Vascular cell adhesion molecule-1
- CUMS:
-
Chronic unpredictable mild stress
- r-fMRI:
-
Resting-state functional magnetic resonance imaging maximal electroshock (MES) model
- PTZ:
-
Pentylenetetrazol test
- SSRIs:
-
Selective serotonin reuptake inhibitors
- 5-HTT:
-
Serotonin
- DSBs:
-
DNA double-strand breaks
- HCC:
-
Hepatocellular carcinoma
- AMP:
-
Activated protein kinase (AMPK) pathway
- (mTOR):
-
Mammalian target of rapamycin pathway
- cCK18:
-
Caspase-cleaved cytokeratin 18
- MMP-9:
-
Matrix metalloprotease 9,
- ROS:
-
Reactive oxygen species,
- DR5:
-
Death receptor 5
- TGF-b:
-
Transforming growth factor beta
- 8-OHdG:
-
8-Hydroxydeoxyguanosine
- MCP-1:
-
Monocyte chemoattractant protein
- CD68:
-
Cluster differentiation
- TNF-α:
-
Tumor necrosis factor alpha
- PKC-α:
-
Protein kinase C alpha
- CCI:
-
Chronic constriction injury
References:
Song KH, Bae SJ, Chang J, Park JH, Jo I, Cho KW, Cho DH (2017) Telmisartan mitigates hyperglycemia-induced vascular inflammation by increasing GSK3β-Ser9 phosphorylation in endothelial cells and mouse aortas. Biochem Biophys Res Commun 491(4):903–911
Guerra GC, Araújo AA, Lira GA, Melo MN, Souto KK, Fernandes D, Silva AL, Júnior RFA (2015) Telmisartan decreases inflammation by modulating TNF-α, IL-10, and RANK/RANKL in a rat model of ulcerative colitis. Pharmacol Rep 67:520–526
Bakheit AH, Abd-Elgalil AA, Mustafa B, Haque A, Wani TA (2015) Telmisartan. Profile Drug Subst Excip Relat Methodol 40:371–429
Li BH, Liao SQ, Yin YW, Long CY, Guo L, Cao XJ, Liu Y, Zhou Y, Gao CY, Zhang LL, Li JC (2015) Telmisartan-induced PPARγ activity attenuates lipid accumulation in VSMCs via induction of autophagy. Mol Biol Rep 42:179–186
Goyal S, Arora S, Mittal R, Joshi S, Nag TC, Ray R, Kumari S, Arya DS (2009) Myocardial salvaging effect of telmisartan in experimental model of myocardial infarction. Eur J Pharmacol 619(1–3):75–84
Rizos CV, Elisaf MS, Liberopoulos EN (2009) Are the pleiotropic effects of telmisartan clinically relevant? Curr Pharm Des 15(24):2815–2832
Hegazy N, Rezq S, Fahmy A (2020) Mechanisms involved in superiority of angiotensin receptor blockade over ACE inhibition in attenuating neuropathic pain induced in rats. Neurotherapeutics 17(3):1031–1047
Dominguez E, Rivat C, Pommier B, Mauborgne A, Pohl M (2008) JAK/STAT3 pathway is activated in spinal cord microglia after peripheral nerve injury and contributes to neuropathic pain development in rat. J Neurochem 107(1):50–60
Chang WT, Cheng JT, Chen ZC (2016) Telmisartan improves cardiac fibrosis in diabetes through peroxisome proliferator activated receptor δ (PPARδ): from bedside to bench. Cardiovasc Diabetol 15(1):1–9
Ahire YS, Kundu S, Bairagi VA (2023) Effect of co-administration of telmisartan with luteolin in dexamethasone-induced insulin resistance in mice. Biol Forum Int J 15(5):1642–1654
Sukumaran V, Veeraveedu PT, Gurusamy N, Yamaguchi KI, Lakshmanan AP, Ma M, Suzuki K, Kodama M, Watanabe K (2011) Cardioprotective effects of telmisartan against heart failure in rats induced by experimental autoimmune myocarditis through the modulation of angiotensin-converting enzyme-2/angiotensin 1–7/mas receptor axis. Int J Biol Sci 7(8):1077
Al-Rejaie SS, Abuohashish HM, Ahmed MM, Arrejaie AS, Aleisa AM, AlSharari SD (2015) Telmisartan inhibits hyperalgesia and inflammatory progression in a diabetic neuropathic pain model of Wistar rats. Neurosci J 20(2):115–123
Saravanan PB, Shanmuganathan MV, Ramanathan M (2015) Telmisartan attenuated LPS-induced neuroinflammation in human IMR-32 neuronal cell line via SARM in AT1R independent mechanism. Life Sci 130:88–96
Oura K, Tadokoro T, Fujihara S, Morishita A, Chiyo T, Samukawa E, Yamana Y, Fujita K, Sakamoto T, Nomura T, Yoneyama H (2017) Telmisartan inhibits hepatocellular carcinoma cell proliferation in vitro by inducing cell cycle arrest. Oncol Rep 38(5):2825–2835
Samukawa E, Fujihara S, Oura K, Iwama H, Yamana Y, Tadokoro T, Chiyo T, Kobayashi K, Morishita A, Nakahara M, Kobara H (2017) Angiotensin receptor blocker telmisartan inhibits cell proliferation and tumor growth of cholangiocarcinoma through cell cycle arrest. Int J Oncol 51(6):1674–1684
Abdel-Wahab A, Mahmoud W, Al-Harizy R (2016) Comparative renal protective effects of canagliflozin and telmisartan in a rat model of diabetic nephropathy. J Nephrol Renal Ther 2(010):1–8
Kasahara Y, Taguchi A, Uno H, Nakano A, Nakagomi T, Hirose H, Stern DM, Matsuyama T (2010) Telmisartan suppresses cerebral injury in a murine model of transient focal ischemia. Brain Res 1340:70–80
Mogi M, Iwanami J, Horiuchi M (2012) Roles of brain angiotensin II in cognitive function and dementia. Int J Hypertens 2012:1–7
Rodriguez-Pallares J, Parga JA, Joglar B, Guerra MJ, Labandeira-Garcia JL (2012) Mitochondrial ATP-sensitive potassium channels enhance angiotensin-induced oxidative damage and dopaminergic neuron degeneration. Relevance for aging-associated susceptibility to Parkinson’s disease. Age 34:863–880
Garrido-Gil P, Joglar B, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL (2012) Involvement of PPAR-γ in the neuroprotective and anti-inflammatory effects of angiotensin type 1 receptor inhibition: effects of the receptor antagonist telmisartan and receptor deletion in a mouse MPTP model of Parkinson’s disease. J Neuroinflammation 9:1–16
Zhang ZH, Yu Y, Wei SG, Felder RB (2010) Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD (P) H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens 28(4):806
