Abstract
Background
Paclitaxel administration is considered a keystone in the management of many types of cancers. However, paclitaxel chemotherapy often leads to peripheral neuropathy which is the most prominent adverse effect that reduces the patient’s quality of life and demands dose reduction leading to decreased disease curing. Paclitaxel induces peripheral neuropathy through disruption of microtubules, distorted function of ion channels, axonal degeneration, and inflammatory events. So far, there is no standard medication to prevent the incidence of paclitaxel-induced peripheral neuropathy (PIPN).
Main body
Numerous preclinical studies in rats and rodents showed that several therapeutic agents have neuroprotective mechanisms and reduce the incidence of PIPN, proving their effectiveness in the prevention of PIPN in animal models. Different mechanisms, such as reduction of the expression of inflammatory mediators, quenching of reactive oxygen species, prevention of neuronal damage, and other mechanisms, have been explored. Moreover, many clinical trials have further established the neuroprotective effect of several investigational drugs on PIPN. Twenty preclinical studies of pharmacological interventions were reviewed for their preventive effect on neuropathy. These medications targeted cannabinoid receptors, oxidative stress, inflammatory response, and ion channels. Additionally, 25 clinical studies with pharmacological preventive interventions of PIPN have been reviewed, of which only 10 showed preventive action in PIPN.
Conclusion
Prevention of PIPN is currently considered an emergent field of research. This review highlights the potential interventions and presents recent findings from both preclinical and clinical studies on the significant prevention of PIPN to help in effective decision-making. However, further well-designed research is required to ascertain recommendations for clinical practice.
Similar content being viewed by others
Background
Taxanes are considered first-line chemotherapeutic agents often used in several cancers including those of the breast, ovaries, prostate, gastric, head and neck, and non-small lung cancers [1]. Paclitaxel is one of the important and commonly used antineoplastic agents in this class due to its microtubule-stabilizing mechanism of action. It is commonly used in the management of several cancer types with extensive evidence proving its antimitotic effect [2, 3].
One of the prime adverse effects of taxanes including paclitaxel is peripheral neurotoxicity known as peripheral neuropathy [4]. Up to 97% of patients receiving paclitaxel will develop paclitaxel-induced peripheral neuropathy (PIPN), which turns into a chronic condition in more than 60% of cases [5]. It decreases the efficacy of the chemotherapy by causing patient discomfort and often resulting in dosage reduction or chemotherapy treatment termination. Significantly, those who suffer from chronic neuropathy have considerably poorer long-term quality of life [4].
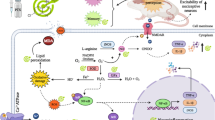
Peripheral neuropathy is a neurological disorder affecting sensory, motor, and autonomic peripheral nerves [6]. Neuropathic pain resulting from paclitaxel administration develops because of somatosensory nerve damage. Mice administered with paclitaxel exhibit elevated activating transcription factor-3 levels in their large and medium dorsal root ganglion (DRG) neurons, which is indicative of neuronal damage [7]. In PIPN, axonal deterioration and depletion of intra-epidermal nerve fibers (IENFs) was reported, showing that DRG injury is the primary cause of taxane-induced nerve damage [8]. Taxane-induced peripheral neuropathy has been linked to several pathophysiological pathways, including oxidative stress, mitochondrial damage, and microtubule disruption [9]. These pathological pathways are demonstrated in Fig. 1.
Paclitaxel alters microtubule dynamics which results in impairment in the passage of nutrients, organelles, and neurotransmitters across the neuronal axon leading to axonal degeneration or axonopathy [10]. Also, mitochondrial dysfunction such as morphological alterations, electrolyte imbalance, and reactive oxygen species (ROS) generation has been considered an important element in PIPN. Furthermore, PIPN in rats was linked to the emergence of behaviors caused by pain and mitochondrial disruption in myelinated fibers and C-fibers [11]. Oxidative stress and inflammatory mediators also have a critical importance in the pathophysiology of PIPN [11, 12]. The number of cycles, length of therapy, patient’s age, use of other neurotoxic medications, as well as the presence of risk factors like diabetes, alcoholism, and previous neuropathy, have been linked to the development of PIPN. Additionally, genetic polymorphism in genes like CYP2C8 (cytochrome P450 family 2 subfamily C member 8) and KCNN3 (potassium calcium-activated channel subfamily N member 3) is also linked to the occurrence of PIPN [13].
The most common clinical presentation of PIPN patients is paranesthesia, tingling, and burning “stock and glove.” In more extreme cases, however, it can lead to loss of sensation, motor deficiencies, and autonomic malfunction. Patients typically exhibit sensory symptoms with a “stocking and glove” description, affecting the extremities and extending toward the proximal body parts. Hyperalgesia and allodynia, due to tactile and heat stimuli, may be experienced [14, 15].
Because of the evolving pathophysiologic pathways mechanisms and the diversity of causes and risk factors, preventive interventions are desperately needed to lower the prevalence of PIPN [16]. Despite emerging evidence, there are no established pharmacological interventions for PIPN prevention. This review aims to map the existing literature on interventions evaluated for the prevention of PIPN. A summary and overview of the types of pharmacological interventions studied through preclinical and clinical trials and their effects on outcomes were reported in this review. Additionally, an insight into the effects of genetic polymorphism on the development of PIPN was also reviewed.
Literature search
We conducted a comprehensive search in several databases, including MEDLINE through PubMed, Web of Science, the Cochrane Library, EBSCOhost, and Scopus. Studies that were published in the English language from year 1999 to year 2023 are included in this review. The search keywords used were “prevention,” “neuropathy,” “paclitaxel,” “neurotoxicity,” “chemotherapy-induced peripheral neuropathy,” “controlled clinical trials,” and “preclinical studies.”
Main text
Preclinical studies for the prevention of paclitaxel-induced peripheral neuropathy
Multiple preclinical studies in rodent models indicate that various pharmacologic agents may offer protective effects against peripheral neurotoxicity induced by paclitaxel.
One study in experimental rats showed that the antianginal trimetazidine reduced apoptosis, oxidative stress, and neuroinflammation which are all effects resulting in axonal degeneration and are linked to recurrent paclitaxel administration. The mode of action was shown to be due to the upregulation of Notch1 and progranulin [17]. Additionally, another study further confirmed PIPN reduction by trimetazidine through modulating toll-like receptor 4 (TLR4)/p38 and Klotho protein expression in Swiss albino mice [18].
The angiotensin-II receptor blocker (ARB) losartan demonstrated protective properties against PIPN in experimental rats. Its administration proved its anti-inflammatory effect on microglia in the central nervous system (CNS) through the reduced expression of inflammatory mediators such as tumor necrosis factor-alpha (TNFα) and interleukin‐6 (IL‐6) and stimulation of peroxisome proliferator‐activated receptor gamma (PPARγ) [19]. Similarly, telmisartan, another ARB, reduced PIPN in mice through its inhibition of cytochrome-p450-epoxygenase (CYP2J6) and the prevention of oxidized lipid synthesis [20]. The involvement of Angiotensin-2 was further demonstrated in the preclinical study where ramipril administration attenuated functional neuropathy secondary to paclitaxel in mouse models [21].
An animal study investigating the potential anti-inflammatory role of hesperidin in PIPN has shown positive results. Hesperidin administration reduced oxidative stress and inflammatory response in nerve tissue secondary to paclitaxel use. This is assumed to be because hesperidin’s antioxidant nature allows it to scavenge reactive oxygen species (ROS) generated from mitochondria, subsequently preventing membrane damage caused by ROS [22].
The antioxidant effects of vitamin C and curcumin have been investigated in suppressing PIPN with demonstrated benefits in the reduction of TNFα and IL‐6 levels in the DRG of rats [23, 24]. Melatonin was found to exhibit similar effects in reducing mitochondrial damage and attenuating PIPN in animal studies [25].
