Abstract
Background
The present review envisages the role of nanovaccines to combat the global challenges of antimicrobial resistance. Nanovaccines are a novel formulation comprised of nanomaterials coupled with an immunogenic component to elicit the immune response and provide protection against the desired infectious disease. The nanovaccines with unique physicochemical properties can be more efficient against targeting the desired tissues in the body, aids in prolong circulation to promote antigen-presenting cells to act upon the target antigens.
Main content
The present review envisages the development of nanovaccines against antimicrobial-resistant pathogens. The use of nanovaccines can exhibit potent antigenicity with prolonged retention and controlled release to induce both cell- and antibody-mediated responses. Nanovaccines usage is still in the early stages and can be next-generation immunisation for prophylactic and therapeutic efficiency. The future development of nanovaccines against multi-drug-resistant pathogens can explore new avenues. Based on these facts, the present review is designed from the previously reported scientific studies and compiled with the fact that nanovaccines can revolutionise vaccine strategies. The articles were extracted from reputed databases like PubMed, Scopus, and ESCI. The size and conjugating chemistry of nanomaterials can be beneficial in developing novel multi-nanovaccine formulations that can target pools of antimicrobial resistance mechanisms.
Conclusion
Overall, the nanovaccines can form one of the best effective modes of targeting multi-drug-resistant pathogens. The nanovaccines can stimulate the innate immune response and generate effective immune-therapeutic novel formulation against infectious pathogens. Based on these facts and considerations, the present article makes an alarming call to develop nanovaccines to counter multi-drug resistance.
Similar content being viewed by others
Background
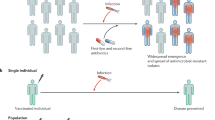
There is growing evidence to confront the problem associated with antimicrobial resistance worldwide [1]. According to World Health Organization (WHO), by 2050 the menace of drug resistance is expected to magnify and cause highest mortality and morbidity rates [2]. The development of drug resistance has conquered all spheres of ecology by affecting both humans and other living species [3]. In the near future, it can have a huge impact and cause imbalances in the socio-economic sector. The impact is higher in developing and low-income countries owing to inadequate healthcare systems and poor sanitary facilities [4]. Drug-resistant pathogens can master their metabolism and physiology to escape the efficacy of most of the available antibiotics [5]. Hence, there is growing interest in combating the situation of drug resistance, which has been recognised as one of the top priorities for research among the scientific communities [6]. There are different drivers that are responsible for expanding drug resistance, for instance, unnecessary prescription of antibiotics for conditions such as viral infections [7]. In most cases, the patient stops the prescribed antibiotics as the symptoms disappear which leads to antimicrobial resistance to the antibiotics. The misuse or overuse of antibiotics in poultry and animal husbandry increases drug resistance [8]. The consumption of undercooked food can lead to food poisoning which is caused by the pathogenic micro-organisms. Faecal contamination from infected individuals can be one of the major sources of transmission of antimicrobial resistance when it enters the ecosystem [9]. The unhygienic medical conditions can lead to nosocomial infections, and sometimes, these pathogens cause secondary infection which often be life-threatening [10]. In order to curb these situations, management strategies such as antibiotic stewardship, usage of antibiotics in livestock, and cluster sites of nosocomial infections are implemented and are under the radar of governmental agencies. Yet, antimicrobial resistance is continually growing [11]. There is pool of gene exchanges among the pathogenic micro-organisms which accelerates the resistance especially among zoonotic and aquatic organisms [8]. To overcome these situations, a large amount of funding support is offered to develop a new wave of antimicrobial agents that can control the afflictions caused by drug resistance [9]. In recent years, principles of nanotechnology have offered significant benefits to the medical sector in the form of developing rapid on-site diagnostic kits, targeted drug delivery, drug designing, and nanomedicines and their usage as antimicrobial agents [10]. To achieve greater heights, researchers are also exploiting the concept of nanotechnology to develop nanovaccines. It has been demonstrated that nanosized particles encapsulated within the carrier molecules are reported to initiate and enhance the immune response, which helps prevent the self-degradation of vaccines [11, 12]. Hence, with these facts and figures, the present mini-review summarises the possible quantification of developing a novel nanovaccine that can uplift the vaccination process. The concept of vaccination can be traced to ancient times before scientific knowledge could evolve, when snake venom was used to initiate the immune response against snake bites by Buddhist monks [13]. It was the efforts of Edward Jenner that demonstrated the use of cow pox to prevent small pox in humans. Broadly, the term "vaccine" can be defined as a biological preparation of an attenuated form of an organism or their products that is formulated to enhance the immune response [14]. There are different ways to construct vaccines, which are presented in Fig. 2. Broadly, these vaccines can be grouped into prophylactic and therapeutic vaccines based on their mode of action. Vaccination is the process of stimulating the immune response and protecting the human body from targeted disease [15]. It can mimic the body’s immune response, which in turn triggers the immune cells and aids in the recognition and subduing of the disease-causing organisms during later contact [16].