Benicky J, Sánchez-Lemus E, Honda M, Pang T, Orecna M, Wang J, Leng Y, Chuang DM, Saavedra JM (2011) Angiotensin II AT1 receptor blockade ameliorates brain inflammation. Neuropsychopharmacology 36(4):857–870
Pinteaux E, Trotter P, Simi A (2009) Cell-specific and concentration-dependent actions of interleukin-1 in acute brain inflammation. Cytokine 45(1):1–7
Pascoe MC, Crewther SG, Carey LM, Crewther DP (2011) Inflammation and depression: why poststroke depression may be the norm and not the exception. Int J Stroke 6(2):128–135
Peng J, Yuan Q, Lin B, Panneerselvam P, Wang X, Luan XL, Lim SK, Leung BP, Ho B, Ding JL (2010) SARM inhibits both TRIF-and MyD88-mediated AP-1 activation. Eur J Immunol 40(6):1738–1747
Kong Y, Le Y (2011) Toll-like receptors in inflammation of the central nervous system. Int Immunopharmacol 11(10):1407–1414
Pang T, Wang J, Benicky J, Sánchez-Lemus E, Saavedra JM (2012) Telmisartan directly ameliorates the neuronal inflammatory response to IL-1β partly through the JNK/c-Jun and NADPH oxidase pathways. J Neuroinflammation 9:1–19
Pang T, Benicky J, Wang J, Orecna M, Sanchez-Lemus E, Saavedra JM (2012) Telmisartan ameliorates lipopolysaccharide-induced innate immune response through peroxisome proliferator-activated receptor-γ activation in human monocytes. J Hypertens 30:87–96
Ghaisas MM, Dandawate PR, Zawar SA, Ahire YS, Gandhi SP (2010) Antioxidant, antinociceptive and anti-inflammatory activities of atorvastatin and rosuvastatin in various experimental models. Inflammopharmacology 18(4):169–177
Rosenbaum JT, McDevitt HO, Guss RB, Egbert P (1980) Endotoxin-induced uveitis in rats as a model for human disease. Nature 286(5773):611–613
Hoekzema R, Verhagen C, Van Haren M, Kijlstra A (1992) Endotoxin-induced uveitis in the rat: the significance of intraocular interleukin-6. Invest Ophthalmol Vis Sci 33(3):532–539
De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger TH (2000) International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol Rev 52(3):415–472
Murphy TJ, Alexander RW, Griendling KK, Runge MS, Bernstein KE (1991) Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature 351(6323):233–236
Moravski CJ, Kelly DJ, Cooper ME, Gilbert RE, Bertram JF, Shahinfar S, Skinner SL, Wilkinson-Berka JL (2000) Retinal neovascularization is prevented by blockade of the renin-angiotensin system. Hypertension 36(6):1099–1104
Chua CC, Hamdy RC, Chua BH (1998) Upregulation of vascular endothelial growth factor by angiotensin II in rat heart endothelial cells. Biochimica Biophysica Acta Mol Cell Res 1401(2):187–194
Candido R, Allen TJ, Lassila M, Cao Z, Thallas V, Cooper ME, Jandeleit-Dahm KA (2004) Irbesartan but not amlodipine suppresses diabetes-associated atherosclerosis. Circulation 109(12):1536–1542
Pastore L, Tessitore A, Martinotti S, Toniato E, Alesse E, Bravi MC, Ferri C, Desideri G, Gulino A, Santucci A (1999) Angiotensin II stimulates intercellular adhesion molecule-1 (ICAM-1) expression by human vascular endothelial cells and increases soluble ICAM-1 release in vivo. Circulation 100(15):1646–1652
Okamura A, Rakugi H, Ohishi M, Yanagitani Y, Takiuchi S, Moriguchi K, Fennessy PA, Higaki J, Ogihara T (1999) Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens 17(4):537–545
Nagai N, Oike Y, Noda K, Urano T, Kubota Y, Ozawa Y, Shinoda H, Koto T, Shinoda K, Inoue M, Tsubota K (2005) Suppression of ocular inflammation in endotoxin-induced uveitis by blocking the angiotensin II type 1 receptor. Invest Ophthalmol Vis Sci 46(8):2925–2931
Niimi R, Nakamura A, Yanagawa Y (2002) Suppression of endotoxin-induced renal tumor necrosis factor-α and interleukin-6 mRNA by renin-angiotensin system inhibitors. Jpn J Pharmacol 88(2):139–145
Lee HY, Noh HJ, Gang JG, Xu ZG, Jeong HJ, Kang SW, Choi KH, Han DS (2002) Inducible nitric oxide synthase (iNOS) expression is increased in lipopolysaccharide (LPS)-stimulated diabetic rat glomeruli: effect of ACE inhibitor and angiotensin II receptor blocker. Yonsei Med J 43(2):183–192
Pa B (1994) Function and activation of NF-kappa B in the immune system. Annu Rev Immunol 12:141–179
Becker MD, Garman K, Whitcup SM, Planck SR, Rosenbaum JT (2001) Inhibition of leukocyte sticking and infiltration, but not rolling, by antibodies to ICAM-1 and LFA-1 in murine endotoxin–induced uveitis. Invest Ophthalmol Vis Sci 42(11):2563–2566
Nappo F, Esposito K, Cioffi M, Giugliano G, Molinari AM, Paolisso G, Marfella R, Giugliano D (2002) Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J Am Coll Cardiol 39(7):1145–1150
Monnink SH, Van Haelst PL, Van Boven AJ, Stroes ES, Tio RA, Plokker TW, Smit AJ, Veeger NJ, Crijns HJ, Van Gilst WH (2002) Endothelial dysfunction in patients with coronary artery disease: a comparison of three frequently reported tests. J Investig Med 50(1):19–24
Zhang L, Zalewski A, Liu Y, Mazurek T, Cowan S, Martin JL, Hofmann SM, Vlassara H, Shi Y (2003) Diabetes-induced oxidative stress and low-grade inflammation in porcine coronary arteries. Circulation 108(4):472–478
Carter AM, Grant PJ (1997) Vascular homeostasis, adhesion molecules, and macrovascular disease in non-insulin-dependent diabetes mellitus. Diabet Med 14(6):423–432
Ross R (1999) Atherosclerosis—an inflammatory disease. N Engl J Med 340:115–126
Andros V, Egger A (2006) Blood pressure goal attainment according to JNC 7 guidelines and utilization of antihypertensive drug therapy in MCO patients with type 1 or type 2 diabetes. J Manag Care Pharm 12(4):303–309