Furthermore, rosuvastatin showed a reduction in pro-inflammatory mediators and oxidative stress in paclitaxel-treated mice [26]. This is speculated to be due to possessing pleiotropic and anti-inflammatory properties, further confirming its anti-neuropathic potential in rat models [27].
Medications used in the treatment of neuropathic pain such as duloxetine, pregabalin, and amitriptyline were found to attenuate PIPN in animal models [28,29,30]. The preventive mechanism was found to be via downregulation of pro-inflammatory biomarkers such as IL-6 and TNFα as well as upregulation of the antioxidant capacity.
Metformin was studied in paclitaxel-treated mice, and the results indicate the effectiveness of metformin in attenuating hyperalgesia priming induced by paclitaxel through its stimulation of adenosine monophosphate-activated protein kinase (AMPK) [31].
On the other hand, the effect of alogliptin, a dipeptidyl peptidase 4 (DPP-4) inhibitor, on chemotherapy-induced peripheral neuropathy was investigated using mice, and its protective effect was demonstrated only against oxaliplatin-induced neurotoxicity but not that induced by paclitaxel nor bortezomib [32].
Moreover, co-treatment with the phosphodiesterase (PDE) inhibitor cilostazol halted the dedifferentiation of Schwann cells, secondary to paclitaxel administration, mediated by cyclic adenosine monophosphate (cAMP) signaling and demyelination in a mixed culture of Schwann cells and DRG neurons [33].
The role of glutamate neurotransmitter and the development of PIPN was confirmed in rat models where the co-administration of valproate suppressed glutamate accumulation and paclitaxel-induced mechanical allodynia [34].
It was previously shown that the acute administration of paclitaxel to neuroblastoma cells in culture increased the binding of the cytoplasmic calcium-binding protein neuronal calcium sensor 1 (NCS-1) to the inositol 1,4,5 tris-phosphate receptor (InsP3R) [35, 36]. A preclinical investigation confirmed that a single prophylactic injection of Ibudilast or lithium might inhibit PIPN in mice before they received paclitaxel therapy. These substances work by interfering with the way paclitaxel, NCS-1, and the InsP3R interact [37].
Clinical studies for the prevention of paclitaxel-induced peripheral neuropathy
Several clinical randomized studies have been reported in the literature demonstrating the use of investigational drugs in the prevention of PIPN showing promising evidence of their effectiveness. Table 1 summarizes the clinical studies investigating the potential role of different agents in the prevention of peripheral neuropathy (PN) secondary to paclitaxel chemotherapy.
Anticonvulsants
Pregabalin and gabapentin are commonly reported to treat different neuropathic pain. A randomized double-blinded clinical pilot study on the use of pregabalin in preventing PIPN reported no significant difference in worst pain scores between the control arm and the pregabalin arm. Moreover, there were no differences across the arms in the worst, average, and least pain area under the curve (AUCs) throughout the first cycle of therapy (p = 0.48, 0.62, 0.22, and 0.07, respectively) or the maximum of average pain. Additionally, the European Organization of Research and Treatment of Cancer Quality of Life Questionnaire (EORTC-QLQ-CIPN20) sensory subscale did not significantly differ between the two arms according to growth curve models or AUC analysis (p = 0.88 and p = 0.46, respectively) [49].
In another double-blinded, placebo-controlled study 40 breast cancer patients were randomly assigned to receive gabapentin or placebo. In all four cycles, the gabapentin group’s neuropathy was primarily grade 1, with no reported cases of ≥ grade 3 neuropathy. In the gabapentin group, the rate of grade 2 and 3 neuropathy was considerably lower (P < 0.001) than in the placebo group. After four cycles of paclitaxel, the gabapentin group’s Nerve Conduction Velocity (NCV) changed to be lower than that of the placebo group (17.7% vs. 61.0% reduction in NCV for sural nerve and 21.9% vs. 62.5% fall in NCV for peroneal nerve) [38].
Biguanides
A double-blinded randomized controlled trial (RCT) assessing the efficacy of metformin in the prevention of PIPN showed that the development of grade two or more peripheral neuropathy (PN) was significantly lower in the metformin group compared to placebo (p = 0.001). Additionally, the time to develop PN was significantly longer in the metformin group. Furthermore, serum nerve growth factor (NGF) was significantly lower in the metformin group, and comparable levels of serum neurotensin were found in the two study groups [41].
Nutritional supplements
Omega 3 fatty acid protective effect in PIPN was assessed in a double-blinded RCT and showed a significant difference in PN occurrence (OR 0.3, 95% CI (0.10–0.88), p = 0.029). The two study groups did not show a significant trend in terms of PIPN severity differences; nevertheless, the placebo group had greater frequencies of PN in all score categories (0.95% CI (− 2.06 − 0.02), p = 0.054). [40]
Vitamin E neuroprotective effect was evaluated in three RCTs, where the incidence of PIPN and modified peripheral neuropathy (PNP) score were significantly lower with Vitamin E. [50] Also, neurotoxicity was more common in the control group than in the vitamin E-supplemented patients [51]. The frequency of ≥ grade 2 neuropathy, was comparable across the two arms; and only a non-significant difference in grade 3 neuropathy was reported. When comparing the two arms, there was a significant difference in the time length of neuropathy, which was measured from the onset of the first ≥ grade 2 or PN to the point at which it resolves to grade 1 neuropathy [52].
Vitamin B complex when used in conjunction with neurotoxic chemotherapy regimens did not prevent CIPN nor was it more effective than a placebo [58].
One RCT indicated the administration of alpha lipoic acid (ALA) in conjunction with the inhibitor of acetylcholinesterase ipidacrine hydrochloride (IPD) significantly reduced the extent of damage to the sural nerves (SNs) and superficial peroneal nerves (SPNs) caused by paclitaxel in patients prescribed for polychemotherapy (PCT). This was done by identifying significant differences in the electroneuromyography (ENMG) indicators of sensory nerve parameters between the studied groups [55]. Another double-blinded RCT showed that the percent of peripheral neuropathy grade 3 was significantly lower in the ALA group. Furthermore, the FACT-GOG-Ntx-12 questionnaire total score was significantly higher in the ALA group. Regarding the biomarkers, ALA group showed lower levels of brain natriuretic peptides (BNP), tumor necrosis factor-alpha (TNF-α), Malondialdehyde (MDA), and NT in comparison with the control group [62].
A controlled study assessed the oral nutritional supplement Eicosatetraenoic (ONS-EPA) acid effect in patients with advanced non-small cell lung cancer, compared to the control group. Patients in the ONS-EPA group showed significantly reduced neuropathy [56].
Another supplement, acetyl-L-carnitine (ALC) has been studied in PIPN. Over two years, CIPN was statistically significantly worse after twenty-four weeks of ALC therapy [47].
Goshajinkigan (GJG), a traditional Japanese herbal medicine, has been evaluated in a randomized controlled trial showing that (GJG) has a neuroprotective effect and nerve-repairing effect in CIPN [60].
Amino-acids and peptides
A randomized controlled study investigating glutamine in PIPN reported an overall frequency of neuropathy across all grades to be 78% at three months and 80% at six months. At six months, the incidences of grade 1 (48%), grade 2 (22%), and grade 3 (10%) neuropathy were recorded and (20%) did not experience neuropathy. Weekly paclitaxel-induced symptoms were mostly of grades 1 and 2, but not grade 4 symptoms. No significant differences were observed across the treatment groups in terms of the symptoms and weekly PIPN was not improved by glutamine [54].
Glutamate has been evaluated in a double-blinded RCT, showing that the selected dosage regimen was ineffective in the prevention of PIPN. Both groups’ frequency of signs and symptoms was similar; however, the glutamate group’s neurotoxicity symptoms tended to manifest at lower severity levels. Additionally, there was similarity between the two groups in the frequency of aberrant electrodiagnostic findings [45].
