Abstract
Background
The long-term outcomes and risk factors of paclitaxel-induced peripheral neuropathy (PIPN) have not yet been fully elucidated.
Methods
We identified 219 breast cancer patients who received paclitaxel as adjuvant chemotherapy between 2002 and 2009. We retrospectively analyzed the incidence, time to onset, duration, and risk factors for PIPN by chart review.
Results
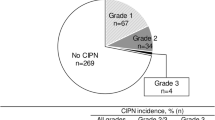
Of the 219 patients, 212 developed PIPN (97%) during a median follow-up time of 57 months (range 5.3–95.5). Median time to PIPN onset was 21 days (range 11–101) for the entire patient population: 35 days (range 14–77) for weekly administration and 21 days (range 11–101) for tri-weekly administration. PIPN caused termination of paclitaxel treatment in 7 patients (4%). Median duration of PIPN was 727 days (range 14–2621 days). PIPN persisted in 64 and 41% of patients at 1 and 3 years after initiating paclitaxel, respectively. Age ≥60 years and severity of PIPN were significantly associated with PIPN duration.
Conclusions
PIPN persists longer in older patients and in those who experience severe neuropathy. Further studies to identify the risk factors for PIPN are warranted.


Similar content being viewed by others
References
Henderson IC, Berry DA, Demetri GD et al (2003) Improved outcomes from adding sequential paclitaxel but not from escalating doxorubicin dose in an adjuvant chemotherapy regimen for patients with node-positive primary breast cancer. J Clin Oncol 21:976–983
Mamounas EP, Bryant J, Lembersky B et al (2005) Paclitaxel after doxorubicin plus cyclophosphamide as adjuvant chemotherapy for node-positive breast cancer: results from NSABP B-28. J Clin Oncol 23:3686–3696
Buzdar AU, Singletary SE, Valero V et al (2002) Evaluation of paclitaxel in adjuvant chemotherapy for patients with operable breast cancer: preliminary data of a prospective randomized trial. Clin Cancer Res 8:1073–1079
De Laurentiis M, Cancello G, D’Agostino D et al (2008) Taxane-based combinations as adjuvant chemotherapy of early breast cancer: a meta-analysis of randomized trials. J Clin Oncol 26:44–53
Rowinsky EK, Donehower RC (1995) Paclitaxel (Taxol). N Engl J Med 332:1004–1014
Akerley W, Herndon JE, Egorin MJ et al (2003) Weekly, high-dose paclitaxel in advanced lung carcinoma: a phase II study with pharmacokinetics by the Cancer and Leukemia Group B. Cancer 97:2480–2486
Winer EP, Berry DA, Woolf S et al (2004) Failure of higher-dose paclitaxel to improve outcome in patients with metastatic breast cancer: Cancer and Leukemia Group B trial 9342. J Clin Oncol 22:2061–2068
Nabholtz JM, Gelmon K, Bontenbal M et al (1996) Multicenter, randomized comparative study of two doses of paclitaxel in patients with metastatic breast cancer. J Clin Oncol 14:1858–1867
Gogas H, Shapiro F, Aghajanian C et al (1996) The impact of diabetes mellitus on the toxicity of therapy for advanced ovarian cancer. Gynecol Oncol 61:22–26
Rowinsky EK, Eisenhauer EA, Chaudhry V et al (1993) Clinical toxicities encountered with paclitaxel (Taxol). Semin Oncol 20:1–15
Rowinsky EK, Chaudhry V, Cornblath DR et al (1993) Neurotoxicity of taxol. J Natl Cancer Inst Monogr 15:107–115
Lee JJ, Swain SM (2006) Peripheral neuropathy induced by microtubule-stabilizing agents. J Clin Oncol 24:1633–1642
Seidman AD, Berry D, Cirrincione C et al (2008) Randomized phase III trial of weekly compared with every-3-weeks paclitaxel for metastatic breast cancer, with trastuzumab for all HER-2 overexpressors and random assignment to trastuzumab or not in HER-2 nonoverexpressors: final results of Cancer and Leukemia Group B protocol 9840. J Clin Oncol 26:1642–1649
Sparano JA, Wang M, Martino S et al (2008) Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med 358:1663–1671
Jones SE, Erban J, Overmoyer B et al (2005) Randomized phase III study of docetaxel compared with paclitaxel in metastatic breast cancer. J Clin Oncol 23:5542–5551
Goldhirsch A, Wood WC, Gelber RD et al (2007) Progress and promise: highlights of the international expert consensus on the primary therapy of early breast cancer 2007. Ann Oncol 18:1133–1144
Goldhirsch A, Ingle JN, Gelber RD et al, Panel members (2009) Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 20:1319–1329
Trotti A, Colevas AD, Setser A et al (2003) CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–181
Chaudhry V, Rowinsky EK, Sartorius SE et al (1994) Peripheral neuropathy from Taxol and cisplatin combination chemotherapy: clinical and electrophysiological studies. Ann Neurol 35:304–311
Wang MS, Davis AA, Culver DG et al (2002) WldS mice are resistant to paclitaxel (Taxol) neuropathy. Ann Neurol 52:442–447
Aplenc R, Glatfelter W, Han P et al (2003) CYP3A genotypes and treatment response in paediatric acute lymphoblastic leukaemia. Br J Haematol 122:240–244
Siau C, Bennett GJ (2006) Dysregulation of cellular calcium homeostasis in chemotherapy-evoked painful peripheral neuropathy. Anaesth Analg 102:1485–1490
Argyriou AA, Koltzenburg M, Polychronopoulos P et al (2008) Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol 66:218–228
Gauchan P, Andoh T, Ikeda K et al (2009) Mechanical allodynia induced by paclitaxel, oxaliplatin and vincristine: Different effectiveness of gabapentin and different expression of voltage dependent calcium channel α2δ-1 subunit. Biol Pharm Bull 32:732–734
Cavaletti G, Frigeni B, Lanzani F et al (2010) Chemotherapy-induced peripheral neurotoxicity assessment: a critical revision of the currently available tools. Eur J Cancer 46:479–494
Postma TJ, Heimans JJ, Muller MJ et al (1998) Pitfalls in grading severity of chemotherapy-induced peripheral neuropathy. Ann Oncol 9:739–744
Acknowledgments
We thank Ms. Nao Nakamura for helping with manuscript revision. This work was supported by a Scientific Research Grant of the Ministry of Health, Labour and Welfare (H21-O21).
Conflict of interest
The authors have declared no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Tanabe, Y., Hashimoto, K., Shimizu, C. et al. Paclitaxel-induced peripheral neuropathy in patients receiving adjuvant chemotherapy for breast cancer. Int J Clin Oncol 18, 132–138 (2013). https://doi.org/10.1007/s10147-011-0352-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10147-011-0352-x