Welch WJ (2008) Angiotensin II–dependent superoxide: effects on hypertension and vascular dysfunction. Hypertension 52(1):51–56
Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW (2004) Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension 43(5):993–1002
Geng DF, Wu W, Jin DM, Wang JF, Wu YM (2006) Effect of peroxisome proliferator-activated receptor γ ligand Rosiglitazone on left ventricular remodeling in rats with myocardial infarction. Int J Cardiol 113(1):86–91
Okoli CO, Akah PA, Onuoha NJ, Okoye TC, Nwoye AC, Nworu CS (2008) Acanthus montanus: An experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement Altern Med 8:1–11
Hua XY, Chen P, Fox A, Myers RR (1996) Involvement of cytokines in lipopolysaccharide-induced facilitation of CGRP release from capsaicin-sensitive nerves in the trachea: Studies with interleukin-1β and tumor necrosis factor-α. J Neurosci 16(15):4742–4748
Nagai N, Oike Y, Izumi-Nagai K, Urano T, Kubota Y, Noda K, Ozawa Y, Inoue M, Tsubota K, Suda T, Ishida S (2006) Angiotensin II type 1 receptor–mediated inflammation is required for choroidal neovascularization. Arterioscler Thromb Vasc Biol 26(10):2252–2259
Morsy M, Ashour O, Amin E, Rofaeil R (2009) Gastroprotective effects of telmisartan on experimentally-induced gastric ulcers in rats. Die Pharmazie Int Pharm Sci 64(9):590–594
Pavel J, Benicky J, Murakami Y, Sanchez-Lemus E, Saavedra JM (2008) Peripherally administered angiotensin II AT1 receptor antagonists are anti-stress compounds in vivo. Ann N Y Acad Sci 1148(1):360–366
Blessing E, Preusch M, Kranzhöfer R, Kinscherf R, Marx N, Rosenfeld ME, Isermann B, Weber CM, Kreuzer J, Gräfe J, Katus HA (2008) Anti-atherosclerotic properties of telmisartan in advanced atherosclerotic lesions in apolipoprotein E deficient mice. Atherosclerosis 199(2):295–303
Al-Hejjaj WK, Numan IT, Al-Sa’ad RZ, Hussain SA (2011) Anti-inflammatory activity of telmisartan in rat models of experimentally-induced chronic inflammation: Comparative study with dexamethasone. Saudi Pharm J 19(1):29–34
Nakano A, Hattori Y, Aoki C, Jojima T, Kasai K (2009) Telmisartan inhibits cytokine-induced nuclear factor-κB activation independently of the peroxisome proliferator-activated receptor-γ. Hypertens Res 32(9):765–769
Araújo AA, Souza TO, Moura LM, Brito GA, Aragão KS, Araújo LS, Medeiros CA, Alves MS, Araújo RF Jr (2013) Effect of telmisartan on levels of IL-1, TNF-α, down-regulated COX-2, MMP-2, MMP-9 and RANKL/RANK in an experimental periodontitis model. J Clin Periodontol 40(12):1104–1111
Closa D, Folch-Puy E (2004) Oxygen free radicals and the systemic inflammatory response. IUBMB Life 56(4):185–191
Shahid M, Raish M, Ahmad A, Bin Jardan YA, Ansari MA, Ahad A, Alkharfy KM, Alaofi AL, Al-Jenoobi FI (2022) Sinapic acid ameliorates acetic acid-induced ulcerative colitis in rats by suppressing inflammation, oxidative stress, and apoptosis. Molecules 27(13):4139
Arab HH, Al-Shorbagy MY, Abdallah DM, Nassar NN (2014) Telmisartan attenuates colon inflammation, oxidative perturbations and apoptosis in a rat model of experimental inflammatory bowel disease. PLoS ONE 9(5):e97193
Desreumaux P, Ernst O, Geboes K, Gambiez L, Berrebi D, Müller-Alouf H, Hafraoui S, Emilie D, Ectors N, Peuchmaur M, Cortot A (1999) Inflammatory alterations in mesenteric adipose tissue in Crohn’s disease. Gastroenterology 117(1):73–81
Crohn BB, Ginzburg L, Oppenheimer GD (1932) Regional ileitis: a pathologic and clinical entity. J Am Med Assoc 99(16):1323–1329
Zulian A, Cancello R, Micheletto G, Gentilini D, Gilardini L, Danelli P, Invitti C (2011) Visceral adipocytes: old actors in obesity and new protagonists in Crohn’s disease? Gut 61(1):86–94
Peyrin-Biroulet L, Gonzalez F, Dubuquoy L, Rousseaux C, Dubuquoy C, Decourcelle C, Saudemont A, Tachon M, Béclin E, Odou MF, Neut C (2012) Mesenteric fat as a source of C reactive protein and as a target for bacterial translocation in Crohn’s disease. Gut 61(1):78–85
Paul G, Schäffler A, Neumeier M, Fürst A, Bataillle F, Buechler C, Müller-Ladner U, Schölmerich J, Rogler G, Herfarth H (2006) Profiling adipocytokine secretion from creeping fat in Crohn’s disease. Inflamm Bowel Dis 12(6):471–477
Chen Y, Siegel F, Kipschull S, Haas B, Fröhlich H, Meister G, Pfeifer A (2013) miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat Commun 4(1):1769
Liu S, Yang Y, Wu J (2011) TNFα-induced up-regulation of miR-155 inhibits adipogenesis by down-regulating early adipogenic transcription factors. Biochem Biophys Res Commun 414(3):618–624
Salmenkari H, Korpela R, Vapaatalo H (2021) Renin–angiotensin system in intestinal inflammation—Angiotensin inhibitors to treat inflammatory bowel diseases? Basic Clin Pharmacol Toxicol 129(3):161–172
Li Y, Zuo L, Zhu W, Gong J, Zhang W, Guo Z, Gu L, Li N, Li J (2015) Telmisartan attenuates the inflamed mesenteric adipose tissue in spontaneous colitis by mechanisms involving regulation of neurotensin/microRNA-155 pathway. Biochem Pharmacol 93(4):461–469
Grønli J, Murison R, Fiske E, Bjorvatn B, Sørensen E, Portas CM, Ursin R (2005) Effects of chronic mild stress on sexual behavior, locomotor activity and consumption of sucrose and saccharine solutions. Physiol Behav 84(4):571–577
Kishi T, Hirooka Y, Sunagawa K (2012) Telmisartan protects against cognitive decline via up-regulation of brain-derived neurotrophic factor/tropomyosin-related kinase B in hippocampus of hypertensive rats. J Cardiol 60(6):489–494