A double-blinded RCT evaluated the use of glutathione for the prevention of paclitaxel/carboplatin-induced peripheral neuropathy. The results revealed no statistical significance difference in determining neurotoxicity with grade 2 using the National Cancer Institute Common Terminology Criteria for Adverse Event (NCI-CTCAE) v4.0 scale or in the time to development of neurotoxicity with grade 2. Moreover, no significant difference in neurotoxicity was measured by the EORTC-QLQ-CIPN20 [63].
Cytokines
Another double-blinded RCT showed that Recombinant Human Leukemia Inhibitory Factor (LIF) was not protective against carboplatin/paclitaxel-induced CIPN. There was a comparable standardized composite peripheral neuropathy electrophysiology (CPNE) score between the baseline and cycle 4, the last cycle, or the post-treatment assessment in both groups [57].
Mucolytic agent
N-acetylcysteine was evaluated for its effect on the prevention of PIPN because of its activity in reducing oxidative stress and elimination of ROS. RCT results demonstrated that when compared to the low-dosage group (61.9%) and the control group (100%), the high-dose group’s occurrence of grades 2 to 3 PN was significantly reduced (28.6%). A significant improvement in QOL and modified total neuropathy score (mTNS) scores was recorded. Additionally, there were significant differences in serum MDA levels between the high-dosage and low-dose groups, as well as significantly greater levels of NGF in the high-dose group [42].
Phosphodiesterase inhibitors
Cilostazol has been evaluated for its preventive effect on PIPN, and when comparing the cilostazol group (40%) to the control group (86.7%), there was a significant difference in the occurrence of grade 2 and 3 peripheral neuropathies. The control group had a greater rate of clinically significant worsening in neuropathy-related quality of life compared to the cilostazol group. The cilostazol group showed a greater percent increase in serum NGF from baseline. At the end of the study, the circulation levels of the neurofilament light chain (NfL) were found to be comparable across the two arms [43].
Tetracycline antibiotics
Minocycline was studied in a multicentric, double-blinded, pilot trial and reported no significant dissimilarity during the first cycle of treatment. Still, there was a significant difference in the daily average area under the curve (AUC) pain score attributable to paclitaxel acute pain syndrome (P-APS), in favor of minocycline. Additionally, there was a tendency toward improvement in the daily worst pain AUC score across the 12 cycles. The overall EORTC-QLQ-CIPN20 sensory subscale did not significantly change between minocycline and placebo, despite the decrease in P-APS linked to minocycline usage [48].
Antidepressant
Over a long time, the antidepressant duloxetine has been evaluated for the management of CIPN, and recently, it was assessed in a double-blinded RCT to prevent PIPN. According to Patient Neurotoxicity Questionnaire (PNQ), 10 (50%) of the 20 participants in the placebo group experienced neurotoxicity (two mild cases, three moderate cases, four severe cases, and one disabled case). Nonetheless, two patients in the duloxetine group experienced moderate neurotoxicity. Median motor, sensory latency, and motor velocity were shown to have significant variations across the groups. The relative risk of polyneuropathy (relative risk: 1), however, was comparable between the two groups. According to the findings, an electrodiagnostic investigation supported the possibility that duloxetine could be a beneficial medication for breast cancer patients in reducing PIPN [53].
H2 antagonist
A small, randomized placebo-controlled study was conducted to evaluate lafutidine in the prevention of PIPN. Due to limited recruitment, the planned total of patients was not attained. Neuralgia of grade 2 or above affected 22.2% of the lafutidine group compared to 14.3% in the control group. In the lafutidine group, 100% of the participants had peripheral sensory neuropathy grade 2 or above, compared to 71.4% in the control group (p = 0.175). In neither group, there was evidence of PN of grade 3 or higher. The two groups’ PNQ scores did not differ significantly from one another. Following the fourth cycle, there was a tendency for the lafutidine group to have lower FACT/GOG-Ntx scores than the control group. Between the two groups, there was no statistically significant difference in progression-free survival (PFS) [59].
Others
A randomized double-blinded placebo-controlled study was conducted to assess the effect of ganglioside monosialic acid (GM1) in the prevention of PIPN, which showed that the GM1 group had a lower incidence of PN grade 1 or higher in CTCAE v4.0 grading. Additionally, the GM1 group had a better significant difference in the FACT-NTX score. Moreover, a lower significant difference in the Eastern Cooperative Oncology Group Neuropathy scale (ENS) in the GM1 group was reported [61].
Assessment tools of PIPN including patient-reported outcome measurements (PROMs)
Patient-reported outcome measures (PROMs) are used more widely as a significant tool for the assessment of PIPN and are considered a valuable tool for collecting PN symptoms. PROMs are mostly used as endpoint measures in PIPN treatment and prevention clinical trials, as well as in research settings to characterize the natural history of neuropathy development and recovery. The most investigated PROMs were the EORTC-QLQ-CIPN20 and FACT-GOG-Ntx.
EORTC-QLQ-CIPN20
EORTC-QLQ-CIPN20 is a 12-item quality-of-life questionnaire that was developed to gather information on patients’ experiences with CIPN-related symptoms and functional limitations. The CIPN20 comprises three subscales motor, sensory, and autonomic. Using a four-point rating system, patients indicate how much they have experienced each symptom (or “item”) over the past seven days (1 = Not at all, 2 = A little bit, 3 = Quite a little, and 4 = Very much). Higher scores indicate a greater symptom burden. The three subscales are each calculated as the sum of component items, linearly transformed to a 0–100 scale [64].
FACT-GOG-Ntx
The FACT/GOG-Ntx was developed in cooperation between the Gynecologic Oncology Group (GOG) and the Functional Assessment of Chronic Illness Therapy group. The original 11-item FACT/GOG-Ntx11 questionnaire was created to assess the magnitude of CIPN and its effects on patients’ quality of life in relation to motor, sensory, and auditory neuropathy and dysfunction. A five-point rating system is used for each item (0 = Not at all, 1 = A little bit, 2 = Somewhat, 3 = Quite a bit, and 4 = alot). The items’ scores are reversed according to the FACIT groups’ scoring convention, with greater total scores indicating better quality of life [65, 66].
Genetic single nucleotide polymorphisms associated with PIPN
Polymorphisms associated with paclitaxel metabolism
PIPN sensitivity may be raised by polymorphisms in genes related to the metabolism of the drug. Increased severity of PIPN has often been linked to single nucleotide polymorphisms (SNPs) in the ABCB1 gene [67,68,69]. In previous reports of patients with breast cancer receiving taxanes, SNPs in CYP2C8 and CYP3A4 were identified as causes of ≥ grade 2 CIPN [68, 70, 71].
Polymorphisms associated with microtubule function
Genes linked to microtubule function have been investigated to anticipate their relationship with PIPN since taxanes alter microtubule function and could contribute to PIPN pathogenesis. In 1303 European patients receiving paclitaxel, a SNP in the β tubulin IIb-encoding gene TUBB2A was linked to PIPN [72]. However, in 454 patients with ovarian cancer treated with paclitaxel and carboplatin, additive SNPs in MAPT and GSK3B were linked to reported neuropathy [73].
Polymorphisms associated with inherited neuropathies
The relationship between CIPN and genes linked to hereditary neuropathies has also been investigated. In African American patients receiving paclitaxel, SBF2, linked to Charcot-Marie-Tooth (CMT) disorder, was linked to CIPN [74]. Another study involving 58 individuals receiving paclitaxel discovered that FZD3 was linked to CIPN but not SBF2 [74]. Also, FGD4 was linked to CIPN in a trial investigating 219 breast cancer patients receiving taxanes [75]. In a larger cohort of 855 patients of European origin treated with paclitaxel, findings confirmed variation in FGD4 gene was linked to the reported sensory CIPN [76]. Another trial examining 269 cancer patients undergoing treatment in Alliance N08C1 found that ARHGEF10 was linked to CIPN when 49 CMT genes were examined in blood samples [77]. These results have been proved in 138 patients receiving paclitaxel in Alliance N08CA [78].