Salim S (2017) Oxidative stress and the central nervous system. J Pharmacol Exp Ther 360(1):201–205
Segev A, Rubin AS, Abush H, Richter-Levin G, Akirav I (2014) Cannabinoid receptor activation prevents the effects of chronic mild stress on emotional learning and LTP in a rat model of depression. Neuropsychopharmacology 39(4):919–933
Li S, Yang C, Wu Z, Chen Y, He X, Liu R, Ma W, Deng S, Li J, Liu Q, Wang Y (2023) Suppressive effects of bilobalide on depression-like behaviors induced by chronic unpredictable mild stress in mice. Food Funct 14(18):8409–8419
Ababei DC, Bild V, Macadan I, Vasincu A, Rusu RN, Blaj M, Stanciu GD, Lefter RM, Bild W (2023) Therapeutic implications of renin-angiotensin system modulators in Alzheimer’s dementia. Pharmaceutics 15(9):2290
Ding L, Zhang X, Guo H, Yuan J, Li S, Hu W, Golden T, Wu N (2015) The functional study of a Chinese herbal compounded antidepressant medicine–Jie Yu Chu Fan capsule on chronic unpredictable mild stress mouse model. PLoS ONE 10(7):e0133405
Li J, Yang R, Xia K, Wang T, Nie B, Gao K, Chen J, Zhao H, Li Y, Wang W (2018) Effects of stress on behavior and resting-state fMRI in rats and evaluation of Telmisartan therapy in a stress-induced depression model. BMC Psychiatry 18(1):1–13
Graus-Nunes F, de Oliveira Santos F, de Souza Marinho T, Miranda CS, Barbosa-da-Silva S, Souza-Mello V (2019) Beneficial effects of losartan or telmisartan on the local hepatic renin-angiotensin system to counter obesity in an experimental model. World J Hepatol 11(4):359
Fox MD, Raichle ME (2007) Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci 8(9):700–711
Marona-Lewicka D, Vetulani J (1989) Neurochemical correlates of differences in responses to psychotropic drugs. I. Apomorphine and morphine effects on locomotor activity of C57/BL and Balb/C mice. Pol J Pharmacol Pharm 41(5):431–438
Nasr SJ, Crayton JW, Agarwal B, Wendt B, Kora R (2011) Lower frequency of antidepressant use in patients on renin-angiotensin-aldosterone system modifying medications. Cell Mol Neurobiol 31:615–618
Ji MJ, Yu XB, Mei ZL, An YQ, Tang SS, Hu M, Long Y, Miao MX, Hu QH, Sun HB, Kong LY (2016) Hippocampal PPARδ overexpression or activation represses stress-induced depressive behaviors and enhances neurogenesis. Int J Neuropsychopharm 19(1):pyv083
Kishi T, Hirooka Y, Sunagawa K (2012) Telmisartan protects against cognitive decline via up-regulation of brain-derived neurotrophic factor/tropomyosin-related kinase B in the hippocampus of hypertensive rats. J Cardiol 60(6):489–494
Devinsky O (2003) Psychiatric comorbidity in patients with epilepsy: implications for diagnosis and treatment. Epilepsy Behav 4:2–10
Li Y, Cheng KC, Liu KF, Peng WH, Cheng JT, Niu HS (2017) Telmisartan activates PPARδ to improve symptoms of unpredictable chronic mild stress-induced depression in mice. Sci Rep 7(1):14021
Brattiya K, Sivaraman M (2020) Evaluation of antidepressant effect of angiotensin receptor blocker telmisartan in albino mice. Int J Basic Clin Pharmacol 9(3):446
Hosseini M, Alaei H, Sharifi MR, Shafei MN (2007) The effects of angiotensin II and captopril on the expression of morphine withdrawal signs in rats. Iran J Pharm Res 6(3):185–191
Pushpa VH, Shetty P, Suresha RN, Jayanthi MK, Ashwini V, Vaibhavi PS (2014) Evaluation and comparison of anticonvulsant activity of telmisartan and olmesartan in experimentally induced animal models of epilepsy. J Clin Diagn Res JCDR 8(10):HC08
Culman J, Blume A, Gohlke P, Unger T (2002) The renin-angiotensin system in the brain: possible therapeutic implications for AT1-receptor blockers. J Hum Hypertens 16(3):S64–S70
Latchford KJ (2008) Electrophysiological effects of angiotensin II on hypothalamic paraventricular nucleus neurons of the rat (Doctoral dissertation)
Sánchez-Lemus E, Honda M, Saavedra JM (2012) Angiotensin II AT1 receptor blocker candesartan prevents the fast up-regulation of cerebrocortical benzodiazepine-1 receptors induced by acute inflammatory and restraint stress. Behav Brain Res 232(1):84–92
Rao RS, Prakash A, Medhi B (2009) Role of different cytokines and seizure susceptibility: a new dimension towards epilepsy research
Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM (2012) Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS ONE 7(7):e41229
Kahle KT, Walcott BP, Simard JM (2013) Continuous hyperosmolar therapy for traumatic brain injury-associated cerebral edema: as good as it gets, or an iatrogenic secondary insult? J Clin Neurosci 20(1):30–31
Tran S, Kuruppu S, Rajapakse NW (2022) Chronic renin-angiotensin System Activation induced neuroinflammation: common mechanisms underlying hypertension and dementia? J Alzheimers Dis 85(3):943–955
Villapol S, Saavedra JM (2015) Neuroprotective effects of angiotensin receptor blockers. Am J Hypertens 28(3):289–299
Kono S, Kurata T, Sato K, Omote Y, Hishikawa N, Yamashita T, Deguchi K, Abe K (2015) Neurovascular protection by telmisartan via reducing neuroinflammation in stroke-resistant spontaneously hypertensive rat brain after ischemic stroke. J Stroke Cerebrovasc Dis 24(3):537–547
Jung KH, Chu K, Lee ST, Kim SJ, Song EC, Kim EH, Park DK, Sinn DI, Kim JM, Kim M, Roh JK (2007) Blockade of AT1 receptor reduces apoptosis, inflammation, and oxidative stress in normotensive rats with intracerebral hemorrhage. J Pharmacol Exp Ther 322(3):1051–1058
Gohlke P, Weiss S, Jansen A, Wienen W, Stangier J, Rascher W, Culman J, Unger T (2001) AT1 receptor antagonist telmisartan administered peripherally inhibits central responses to angiotensin II in conscious rats. J Pharmacol Exp Ther 298(1):62–70
Wei X, Hu CC, Zhang YL, Yao SL, Mao WK (2016) Telmisartan reduced cerebral edema by inhibiting NLRP3 inflammasome in mice with cold brain injury. J Huazhong Univ Sci Technol Med Sci 36(4):576–583
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219
Abdul-Muneer PM, Chandra N, Haorah J (2015) Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Mol Neurobiol 51:966–979
Fann YW, Lee SY, Manzanero S, Tang SC, Gelderblom M, Chunduri P, Bernreuther C, Glatzel M, Cheng YL, Thundyil J, Widiapradja A (2013) Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis 4(9):e790–e790
Ismael S, Ahmed HA, Adris T, Parveen K, Thakor P, Ishrat T (2021) The NLRP3 inflammasome: a potential therapeutic target for traumatic brain injury. Neural Regen Res 16(1):49
Baxter AJ, Scott KM, Vos T, Whiteford HA (2013) Global prevalence of anxiety disorders: a systematic review and meta-regression. Psychol Med 43(5):897–910
Okuyama S, Sakagawa T, Inagami T (1999) Role of the angiotensin II type-2 receptor in the mouse central nervous system. Jpn J Pharm 81(3):259–263
Braszko JJ, Winnicka MM (2003) Effects of angiotensin II and its receptor antagonists on. Control 2:271–281
Sánchez-Lemus E, Benicky J, Pavel J, Saavedra JM (2009) In vivo Angiotensin II AT1 receptor blockade selectively inhibits LPS-induced innate immune response and ACTH release in rat pituitary gland. Brain Behav Immun 23(7):945–957
Toba H, Tojo C, Wang J, Noda K, Kobara M, Nakata T (2012) Telmisartan inhibits vascular dysfunction and inflammation via activation of peroxisome proliferator-activated receptor-γ in subtotal nephrectomized rat. Eur J Pharmacol 685(1–3):91–98
Evans T, Sany O, Pearmain P, Ganesan R, Blann A, Sundar S (2011) Differential trends in the rising incidence of endometrial cancer by type: data from a UK population-based registry from 1994 to 2006. Br J Cancer 104(9):1505–1510
Obel JC, Friberg G, Fleming GF (2006) Chemotherapy in endometrial cancer. Clin adv hematol Oncol HO 4(6):459–468
Ota K, Ito K, Suzuki T, Saito S, Tamura M, Hayashi SI, Okamura K, Sasano H, Yaegashi N (2006) Peroxisome proliferator-activated receptor γ and growth inhibition by its ligands in uterine endometrial carcinoma. Clin Cancer Res 12(14):4200–4208
Ren P, Zhang Y, Huang Y, Yang Y, Jiang M (2015) Functions of peroxisome proliferator-activated receptor gamma (PPARγ) in gynecologic disorders. Clin Med Insights Oncol 9:23527
Bernstein LM, Kvatchevskaya J, Poroshina TE, Kovalenko IG, Tsyrlina EV, Zimarina TS, Ourmantcheeva AF, Ashrafian L, Thijssen JHH (2004) Insulin resistance, its consequences for the clinical course of the disease, and possibilities of correction in endometrial cancer. J Cancer Res Clin Oncol 130:687–693
Chen LM, Berek JS (2013) Endometrial carcinoma: Clinical features and diagnosis. UpToDate. In: Goff B, editor. UpToDate. MA: Waltham
Koyama N, Nishida Y, Ishii T, Yoshida T, Furukawa Y, Narahara H (2014) Telmisartan induces growth inhibition, DNA double-strand breaks, and apoptosis in human endometrial cancer cells. PLoS ONE 9(3):e93050
Sarraf P, Mueller E, Jones D, King FJ, DeAngelo DJ, Partridge JB, Holden SA, Chen LB, Singer S, Fletcher C, Spiegelman BM (1998) Differentiation and reversal of malignant changes in colon cancer through PPARγ. Nat Med 4(9):1046–1052
Lee LD, Mafura B, Lauscher JC, Seeliger H, Kreis ME, Gröne J (2014) Antiproliferative and apoptotic effects of telmisartan in human colon cancer cells. Oncol Lett 8(6):2681–2686
Fajas L, Fruchart JC, Auwerx J (1998) PPARγ3 mRNA: a distinct PPARγ mRNA subtype transcribed from an independent promoter. FEBS Lett 438(1–2):55–60
Mielczarek-Puta M, Otto-Ślusarczyk D, Chrzanowska A, Filipek A, Graboń W (2020) Telmisartan influences the antiproliferative activity of linoleic acid in human colon cancer cells. Nutr Cancer 72(1):98–109
Kato M, Kusumi T, Tsuchida S, Tanaka M, Sasaki M, Kudo H (2004) Induction of differentiation and peroxisome proliferator-activated receptor γ expression in colon cancer cell lines by troglitazone. J Cancer Res Clin Oncol 130:73–79
Forner A, Llovet JM, Bruix J (2012) Hepatocellularcarcinoma. Lancet 379(9822):1245
Altekruse SF, Henley JS, Cucinelli JE, McGlynn KA (2014) Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Off J Am Coll Gastroenterol 109(4):542–553
Morishita A, Iwama H, Fujihara S, Sakamoto T, Fujita K, Tani J, Miyoshi H, Yoneyama H, Himoto T, Masaki T (2016) MicroRNA profiles in various hepatocellular carcinoma cell lines. Oncol Lett 12(3):1687–1692
Kozako T, Soeda S, Yoshimitsu M, Arima N, Kuroki A, Hirata S, Tanaka H, Imakyure O, Tone N, Honda SI, Soeda S (2016) Angiotensin II type 1 receptor blocker telmisartan induces apoptosis and autophagy in adult T-cell leukemia cells. FEBS Open Bio 6(5):442–460
Hou Y, Liu R, Xia M, Sun C, Zhong B, Yu J, Ai N, Lu JJ, Ge W, Liu B, Chen X (2021) Nannocystin ax, an eEF1A inhibitor, induces G1 cell cycle arrest and caspase-independent apoptosis through cyclin D1 downregulation in colon cancer in vivo. Pharmacol Res 173:105870
Kurokawa H, Sugiyama S, Nozaki T, Sugamura K, Toyama K, Matsubara J, Fujisue K, Ohba K, Maeda H, Konishi M, Akiyama E (2015) Telmisartan enhances mitochondrial activity and alters cellular functions in human coronary artery endothelial cells via AMP-activated protein kinase pathway. Atherosclerosis 239(2):375–385