Polymorphisms associated with inflammatory pathways
An increasing corpus of research indicates that PIPN is influenced by inflammation, and changes in inflammatory pathways were linked to neuropathic pain [9, 79]. SNP in FCAMR, which encodes the FC receptor, led to a substantial relationship with CIPN in 3,431 breast cancer patients receiving paclitaxel treatment [80].
Polymorphisms associated with ion channels
Moreover, PIPN may potentially result from disruption of neuronal function via ion channels [9, 81] and SCN10A encode sodium channels located in the dorsal root ganglia, specifically Nav1.7 and Nav1.8. Among 186 Japanese patients with taxanes-treated breast and ovarian cancer, a SNP in the encoding gene SCN9A was linked to the development of ≥ grade 2 CIPN [82].
Polymorphisms associated with neuronal function
Genes related to cellular repair pathways and nervous system development and function have been connected to PIPN. In patients receiving taxanes, CIPN is linked to changes in the genes coding the Eph receptors (EPHA4, EPHA5, EPHA6, EPHA8), a class of tyrosine kinase receptors responsible for nerve growth and regulation [83,84,85,86,87]. Also, in 107 patients of gynecologic malignancies undergoing taxane or platinum chemotherapy, polymorphisms in the neural development-related genes SOX10 and GPX7 were linked to CIPN [88].
Future perspectives
Potential difficulties in many clinical trials investigating PIPN prevention include small sample sizes, the absence of placebo control groups, varying dosing and treatment regimens of investigational medications, non-standardized definitions and assessments of neuropathy, and inclusion of cancer patients receiving different chemotherapy treatment protocols. These factors limit the generalizability and interpretation of study findings. To manage these obstacles, future trials should ensure randomized placebo-controlled designs with adequate statistical power. Standardizing objective neuropathy assessments and definitions of clinically significant PIPN across studies would enable cross-trial comparisons. Additionally, narrowing eligibility criteria to specific chemotherapy protocols may yield more homogeneous cohorts for evaluating preventive interventions.
Conclusion
Preventing paclitaxel-induced neuropathy is a complex and evolving field. The literature on PIPN prevention is characterized by the heterogeneity of study designs, patient populations, and outcome measures, making conclusive evidence challenging. The literature suggests a variety of potential approaches such as pharmacological interventions; however, more high-quality research is needed to establish clear recommendations for clinical practice.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Abbreviations
- ABCB1:
-
Adenosine 5′ triphosphate binding cassette subfamily B member 1
- ALA:
-
Alpha lipoic acid
- ALC:
-
Acetyl-L-carnitine
- AMPK:
-
Adenosine monophosphate protein kinase
- ARB:
-
Angiotensin II receptor blocker
- ARHGEF10:
-
Rho guanine nucleotide exchange factor 10
- AT:
-
Paclitaxel-doxorubicin regimen
- AUC:
-
Area under the curve
- BID:
-
Twice daily
- BPI-SF:
-
Brief pain inventory
- BNP:
-
Brain natriuretic peptides
- cAMP:
-
Cyclic adenosine monophosphate
- CI:
-
Confidence interval
- CIPN:
-
Chemotherapy-induced peripheral neuropathy
- CMT:
-
Charcot-Marie-Tooth
- CNS:
-
Central nervous system
- CPNE:
-
Standardized composite peripheral neuropathy electrophysiology score
- CPT:
-
Current perception threshold
- CTCAE:
-
Common terminology criteria for adverse events
- CYP2C8:
-
Cytochrome P450 family 2 subfamily C member 8
- CYP2C8:
-
Cytochrome P450 family 2 subfamily C member 8
- CYP2J6:
-
Cytochrome P450 family 2 subfamily J member 6
- CYP3A4:
-
Cytochrome P450 family 3 subfamily A member 4
- DPP-4:
-
Dipeptidyl peptidase inhibitor-4
- DRG:
-
Dorsal root ganglia
- ENMG:
-
Electroneuromyography
- ENS:
-
Eastern cooperative oncology group neuropathy scale
- EORTC-QLQ-CIPN20:
-
European Organization of Research and Treatment of Cancer Quality of Life, chemotherapy-induced peripheral neuropathy 20
- EPHA4:
-
Ephrin Type A receptor 4
- EPHA5,:
-
Ephrin Type A receptor 5
- EPHA6,:
-
Ephrin Type A receptor 6
- EPHA8:
-
Ephrin Type A receptor 8
- ET:
-
Paclitaxel-epirubicin regimen
- FACIT:
-
Functional assessment of chronic illness therapy
- FACT-GOG-NTX:
-
Functional assessment of cancer therapy/gynecologic oncology group-neurotoxicity
- FACT-NTX:
-
Functional assessment of chemotherapy-taxane
- FC:
-
Fc alpha and mu receptor
- FCAMR:
-
Fc alpha and mu receptor
- FDG3:
-
Facio-genital dysplasia gene 3
- FGD4:
-
FYVE-RhoGEF and PH domain containing 4
- FZD3:
-
Frizzled class receptor 3
- GJG:
-
Goshajinkigan
- GM1:
-
Ganglioside monosialic acid
- GPX7:
-
Glutathione peroxidase 7
- GSK3B:
-
Glycogen synthase kinase 3 beta
- HROL:
-
Health related quality of life
- IENF:
-
Intra-epidermal nerve fibers
- IL-6:
-
Interlukin-6
- InsP3R:
-
Inositol 1,4,5 tris-phosphate receptor
- IPD:
-
Ipidacrine hydrochloride
- IV:
-
Intravenous
- KCNN3:
-
Potassium calcium-activated channel subfamily N member 3
- LIF:
-
Leukemia inhibitory factor
- MAPT:
-
Microtubule-associated protein tau
- MDA:
-
Malondialdehyde
- mTNS:
-
Modified total neuropathy score
- NCI-CTCAE:
-
National cancer institute’s common toxicity criteria for adverse event
- NCS-1:
-
Neuronal calcium sensor 1
- NCV:
-
Nerve conduction velocity
- Nfl:
-
Neurofilament chain light
- NGF:
-
Nerve growth factor
- NSCLC:
-
Non-small cell lung cancer
- NT:
-
Neurotensin
- ONS-EPA:
-
Oral nutritional supplement eicosatetraenoic
- OR:
-
Odds ratio
- P-APS:
-
Paclitaxel acute pain syndrome
- PCT:
-
Polychemotherapy
- PDE:
-
Phosphodiesterase
- PIPN:
-
Paclitaxel-induced peripheral neuropathy
- PN:
-
Peripheral neuropathy
- PNP:
-
Peripheral neuropathy score
- PNQ:
-
Patient neurotoxicity questionnaire
- PNQ:
-
Patient neurotoxicity questionnaire
- PO:
-
Per oral
- PPARγ:
-
Peroxisome proliferator‐activated receptor gamma
- PROM:
-
Patient-reported outcome measurements
- PTX:
-
Paclitaxel
- QLQ:
-
Quality-of-life questionnaire
- QOL:
-
Quality of life
- ROS:
-
Reactive oxygen species
- rTNS:
-
Total neuropathy score
- SCN10A:
-
Sodium voltage-gated channel alpha subunit 10
- SCN9A:
-
Sodium voltage-gated channel alpha subunit 9
- SN:
-
Sural nerves
- SNP:
-
Single nucleotide polymorphism
- SOX10:
-
Sky box transcription factor 10
- SPN:
-
Superficial peroneal nerves
- TLR4:
-
Toll-like receptor 4
- TNFα:
-
Tumor necrosis factor α
- TUBB2A:
-
Tubulin beta 2A
- VAS:
-
Visual analogue scale
References
Ibrahim EY, Ehrlich BE (2020) Prevention of chemotherapy-induced peripheral neuropathy: a review of recent findings. Crit Rev Oncol Hematol 145:102831. https://doi.org/10.1016/J.CRITREVONC.2019.102831
Van Vuuren RJ, Visagie MH, Theron AE, Joubert AM (2015) Antimitotic drugs in the treatment of cancer. Cancer Chemother Pharmacol 76(6):1101–1112. https://doi.org/10.1007/S00280-015-2903-8/TABLES/1
McGrogan BT, Gilmartin B, Carney DN, McCann A (2008) Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta Rev Cancer 1785(2):96–132. https://doi.org/10.1016/j.bbcan.2007.10.004
da Costa R, Passos GF, Quintão NLM, Fernandes ES, Maia JRLCB, Campos MM, Calixto JB (2020) Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol 177:3127–3146
Tanabe Y, Hashimoto K, Shimizu C, Hirakawa A, Harano K, Yunokawa M, Yonemori K, Katsumata N, Tamura K, Ando M, Kinoshita T, Fujiwara Y (2013) Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol 18(1):132–138. https://doi.org/10.1007/s10147-011-0352-x
Katona I, Weis J (2018) Diseases of the peripheral nerves. Handb Clin Neurol 145:453–474. https://doi.org/10.1016/B978-0-12-802395-2.00031-6
Jimenez-Andrade JM, Peters CM, Mejia NA, Ghilardi JR, Kuskowski MA, Mantyh PW (2006) Sensory neurons and their supporting cells located in the trigeminal, thoracic and lumbar ganglia differentially express markers of injury following intravenous administration of paclitaxel in the rat. Neurosci Lett 405(1–2):62–67. https://doi.org/10.1016/J.NEULET.2006.06.043
Liu CC, Lu N, Cui Y, Yang T, Zhao ZQ, Xin WJ, Liu XG (2010) Prevention of paclitaxel-induced allodynia by minocycline: effect on loss of peripheral nerve fibers and infiltration of macrophages in rats. Mol Pain. https://doi.org/10.1186/1744-8069-6-76
Staff NP, Fehrenbacher JC, Caillaud M, Damaj MI, Segal RA, Rieger S (2020) Pathogenesis of paclitaxel-induced peripheral neuropathy: a current review of in vitro and in vivo findings using rodent and human model systems. Exp Neurol 324:113121. https://doi.org/10.1016/J.EXPNEUROL.2019.113121
Fukuda Y, Li Y, Segal RA (2017) A mechanistic understanding of axon degeneration in chemotherapy-induced peripheral neuropathy. Front Neurosci. https://doi.org/10.3389/FNINS.2017.00481
Xiao WH, Bennett GJ (2012) Effects of mitochondrial poisons on the neuropathic pain produced by the chemotherapeutic agents, paclitaxel and oxaliplatin. Pain 153(3):704–709. https://doi.org/10.1016/J.PAIN.2011.12.011
Al-Mazidi S, Alotaibi M, Nedjadi T, Chaudhary A, Alzoghaibi M, Djouhri L (2018) Blocking of cytokines signalling attenuates evoked and spontaneous neuropathic pain behaviours in the paclitaxel rat model of chemotherapy-induced neuropathy. Eur J Pain 22(4):810–821. https://doi.org/10.1002/EJP.1169
da Costa R, Passos GF, Quintão NLM, Fernandes ES, Maia JRLCB, Campos MM, Calixto JB (2020) Taxane-induced neurotoxicity: pathophysiology and therapeutic perspectives. Br J Pharmacol 177(14):3127–3146. https://doi.org/10.1111/bph.15086
Grisold W, Cavaletti G, Windebank AJ (2012) Peripheral neuropathies from chemotherapeutics and targeted agents: diagnosis, treatment, and prevention. Neuro Oncol. https://doi.org/10.1093/neuonc/nos203
Velasco R, Bruna J (2015) Taxane-induced peripheral neurotoxicity. Toxics 3(2):152. https://doi.org/10.3390/TOXICS3020152
Tamburin S, Park SB, Alberti P, Demichelis C, Schenone A, Argyriou AA (2019) Taxane and epothilone-induced peripheral neurotoxicity: From pathogenesis to treatment. J Peripher Nerv Syst 24:S40–S51. https://doi.org/10.1111/JNS.12336
Nasser AH, Gendy AM, El-Yamany MF, El-Tanbouly DM (2022) Upregulation of neuronal progranulin mediates the antinociceptive effect of trimetazidine in paclitaxel-induced peripheral neuropathy: role of ERK1/2 signaling. Toxicol Appl Pharmacol 448:116096. https://doi.org/10.1016/J.TAAP.2022.116096
Hammad ASA, Sayed-Ahmed MM, Abdel Hafez SMN, Ibrahim ARN, Khalifa MMA, El-Daly M (2023) Trimetazidine alleviates paclitaxel-induced peripheral neuropathy through modulation of TLR4/p38/NF-κB and klotho protein expression. Chem Biol Interact 376:110446. https://doi.org/10.1016/J.CBI.2023.110446
Kalynovska N, Diallo M, Sotakova-Kasparova D, Palecek J (2020) Losartan attenuates neuroinflammation and neuropathic pain in paclitaxel-induced peripheral neuropathy. J Cell Mol Med 24(14):7949–7958. https://doi.org/10.1111/JCMM.15427
Sisignano M, Angioni C, Park CK, Dos SSM, Jordan H, Kuzikov M, Liu D, Zinn S, Hohman SW, Schreiber Y, Zimmer B, Schmidt M, Lu R, Suo J, Zhang DD, Schäfer SMG, Hofmann M, Yekkirala AS, De Bruin N, Parnham MJ, Woolf CJ, Ji RR, Scholich K, Geisslinger G (2016) Targeting CYP2J to reduce paclitaxel-induced peripheral neuropathic pain. Proc Natl Acad Sci U S A 113(44):12544–12549. https://doi.org/10.1073/PNAS.1613246113
Bouchenaki H, Bernard A, Bessaguet F, Frachet S, Richard L, Sturtz F, Magy L, Bourthoumieu S, Demiot C, Danigo A (2022) Neuroprotective effect of ramipril is mediated by AT2 in a mouse MODEL of paclitaxel-induced peripheral neuropathy. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14040848
Semis HS, Kandemir FM, Kaynar O, Dogan T, Arikan SM (2021) The protective effects of hesperidin against paclitaxel-induced peripheral neuropathy in rats. Life Sci 287:120104. https://doi.org/10.1016/J.LFS.2021.120104
Miao H, Xu J, Xu D, Ma X, Zhao X, Liu L (2019) Nociceptive behavior induced by chemotherapeutic paclitaxel and beneficial role of antioxidative pathways. Physiol Res 68(3):491–500. https://doi.org/10.33549/PHYSIOLRES.933939
Yardım A, Kandemir FM, Çomaklı S, Özdemir S, Caglayan C, Kucukler S, Çelik H (2021) Protective effects of curcumin against paclitaxel-induced spinal cord and sciatic nerve injuries in rats. Neurochem Res 46(2):379–395. https://doi.org/10.1007/S11064-020-03174-0/FIGURES/11
Galley HF, McCormick B, Wilson KL, Lowes DA, Colvin L, Torsney C (2017) Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J Pineal Res 63(4):e12444. https://doi.org/10.1111/JPI.12444
Miranda HF, Sierralta F, Aranda N, Poblete P, Castillo RL, Noriega V, Prieto JC (2018) Antinociception induced by rosuvastatin in murine neuropathic pain. Pharmacol Rep 70(3):503–508. https://doi.org/10.1016/J.PHAREP.2017.11.012
El-Sawaf ES, Saleh S, Abdallah DM, Ahmed KA, El-Abhar HS (2021) Vitamin D and rosuvastatin obliterate peripheral neuropathy in a type-2 diabetes model through modulating Notch1, Wnt-10α, TGF-β and NRF-1 crosstalk. Life Sci 279:119697. https://doi.org/10.1016/j.lfs.2021.119697