Myojo M, Nagata D, Fujita D, Kiyosue A, Takahashi M, Satonaka H, Morishita Y, Akimoto T, Nagai R, Komuro I, Hirata Y (2014) Telmisartan activates endothelial nitric oxide synthase via Ser1177 phosphorylation in vascular endothelial cells. PLoS ONE 9(5):e96948
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24(14):2137–2150
Vo C, Carney ME (2007) Ovarian cancer hormonal and environmental risk effect. Obstet Gynecol Clin North Am 34(4):687–700
Jiang WG, Redfern A, Bryce RP, Mansel RE (2000) Peroxisome proliferator-activated receptor-γ (PPAR-γ) mediates the action of gamma linolenic acid in breast cancer cells. Prostaglandins Leukotrienes and Essential Fatty Acids (PLEFA) 62(2):119–127
De Araujo Junior RF, Leitão Oliveira ALC, de Melo Silveira RF, de Oliveira Rocha HA, de França Cavalcanti P, de Araujo AA (2015) Telmisartan induces apoptosis and regulates Bcl-2 in human renal cancer cells. Exp Biol Med 240(1):34–44
Mozzetti S, Ferlini C, Concolino P, Filippetti F, Raspaglio G, Prislei S, Gallo D, Martinelli E, Ranelletti FO, Ferrandina G, Scambia G (2005) Class III β-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin Cancer Res 11(1):298–305
Komar CM (2005) Peroxisome proliferator-activated receptors (PPARs) and ovarian function–implications for regulating steroidogenesis, differentiation, and tissue remodeling. Reprod Biol Endocrinol 3(1):1–14
Maejima Y, Okada H, Haraguchi G, Onai Y, Kosuge H, Suzuki JI, Isobe M (2011) Telmisartan, a unique ARB, improves left ventricular remodeling of infarcted heart by activating PPAR gamma. Lab Invest 91(6):932–944
Zafiriou S, Stanners SR, Saad S, Polhill TS, Poronnik P, Pollock CA (2005) Pioglitazone inhibits cell growth and reduces matrix production in human kidney fibroblasts. J Am Soc Nephrol 16(3):638–645
Yokota T, Osanai T, Hanada K, Kushibiki M, Abe N, Oikawa K, Tomita H, Higuma T, Yokoyama J, Hanada H, Okumura K (2010) Effects of telmisartan on markers of ventricular remodeling in patients with acute myocardial infarction: comparison with enalapril. Heart Vessels 25:460–468
Pu Z, Zhu M, Kong F (2016) Telmisartan prevents proliferation and promotes apoptosis of human ovarian cancer cells through upregulating PPARγ and downregulating MMP 9 expression. Mol Med Rep 13(1):555–559
Pathade PA, Ahire YS, Bairagi VA, Abhang DR (2011) Antioxidants therapy in cognitive dysfunction associated with diabetes mellitus: an overview. Res J Pharmacol Pharmacodyn 3(2):39–44
Bairagi VA, Sadu N, Senthilkumar KL, Ahire Y (2012) Anti-diabetic potential of Quisqualis indica linn in rats. Int J Pharm Phytopharmacol Res 1:166–171
Pawar AY, Hiray AP, Sonawane DD, Bhambar RS, Derle DV, Ahire YS (2020) Convalescent plasma: a possible treatment protocol for COVID-19 patients suffering from diabetes or underlying liver diseases. Diabetes Metab Syndr 14(4):665–669
Ghaisas MM, Ahire YS, Dandawate PR, Gandhi SP, Mule M (2011) Effects of combination of thiazolidinediones with melatonin in dexamethasone-induced insulin resistance in mice. Indian J Pharm Sci 73(6):601
Ahire YS, Ghaisas MM, Dandawate PR, Gandhi SP (2013) Beneficial effects of co-administration of PPAR-γ agonist with melatonin on cardiovascular complications associated with diabetes. Chron Young Sci 4(1):59
Griendling KK, Ushio-Fukai M (2000) Reactive oxygen species as mediators of angiotensin II signaling. Regul Pept 91:21–27
Reutens AT, Atkins RC (2011) Epidemiology of diabetic nephropathy. Contrib Nephrol. Basel, Karger 170:1–7
Ruggenenti P, Cravedi P, Remuzzi G (2010) The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 6:319–330
Khundmiri SJ, Dean WL, McLeish KR, Lederer ED (2005) Parathyroid hormone-mediated regulation of Na+ -K+ -ATPase requires ERK-dependent translocation of protein kinase Calpha. J Biol Chem 280:8705–8713
Liang M, Knox FG (1999) Nitric oxide activates PKC-alpha and inhibits Na+ -K+ -ATPase in opossum kidney cells. Am J Physiol 277:F859–F865
Menne J, Park JK, Boehne M, Elger M, Lindschau C, Kirsch T et al (2004) Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-α-deficient diabetic mice. Diabetes 53:2101–2109
Yao L, Huang DY, Pfaff IL, Nie X, Leitges M, Vallon V (2004) Evidence for a role of protein kinase C alpha in urine concentration. Am J Physiol Renal Physiol 287:F299-304
Wang Y, Xue J, Li Y, Zhou X, Qiao S, Han D (2019) Telmisartan protects against high glucose/high lipid-induced apoptosis and insulin secretion by reducing the oxidative and ER stress. Cell Biochem Funct 37(3):161–168
Sato-Horiguchi C, Ogawa D, Wada J, Tachibana H, Kodera R, Eguchi J, Nakatsuka A, Terami N, Shikata K, Makino H (2013) Telmisartan attenuates diabetic nephropathy by suppressing oxidative stress in db/db mice. Nephron Exp Nephrol 121(3–4):e97–e108
Brezniceanu ML, Lau CJ, Godin N, Chénier I, Duclos A, Éthier J, Filep JG, Ingelfinger JR, Zhang SL, Chan JS (2010) Reactive oxygen species promote caspase-12 expression and tubular apoptosis in diabetic nephropathy. J Am Soc Nephrol 21(6):943
Lin M, Yiu WH, Wu HJ, Chan LY, Leung JC, Au WS, Chan KW, Lai KN, Tang SC (2012) Toll-like receptor 4 promotes tubular inflammation in diabetic nephropathy. J Am Soc Nephrol 23(1):86
Korsunov SS, Skulachev VP, Starkov AA (1997) High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett 416:15–18. https://doi.org/10.1016/S0014-5793(97)01159-9
Du XL, Edelstein D, Dimmeler S, Ju Q, Sui C, Brownlee M (2001) Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. J Clin Investig 108:1341–1348. https://doi.org/10.1172/JCI11235
Doublier S, Salvidio G, Lupia E, Ruotsalainen V, Verzola D, Deferrari G, Camussi G (2003) Nephrin expression is reduced in human diabetic nephropathy: evidence for a distinct role for glycated albumin and angiotensin II. Diabetes 52:1023–1030. https://doi.org/10.2337/diabetes.52.4.1023
Zhang Q, Xiao X, Li M, Li W, Yu M, Zhang H, Sun X, Mao L, Xiang H (2012) Telmisartan improves kidney function through inhibition of the oxidative phosphorylation pathway in diabetic rats. J Mol Endocrinol 49(1):35
Lundborg G (2000) A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg 25(3):391–414
Akishita M, Nagai K, Xi H, Yu W, Sudoh N, Watanabe T, Ohara-Imaizumi M, Nagamatsu S, Kozaki K, Horiuchi M, Toba K (2005) Renin-angiotensin system modulates oxidative stress–induced endothelial cell apoptosis in rats. Hypertension 45(6):1188–1193
Culman J, Nguyen-Ngoc M, Glatz T, Gohlke P, Herdegen T, Zhao Y (2012) Treatment of rats with pioglitazone in the reperfusion phase of focal cerebral ischemia: a preclinical stroke trial. Exp Neurol 238(2):243–253
Schiffrin EL, Amiri F, Benkirane K, Iglarz M, Diep QN (2003) Peroxisome proliferator-activated receptors: vascular and cardiac effects in hypertension. Hypertension 42(4):664–668
Yuksel TN, Halici Z, Demir R, Cakir M, Calikoglu C, Ozdemir G, Unal D (2015) Investigation of the effect of telmisartan on experimentally induced peripheral nerve injury in rats. Int J Neurosci 125(6):464–473
Muroya M, Chang K, Uchida K, Bougaki M, Yamada Y (2012) Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci Trends 6(2):70–80
Davies NM, Kehoe PG, Ben-Shlomo Y, Martin RM (2011) Associations of anti-hypertensive treatments with Alzheimer’s disease, vascular dementia, and other dementias. J Alzheimers Dis 26(4):699–708
Haack KK, Mitra AK, Zucker IH (2013) NF-κB and CREB are required for angiotensin II type 1 receptor upregulation in neurons. PLoS ONE 8(11):e78695
Clark MA, Gonzalez N (2007) Angiotensin II stimulates rat astrocyte mitogen-activated protein kinase activity and growth through EGF and PDGF receptor transactivation. Regul Pept 144(1–3):115–122
Hegazy N, Rezq S, Fahmy A (2020) Mechanisms involved in the superiority of angiotensin receptor blockade over ACE inhibition in attenuating neuropathic pain induced in rats. Neurotherapeutics 17:1031–1047
Freeman RS, Burch RL, Crowder RJ, Lomb DJ, Schoell MC, Straub JA, Xie L (2004) NGF deprivation-induced gene expression: after ten years, where do we stand? Prog Brain Res 146:111–126
Ola MS, Ahmed MM, Abuohashish HM, Al-Rejaie SS, Alhomida AS (2013) Telmisartan ameliorates neurotrophic support and oxidative stress in the retina of streptozotocin-induced diabetic rats. Neurochem Res 38:1572–1579
Maier R, Weger M, Haller-Schober EM, El-Shabrawi Y, Wedrich A, Theisl A, Aigner R, Barth A, Haas A (2008) Multiplex bead analysis of vitreous and serum concentrations of inflammatory and proangiogenic factors in diabetic patients. Mol Vis 14:637
Behl T, Kotwani A (2017) Potential of angiotensin II receptor blockers in the treatment of diabetic retinopathy. Life Sci 176:1–9
Cheng J, Zhang W, Zhang X, Han F, Li X, He X, Li Q, Chen J (2014) Effect of angiotensin-converting enzyme inhibitors and angiotensin II receptor blockers on all-cause mortality, cardiovascular deaths, and cardiovascular events in patients with diabetes mellitus: a meta-analysis. JAMA Intern Med 174(5):773–785
Nagai N, Izumi-Nagai K, Oike Y, Koto T, Satofuka S, Ozawa Y, Yamashiro K, Inoue M, Tsubota K, Umezawa K, Ishida S (2007) Suppression of diabetes-induced retinal inflammation by blocking the angiotensin II type 1 receptor or its downstream nuclear factor-κB pathway. Invest Ophthalmol Vis Sci 48(9):4342–4350
Kurihara T, Ozawa Y, Nagai N, Shinoda K, Noda K, Imamura Y, Tsubota K, Okano H, Oike Y, Ishida S (2008) Angiotensin II type 1 receptor signaling contributes to synaptophysin degradation and neuronal dysfunction in the diabetic retina. Diabetes 57(8):2191–2198
Grammatopoulos TN, Jones SM, Ahmadi FA, Hoover BR, Snell LD, Skoch J, Jhaveri VV, Poczobutt AM, Weyhenmeyer JA, Zawada WM (2007) Angiotensin type 1 receptor antagonist losartan, reduces MPTP-induced degeneration of dopaminergic neurons in substantia nigra. Mol Neurodegener 2:1–17
Mertens B, Varcin M, Michotte Y, Sarre S (2011) The neuroprotective action of candesartan is related to interference with the early stages of 6-hydroxydopamine-induced dopaminergic cell death. Eur J Neurosci 34(7):1141–1148
Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K (2010) Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia 53:971–979
Seki M, Tanaka T, Nawa H, Usui T, Fukuchi T, Ikeda K, Abe H, Takei N (2004) Involvement of brain-derived neurotrophic factor in early retinal neuropathy of streptozotocin-induced diabetes in rats: therapeutic potential of brain-derived neurotrophic factor for dopaminergic amacrine cells. Diabetes 53(9):2412–2419
Cao Z, Zong Y, Yang C, Yang X, Wang Y, Sun Z, Jiang T (2019) Inhibitory effect of Telmisartan on STZ-induced early retinopathy in diabetic mice
Garcia PJM, Marin-Castaño ME (2014) Angiotensin II-related hypertension and eye diseases. World J Cardiol 6(9):968
Pietzsch M, Theuer S, Haase G, Plath F, Keyser M, Riethling AK (2002) Results of systematic screening for serious gastrointestinal bleeding associated with NSAIDs in Rostock hospitals. Int J Clin Pharmacol Ther 40:111–115