Meng J, Zhang Q, Yang C, Xiao L, Xue Z, Zhu J (2019) Duloxetine, a balanced serotonin-norepinephrine reuptake inhibitor, improves painful chemotherapy-induced peripheral neuropathy by inhibiting activation of p38 MAPK and NF-κB. Front Pharmacol. https://doi.org/10.3389/fphar.2019.00365
Lu Y, Zhang P, Zhang Q, Yang C, Qian Y, Suo J, Tao X, Zhu J (2020) Duloxetine attenuates paclitaxel-induced peripheral nerve injury by inhibiting p53-related pathways. J Pharmacol Exp Ther 373(3):453–462. https://doi.org/10.1124/jpet.120.265082
Al-Massri KF, Ahmed LA, El-Abhar HS (2018) Pregabalin and lacosamide ameliorate paclitaxel-induced peripheral neuropathy via inhibition of JAK/STAT signaling pathway and Notch-1 receptor. Neurochem Int 120:164–171. https://doi.org/10.1016/J.NEUINT.2018.08.007
Inyang KE, McDougal TA, Ramirez ED, Williams M, Laumet G, Kavelaars A, Heijnen CJ, Burton M, Dussor G, Price TJ (2019) Alleviation of paclitaxel-induced mechanical hypersensitivity and hyperalgesic priming with AMPK activators in male and female mice. Neurobiol Pain 6:100037. https://doi.org/10.1016/J.YNPAI.2019.100037
Shigematsu N, Kawashiri T, Kobayashi D, Shimizu S, Mine K, Hiromoto S, Uchida M, Egashira N, Shimazoe T (2020) Neuroprotective effect of alogliptin on oxaliplatin-induced peripheral neuropathy in vivo and in vitro. Sci Rep 10(1):1–8. https://doi.org/10.1038/s41598-020-62738-w
Koyanagi M, Imai S, Iwamitsu Y, Matsumoto M, Saigo M, Moriya A, Ogihara T, Nakazato Y, Yonezawa A, Nakagawa S, Nakagawa T, Matsubara K (2021) Cilostazol is an effective causal therapy for preventing paclitaxel-induced peripheral neuropathy by suppression of Schwann cell dedifferentiation. Neuropharmacology 188:108514. https://doi.org/10.1016/J.NEUROPHARM.2021.108514
Wang XM, Gu P, Saligan L, Iadarola M, Wong SSC, Ti LK, Cheung CW (2020) Dysregulation of EAAT2 and VGLUT2 spinal glutamate transports via histone deacetylase 2 (HDAC2) contributes to paclitaxel-induced painful neuropathy. Mol Cancer Ther 19(10):2196–2209. https://doi.org/10.1158/1535-7163.MCT-20-0006/87847/AM/DYSREGULATION-OF-EAAT2-AND-VGLUT2-SPINAL-GLUTAMATE
Hernández-Cruz A (2010) Ion channels and neuroendocrine cells: old partners in the investigation of each other’s function. Commentary on the “ion Channels” chapter. In: Cellular and molecular neurobiology. pp 1197–1199
Boehmerle W, Splittgerber U, Lazarus MB, Mckenzie KM, Johnston DG, Austin DJ, Ehrlich BE (2006) Paclitaxel induces calcium oscillations via an inositol 1,4,5-trisphosphate receptor and neuronal calcium sensor 1-dependent mechanism. Proc Natl Acad Sci 103(48):18356–18361
Mo M, Erdelyi I, Szigeti-Buck K, Benbow JH, Ehrlich BE (2012) Prevention of paclitaxel-induced peripheral neuropathy by lithium pretreatment. FASEB J 26(11):4696–4709. https://doi.org/10.1096/fj.12-214643
Aghili M, Zare M, Mousavi N, Ghalehtaki R, Sotoudeh S, Kalaghchi B, Akrami S, Esmati E (2019) Efficacy of gabapentin for the prevention of paclitaxel induced peripheral neuropathy: a randomized placebo controlled clinical trial. Breast J 25(2):226–231. https://doi.org/10.1111/tbj.13196
Pandey P, Kumar A, Pushpam D, Khurana S, Malik PS, Gogia A, Arunmozhimaran E, Singh MB, Chandran DS, Batra A (2023) Randomized double-blind, placebo-controlled study of oral gabapentin for prevention of neuropathy in patients receiving paclitaxel. Trials 24(1):1–9. https://doi.org/10.1186/S13063-023-07126-1/FIGURES/1
Ghoreishi Z, Esfahani A, Djazayeri A, Djalali M, Golestan B, Ayromlou H, Hashemzade S, Asghari Jafarabadi M, Montazeri V, Keshavarz SA, Darabi M (2012) Omega-3 fatty acids are protective against paclitaxel-induced peripheral neuropathy: a randomized double-blind placebo controlled trial. BMC Cancer. https://doi.org/10.1186/1471-2407-12-355
Bakry HM, Mansour NO, ElKhodary TR, Soliman MM (2023) Efficacy of metformin in prevention of paclitaxel-induced peripheral neuropathy in breast cancer patients: a randomized controlled trial. Front Pharmacol. https://doi.org/10.3389/fphar.2023.1181312
Khalefa HG, Shawki MA, Aboelhassan R, El Wakeel LM (2020) Evaluation of the effect of N-acetylcysteine on the prevention and amelioration of paclitaxel-induced peripheral neuropathy in breast cancer patients: a randomized controlled study. Breast Cancer Res Treat 183(1):117–125. https://doi.org/10.1007/s10549-020-05762-8
Haroun EA, Mansour NO, Eltantawy A, Shams MEE (2023) Effect of cilostazol on preventing paclitaxel-induced neuropathy in patients with breast cancer: a randomized controlled trial. Pharmacotherapy 43(9):872–882. https://doi.org/10.1002/phar.2830
Gelmon K, Eisenhauer E, Bryce C, Tolcher A, Mayer L, Tomlinson E, Zee B, Blackstein M, Tomiak E, Yau J, Batist G, Fisher B, Iglesias J (1999) Randomized phase II study of high-dose paclitaxel with or without amifostine in patients with metastatic breast cancer. J Clin Oncol 17(10):3038–3047. https://doi.org/10.1200/JCO.1999.17.10.3038
Loven D, Levavi H, Sabach G, Zart R, Andras M, Fishman A, Karmon Y, Levi T, Dabby R, Gadoth N (2009) Long-term glutamate supplementation failed to protect against peripheral neurotoxicity of paclitaxel. Eur J Cancer Care (Engl) 18(1):78–83. https://doi.org/10.1111/J.1365-2354.2008.00996.X
Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL (2014) North Central Cancer Treatment Group/Alliance trial N08CA - the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer 120(12):1890–1897. https://doi.org/10.1002/cncr.28654
Hershman DL, Unger JM, Crew KD, Till C, Greenlee H, Minasian LM, Moinpour CM, Lew DL, Fehrenbacher L, Wade JL, Wong SF, Fisch MJ, Henry NL, Albain KS (2018) Two-year trends of taxane-induced neuropathy in women enrolled in a randomized trial of Acetyl-L-Carnitine (SWOG S0715). J Natl Cancer Inst 110(6):669–676. https://doi.org/10.1093/JNCI/DJX259
Pachman DR, Dockter T, Zekan PJ, Fruth B, Ruddy KJ, Ta LE, Lafky JM, Dentchev T, Le-Lindqwister NA, Sikov WM, Staff N, Beutler AS, Loprinzi CL (2017) A pilot study of minocycline for the prevention of paclitaxel-associated neuropathy: ACCRU study RU221408I. Support Care Cancer 25(11):3407–3416. https://doi.org/10.1007/s00520-017-3760-2
Shinde SS, Seisler D, Soori G, Atherton PJ, Pachman DR, Lafky J, Ruddy KJ, Loprinzi CL (2016) Can pregabalin prevent paclitaxel-associated neuropathy?–an ACCRU pilot trial. Support Care Cancer 24(2):547–553. https://doi.org/10.1007/S00520-015-2807-5
Argyriou AA, Chroni E, Koutras A, Iconomou G, Papapetropoulos S, Polychronopoulos P, Kalofonos HP (2006) Preventing paclitaxel-induced peripheral neuropathy: a phase II trial of vitamin E supplementation. J Pain Symptom Manag 32(3):237–244. https://doi.org/10.1016/j.jpainsymman.2006.03.013
Argyriou AA, Chroni E, Koutras A, Ellul J, Papapetropoulos S, Katsoulas G, Iconomou G, Kalofonos HP (2005) Vitamin E for prophylaxis against chemotherapy-induced neuropathy: a randomized controlled trial. Neurology 64(1):26–31. https://doi.org/10.1212/01.WNL.0000148609.35718.7D
Heiba MA, Ismail SS, Sabry M, Bayoumy WAE, Kamal KAA (2021) The use of vitamin E in preventing taxane-induced peripheral neuropathy. Cancer Chemother Pharmacol 88(6):931–939. https://doi.org/10.1007/S00280-021-04347-6/FIGURES/2
Aghabozorgi R, Hesam M, Zahed G, Babaee M, Hashemi M, Rayegani SM (2023) Efficacy of Duloxetine on electrodiagnostic findings of Paclitaxel-induced peripheral neuropathy, does it have a prophylactic effect? A randomized clinical trial. Anticancer Drugs 34(5):680–685. https://doi.org/10.1097/CAD.0000000000001429
Abbas W, Rao RR, Agarwal A, Saha R, Bajpai P, Qureshi S, Mittal A (2018) Incidence of neuropathy with weekly paclitaxel and role of oral glutamine supplementation for prevention of paclitaxel induced peripheral neuropathy randomized controlled trial. Indian J Med Paediatr Oncol. https://doi.org/10.4103/ijmpo.ijmpo_38_17
Holotiuk IS, Kryzhanivska AY, Holotiuk SI, Holotiuk VV, Maliborska SV (2022) Effectiveness of alpha-lipoic acid and ipidacrine hydrochloride in prevention of paclitaxel-induced peripheral neuropathy assessed by electroneuromyography of superficial peroneal and sural nerves. Exp Oncol 44(4):300–306. https://doi.org/10.32471/exp-oncology
Sánchez-Lara K, Turcott JG, Juárez-Hernández E, Nuñez-Valencia C, Villanueva G, Guevara P, De la Torre-Vallejo M, Mohar A, Arrieta O (2014) Effects of an oral nutritional supplement containing eicosapentaenoic acid on nutritional and clinical outcomes in patients with advanced non-small cell lung cancer: randomised trial. Clin Nutr 33(6):1017–1023. https://doi.org/10.1016/J.CLNU.2014.03.006
Davis ID, Kiers L, MacGregor L, Quinn M, Arezzo J, Green M, Rosenthal M, Chia M, Michael M, Bartley P, Harrison L, Daly M (2005) A randomized, double-blinded, placebo-controlled phase ii trial of recombinant human leukemia inhibitory factor (rhuLIF, Emfilermin, AM424) to prevent chemotherapy-induced peripheral neuropathy. Clin Cancer Res 11(5):1890–1898. https://doi.org/10.1158/1078-0432.CCR-04-1655
Schloss JM, Colosimo M, Airey C, Masci P, Linnane AW, Vitetta L (2017) A randomised, placebo-controlled trial assessing the efficacy of an oral B group vitamin in preventing the development of chemotherapy-induced peripheral neuropathy (CIPN). Support Care Cancer 25(1):195–204. https://doi.org/10.1007/S00520-016-3404-Y/TABLES/4
Cho K, Saikawa H, Hashimoto T, Katagiri H, Owada Y, Yakuwa K, Fujimura I, Utsumi Y, Akiyama M, Nagashima H, Takahashi F, Maemondo M (2023) A randomized trial to evaluate the preventive effect of lafutidine on chemotherapy-induced peripheral neuropathy in patients treated with carboplatin and paclitaxel for lung cancer. Ann Palliat Med 12(6):1136–1145. https://doi.org/10.21037/APM-23-90
Kaku H, Kumagai S, Onoue H, Takada A, Shoji T, Miura F, Yoshizaki A, Sato S, Kigawa J, Arai T, Tsunoda S, Tominaga E, Aoki D, Sugiyama T (2012) Objective evaluation of the alleviating effects of Goshajinkigan on peripheral neuropathy induced by paclitaxel/carboplatin therapy: a multicenter collaborative study. Exp Ther Med 3(1):60. https://doi.org/10.3892/ETM.2011.375
Su Y et al (2020) The effects of ganglioside-monosialic acid in taxane-induced peripheral neurotoxicity in patients with breast cancer: a randomized trial. JNCI J Natl Cancer Inst 112(1):55–62. https://doi.org/10.1093/JNCI/DJZ086
Werida RH, Elshafiey RA, Ghoneim A, Elzawawy S, Mostafa TM (2022) Role of alpha-lipoic acid in counteracting paclitaxel- and doxorubicin-induced toxicities: a randomized controlled trial in breast cancer patients. Support Care Cancer 30(9):7281. https://doi.org/10.1007/S00520-022-07124-0
Leal AD, Qin R, Atherton PJ, Haluska P, Behrens RJ, Tiber CH, Watanaboonyakhet P, Weiss M, Adams PT, Dockter TJ, Loprinzi CL (2014) North Central Cancer Treatment Group/Alliance trial N08CA-the use of glutathione for prevention of paclitaxel/carboplatin-induced peripheral neuropathy: a phase 3 randomized, double-blind, placebo-controlled study. Cancer 120(12):1890–1897. https://doi.org/10.1002/CNCR.28654
Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lantéri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R (2005) The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer 41(8):1135–1139. https://doi.org/10.1016/j.ejca.2005.02.012
Huang HQ, Brady MF, Cella D, Fleming G, Mackey D (2007) Validation and reduction of FACT/GOG-Ntx subscale for platinum/paclitaxel-induced neurologic symptoms: a gynecologic oncology group study. Int J Gynecol Cancer 17(2):387–393. https://doi.org/10.1111/J.1525-1438.2007.00794.X
Webster K, Cella D, Yost K (2003) The functional assessment of chronic illness therapy (FACIT) measurement system: properties, applications, and interpretation. Health Qual Life Outcomes 1(1):1–7. https://doi.org/10.1186/1477-7525-1-79/TABLES/1
Abdelfattah NM, Solayman MH, Elnahass Y, Sabri NA (2021) ABCB1 single nucleotide polymorphism genotypes as predictors of paclitaxel-induced peripheral neuropathy in breast cancer. Genet Test Mol Biomark. https://doi.org/10.1089/gtmb.2021.0014
Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier AL, Hiller L, Burns R, Jones L, Bowden SJ, Dunn JA, Poole CJ, Caldas C, Pharoah PPD, Earl HM (2014) Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin Cancer Res 20(9):2466–2475. https://doi.org/10.1158/1078-0432.CCR-13-3232/86320/AM/REPLICATION-OF-GENETIC-POLYMORPHISMS-REPORTED-TO
Tanabe Y, Shimizu C, Hamada A, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yamamoto H, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y (2017) Paclitaxel-induced sensory peripheral neuropathy is associated with an ABCB1 single nucleotide polymorphism and older age in Japanese. Cancer Chemother Pharmacol 79(6):1179–1186. https://doi.org/10.1007/S00280-017-3314-9/TABLES/4
Kus T, Aktas G, Emin Kalender M, Demiryurek AT, Ulasli M, Oztuzcu S, Kul S, Camci C (2016) OncoTargets and therapy dovepress polymorphism of CYP3A4 and ABCB1 genes increase the risk of neuropathy in breast cancer patients treated with paclitaxel and docetaxel. OncoTargets Ther. https://doi.org/10.2147/OTT.S106574