Boehme MW, Autschbach F, Ell C, Raeth U (2007) Prevalence of silent gastric ulcer, erosions or severe acute gastritis in patients with type 2 diabetes mellitus—a cross sectional study. Hepatogastroenterology 54:643–648
Perdichizzi G, Bottari M, Pallio S, Fera MT, Carbone M, Barresi G (1996) Gastric infection by Helicobacter pylori and antral gastritis in hyperglycemic obese and in diabetic subjects. New Microbiol 19:149–154
Tashima K, Korolkiewicz R, Kubomi M, Takeuchi K (1998) Increased susceptibility of gastric mucosa to ulcerogenic stimulation in diabetic rats–role of capsaicin-sensitive sensory neurons. Br J Pharmacol 124(7):1395–1402
Cai H, Griendling KK, Harrison DG (2003) The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci 24:471–478
Nakamura T, Keep RF, Hua Y, Hoff JT, Xi G (2005) Oxidative DNA injury after experimental intracerebral hemorrhage. Brain Res 1039:30–36
Blessing E, Preusch M, Kranzhöfer R, Kinscherf R, Marx N, Rosenfeld ME, Isermann B, Weber CM, Kreuzer J, Gräfe J, Katus HA (2008) Anti-atherosclerotic properties of telmisartan in advanced atherosclerotic lesions in apolipoprotein E deficient mice. Atherosclerosis 199:295–303
Konturek PC, Brzozowski T, Kania J, Kukharsky V, Bazela K, Kwiecien S, Harsch I, Konturek SJ (2003) Pioglitazone, a specific ligand of the peroxisome proliferator-activated receptor gamma reduces gastric mucosal injury induced by ischaemia/reperfusion in rat. Scand J Gastroenterol 38:468–476
Faubion WA, Gores GJ (1999) Death receptors in liver biology and pathobiology. Hepatology 29:1–4
Kaschina E, Schrader F, Sommerfeld M, Kemnitz UR, Grzesiak A, Krikov M, Unger T (2008) Telmisartan prevents aneurysm progression in the rat by inhibiting proteolysis, apoptosis, and inflammation. J Hypertens 26:2361–2373
Li Y, Zuo L, Zhu W, Gong J, Zhang W, Guo Z, Gu L, Li N, Li J (2015) Telmisartan attenuates the inflamed mesenteric adipose tissue in spontaneous colitis by mechanisms involving regulation of neurotensin/microRNA-155 pathway. Biochem pharmacol 93(4):461–469
Wild J, Schüler R, Knopp T, Molitor M, Kossmann S, Münzel T, Daiber A, Waisman A, Wenzel P, Karbach SH (2019) Telmisartan lowers elevated blood pressure in psoriatic mice without attenuating vascular dysfunction and inflammation. Int J Mol Sci 20(17):4261
Balaji SP, Chand CV, Justin A, Ramanathan M (2015) Telmisartan mediates anti-inflammatory and not cognitive function through PPAR-γ agonism via SARM and MyD88 signaling. Pharmacol Biochem Behav 137:60–68
Arumugam S, Sreedhar R, Thandavarayan RA, Giridharan VV, Karuppagounder V, Pitchaimani V, Afrin MR, Miyashita S, Nomoto M, Harima M, Suzuki H (2015) Telmisartan treatment targets inflammatory cytokines to suppress the pathogenesis of acute colitis induced by dextran sulphate sodium. Cytokine 74(2):305–312
Al-Hejjaj WK, Numan IT, Al-Sa’ad RZ, Hussain SA (2011) Anti-inflammatory activity of telmisartan in rat models of experimentally-induced chronic inflammation: Comparative study with dexamethasone. Saudi Pharma J 19(1):29–34
Swetha ES. Research article evaluation of antianxiety activity of angiotensin receptor blockers in Albino Mice
Mielczarek-Puta M, Otto-Ślusarczyk D, Chrzanowska A, Filipek A, Graboń W (2020) Telmisartan influences the antiproliferative activity of linoleic acid in human colon cancer cells. Nutr Cancer 72(1):98–109
Rasheduzzaman M, Moon JH, Lee JH, Nazim UM, Park SY (2018) Telmisartan generates ROS-dependent upregulation of death receptor 5 to sensitize TRAIL in lung cancer via inhibition of autophagy flux. Int J Biochem Cell Biol 102:20–30
Pu Z, Zhu M, Kong F (2016) Telmisartan prevents proliferation and promotes apoptosis of human ovarian cancer cells through upregulating PPARγ and downregulating MMP 9 expression. Mol Med Reports 13(1):555–559
Yao LJ, Wang JQ, Zhao H, Liu JS, Deng AG (2007) Effect of telmisartan on expression of protein kinase C-α in kidneys of diabetic mice 1. Acta Pharmacologica Sinica 28(6):829–838
Fouad AA, Al-Sultan AI, Yacoubi MT, Gomaa W (2010) Ameliorative effects of telmisartan in diabetic rats with indomethacin-induced gastric ulceration. Euro J Pharmacol 637(1–3):162–170
Cianchetti S, Del Fiorentino A, Colognato R, Di Stefano R, Franzoni F, Pedrinelli R (2008) Anti-inflammatory and anti-oxidant properties of telmisartan in cultured human umbilical vein endothelial cells. Atherosclerosis 198(1):22–28
Kurihara T, Ozawa Y, Shinoda K, Nagai N, Inoue M, Oike Y, Tsubota K, Ishida S, Okano H (2006) Neuroprotective effects of angiotensin II type 1 receptor (AT1R) blocker, telmisartan, via modulating AT1R and AT2R signaling in retinal inflammation. Invest Ophthalmol Vis Sci 47(12):5545–5552
Acknowledgements
Not applicable.
Funding
No funding in design of the study and collection, analysis, and interpretation of data and in writing the manuscript has been utilized.
Author information
Authors and Affiliations
Contributions
Each author has made substantial contributions to the acquisition, analysis, and interpretation of data, and all authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable for this work.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ahire, Y.S., Bairagi, V.A., Somavanshi, D.B. et al. Expanding telmisartan’s therapeutic horizon: exploring its multifaceted mechanisms beyond cardiovascular disorders. Futur J Pharm Sci 10, 84 (2024). https://doi.org/10.1186/s43094-024-00655-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00655-9