Hertz D, Roy S, Motsinger-Reif A, Drobish A, Clark L, McLeod H, Carey L, Dees E (2013) CYP2C8*3 increases risk of neuropathy in breast cancer patients treated with paclitaxel. Ann Oncol. https://doi.org/10.1093/annonc/mdt018
Abraham JE, Guo Q, Dorling L, Tyrer J, Ingle S, Hardy R, Vallier AL, Hiller L, Burns R, Jones L, Bowden SJ, Dunn JA, Poole CJ, Caldas C, Pharoah PPD, Earl HM (2014) Replication of genetic polymorphisms reported to be associated with taxane-related sensory neuropathy in patients with early breast cancer treated with paclitaxel. Clin Cancer Res 20(9):2466–2475. https://doi.org/10.1158/1078-0432.CCR-13-3232
Park SB, Kwok JB, Asher R, Lee CK, Beale P, Selle F, Friedlander M (2017) Clinical and genetic predictors of paclitaxel neurotoxicity based on patient- versus clinicianreported incidence and severity of neurotoxicity in the ICON7 trial. Ann Oncol 28(11):2733–2740. https://doi.org/10.1093/annonc/mdx491
Chen Y, Fang F, Kidwell KM, Vangipuram K, Marcath LA, Gersch CL, Rae JM, Hayes DF, Lavoie Smith EM, Henry NL, Beutler AS, Hertz DL (2020) Genetic variation in Charcot-Marie-Tooth genes contributes to sensitivity to paclitaxel-induced peripheral neuropathy. Pharmacogenomics 21(12):841–851. https://doi.org/10.2217/pgs-2020-0053
Kus T, Aktas G, Kalender M, Demiryurek AT, Ulasli M, Oztuzcu S, Sevinc A, Kul S, Camci C (2016) Polymorphism of CYP3A4 and ABCB1 genes increase the risk of neuropathy in breast cancer patients treated with paclitaxel and docetaxel. Onco Targets Ther 9:5073–5080. https://doi.org/10.2147/OTT.S106574
Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL (2012) A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 18(18):5099–5109. https://doi.org/10.1158/1078-0432.CCR-12-1590
Beutler AS, Kulkarni AA, Kanwar R, Klein CJ, Therneau TM, Qin R, Banck MS, Boora GK, Ruddy KJ, Wu Y, Smalley RL, Cunningham JM, Le-Lindqwister NA, Beyerlein P, Schroth GP, Windebank AJ, Züchner S, Loprinzi CL (2014) Sequencing of Charcot-Marie-Tooth disease genes in a toxic polyneuropathy. Ann Neurol 76(5):727–737. https://doi.org/10.1002/ANA.24265
Boora GK, Kulkarni AA, Kanwar R, Beyerlein P, Qin R, Banck MS, Ruddy KJ, Pleticha J, Lynch CA, Behrens RJ, Züchner S, Loprinzi CL, Beutler AS (2015) Association of the Charcot–Marie–Tooth disease gene ARHGEF10 with paclitaxel induced peripheral neuropathy in NCCTG N08CA (Alliance). J Neurol Sci 357(1–2):35–40. https://doi.org/10.1016/j.jns.2015.06.056
Peters CM, Jimenez-Andrade JM, Jonas BM, Sevcik MA, Koewler NJ, Ghilardi JR, Wong GY, Mantyh PW (2007) Intravenous paclitaxel administration in the rat induces a peripheral sensory neuropathy characterized by macrophage infiltration and injury to sensory neurons and their supporting cells. Exp Neurol 203(1):42–54. https://doi.org/10.1016/J.EXPNEUROL.2006.07.022
Schneider BP, Li L, Radovich M, Shen F, Miller KD, Flockhart DA, Jiang G, Vance G, Gardner L, Vatta M, Bai S, Lai D, Koller D, Zhao F, O’Neill A, Lou SM, Railey E, White C, Partridge A, Sparano J, Davidson NE, Foroud T, Sledge GW (2015) Genome-wide association studies for taxane-induced peripheral neuropathy in ECOG-5103 and ECOG-1199. Clin Cancer Res 21(22):5082–5091. https://doi.org/10.1158/1078-0432.CCR-15-0586
Zajaczkowską R, Kocot-Kępska M, Leppert W, Wrzosek A, Mika J, Wordliczek J (2019) Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. https://doi.org/10.3390/IJMS20061451
Tanabe Y, Shiraishi S, Hashimoto K, Ikeda K, Nishizawa D, Hasegawa J, Shimomura A, Ozaki Y, Tamura N, Yunokawa M, Yonemori K, Takano T, Kawabata H, Tamura K, Fujiwara Y, Shimizu C (2020) Taxane-induced sensory peripheral neuropathy is associated with an SCN9A single nucleotide polymorphism in Japanese patients. BMC Cancer. https://doi.org/10.1186/s12885-020-06834-0
Baldwin RM, Owzar K, Zembutsu H, Chhibber A, Kubo M, Jiang C, Watson D, Eclov RJ, Mefford J, McLeod HL, Friedman PN, Hudis CA, Winer EP, Jorgenson EM, Witte JS, Shulman LN, Nakamura Y, Ratain MJ, Kroetz DL (2012) A genome-wide association study identifies novel loci for paclitaxel-induced sensory peripheral neuropathy in CALGB 40101. Clin Cancer Res 18(18):5099–5109. https://doi.org/10.1158/1078-0432.CCR-12-1590/286451/AM/A-GENOME-WIDE-ASSOCIATION-STUDY-IDENTIFIES-NOVEL
Leandro-García LJ, Inglada-Pérez L, Pita G, Hjerpe E, Leskelä S, Jara C, Mielgo X, González-Neira A, Robledo M, Åvall-Lundqvist E, Gréen H, Rodríguez-Antona C (2013) Genome-wide association study identifies ephrin type A receptors implicated in paclitaxel induced peripheral sensory neuropathy. J Med Genet 50(9):599–605. https://doi.org/10.1136/JMEDGENET-2012-101466
Marcath LA, Kidwell KM, Vangipuram K, Gersch CL, Rae JM, Burness ML, Griggs JJ, Van Poznak C, Hayes DF, Smith EML, Henry NL, Beutler AS, Hertz DL (2020) Genetic variation in EPHA contributes to sensitivity to paclitaxel-induced peripheral neuropathy. Br J Clin Pharmacol 86(5):880–890. https://doi.org/10.1111/BCP.14192
Apell Aniz-Ruiz M et al (2017) Personalized medicine and imaging targeted sequencing reveals low-frequency variants in EPHA genes as markers of paclitaxel-induced peripheral neuropathy. Clin Cancer Res 23(5):1227–1235. https://doi.org/10.1158/1078-0432.CCR-16-0694
Flanagan JG, Vanderhaeghen P (2003) The ephrins and eph receptors in neural development. Ann Rev Neurosci 21(1):309–345. https://doi.org/10.1146/annurev.neuro211309
Thomaier L, Darst BF, Jewett P, Hoffmann C, Brown K, Makaram A, Blaes A, Argenta P, Teoh D, Vogel RI (2021) Genetic variants predictive of chemotherapy-induced peripheral neuropathy symptoms in gynecologic cancer survivors. Gynecol Oncol 163(3):578–582. https://doi.org/10.1016/j.ygyno.2021.10.006
Acknowledgements
None.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Author information
Authors and Affiliations
Contributions
AMASM collected, distributed, and organized the data sets and prepared the first draft of the manuscript. NOES contributed to the conception and design of the study. The final manuscript was revised by ES, NOES, and HA. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mahmoud, A.M.A.S., El Said, N.O., Shash, E. et al. Prevention of paclitaxel-induced peripheral neuropathy: literature review of potential pharmacological interventions. Futur J Pharm Sci 10, 67 (2024). https://doi.org/10.1186/s43094-024-00638-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00638-w