Main text
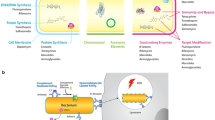
Nanovaccine
Nanovaccines are a novel formulation composed of nanoscale particles that can be solely or attached to microparticles for intentional stimulation of the immune response against a particular disease [17]. The nanovaccines are designed to harness the body’s ability to fight and suppress the desired disease. The use of nanovaccines could offer better advantages owing to their physicochemical properties like size, shape, inertness, functional moieties, and biodegradation, which can offer higher biocompatibility and bioavailability [18]. Nanovaccines can generate site-specific profiles and minimise the potential side effects. They can be used at low concentrations and tuned to trigger immune-stimulatory properties that generate antigenicity and activate the immune cells [19]. The size of the nanovaccines is similar to that of many cellular components, which can easily enter the cellular mechanism via a process called endocytosis. Also, they are designed to induce a robust and long-lasting response as the first line of defence [20]. Studies have also reported that nanovaccines can enter antigen-presenting cells via different modes, which is critical to generating an immune response to intracellular pathogens [21]. There are different modes via which nanovaccines can elicit immune response (Fig. 1). The process of developing nanovaccination requires minimal formulation, which can be more stable than conventional vaccines and offer long-term benefits. Furthermore, the processes of nanoemulsion and encapsulation can generate higher affinity rates [22]. The nanovaccines can also be conjugated with the desired antigen to mirror and trigger the B-cell response. It has been reported that during the development of nanovaccines, conjugated antigens create surface modifications that can offer stability and reach the desired location in the organs. These nanovaccines can be modified to enhance both humoral and cellular immune systems [23]. Hence, in order to develop nanovaccines, various parameters are accounted for, including the type of nanovaccine, mode of preparation, mode of action, properties, and stability of the nanovaccines in comparison with conventional vaccines [24]. Furthermore, availability and reaching the masses at affordable rates are also important parameters to be considered. Hence, some of these considerations are briefly described in the present review.
Properties and mechanism of nanovaccines
The size-dependent properties of nanovaccines play an important role in immunomodulation and biodistribution within the body. In a recent study, nanosized particles measuring 146 nm in size generated a stronger response in mice as compared to 64-nm-sized particles. The nanovaccines generate antigenicity by triggering a specific immune response generated by B cells to produce antibodies [25]. These antibodies recognise both nanoscale particles and their conjugates as foreign particles and induce antibody production. The production of antibodies also depends on the type of composition of the nanovaccines, the surface coating, the uptake, and the processing by cells of the immune system [26]. Further, the adjuvanticity of nanovaccines is a crucial factor in gaining the efficacy and effectiveness of vaccination. The exact mechanism of nanovaccines' adjuvanticity is yet to be completely elucidated, but a perusal of studies has revealed that nanovaccines can stimulate the antigen-presenting cells to enhance the antigen uptake [27]. Interestingly, the size of the nanovaccines allows them to easily penetrate through cells and travel via lymphatic nodes [28]. Nanovaccines can be designed to target-specific immune cells or tissues. Modifying the nanocarrier surface with ligands or antibodies enables the binding of specific receptors on immune cells, increasing the efficiency of the vaccines. The size of the nanoparticles enhances tissue penetration and enables immune cell interaction and activation at the infected site [29]. The nanovaccines are usually designed to target dendritic cells and induce an inflammatory response, which involves the secretion of cytokines by effector immune cells to act on foreign bodies [30]. To initiate an inflammatory response, the physicochemical properties of nanoscale particles play a vital role. It has been demonstrated that cationic-based nanovaccines are reported to induce more cytokine production than anionic nanovaccines. The functional groups and surface modifications of nanovaccines can influence the expression of dendritic cells [31]. The use of nanovaccines can be more advantageous in comparison with conventional vaccines. The incorporation of the antigen into nanocarriers protects the components from degradation and can increase their shelf life. In contrast, conventional vaccines require strict temperature control and maintenance [11]. On a similar front, the dosage of vaccines also plays an important role; in the case of conventional vaccines, the dosage depends on factors such as the target population, the specific vaccine, and the level of immune response [32]. In some cases, large or multiple dosages are required to protect against infections. Nanovaccines have a high surface area, which influences the dosage level by conjugating the nanoparticles with components like liposomes and viral vectors, which enhance the efficiency of the vaccines and minimise the administration of multiple dosages [33].
Types of nanovaccines
The different types of nanovaccines are grouped based on various parameters such as composition, type of construction, shape, and their mode of action against desired diseases [33]. These nanomaterials are often small, ranging in size from 1 to 200 nm, and have myriad shapes. In most cases, nanomaterials are used to act as carrier molecules to achieve the desired delivery system [34]. Some of the nanovaccines are discussed below:
Metal and metal oxide-based nanomaterials
Metallic nanomaterials are regarded as one of the most diverse classes of nanomaterials, with a large number of useful properties [35]. The unique properties offer functional possibilities for tuning their applications. Scientific studies have demonstrated that metallic nanoparticles, such as silver nanoparticles, are able to activate leukocytes such as macrophages [36]. Similarly, gold nanoparticles are reported to induce the secretion of cytokines and can efficiently penetrate cells and activate the first line of defence mechanisms by triggering antigen-presenting cells [37]. Furthermore, aluminium-based nanomaterials are widely used as vaccine adjuvants and are regarded as one of the safest for human vaccination [38]. In recent years, mesoporous nanosilica rod has been employed in developing nanoformulations owing to its biocompatibility. It has been reported that these nanosilica rods create a microenvironment in the host system that initiates the recruitment of dendritic cells and triggers inflammatory signals [39].
Liposomes
Liposomes are composed of lipid bilayers with different sizes, usually ranging from 50 to 500 nm. The applications of liposomes are well demonstrated in biomedical sectors, especially in drug delivery systems [40]. They are also being employed in the formulation of nanovaccines as an adjuvant that has displayed prolonged stability and triggers the antigen-presenting cells to initiate the immune response. Liposomes are tailored with immune-stimulating ligands to initiate more specific activity [41].
Exosomes
It is composed of a lipophilic bilayer composed of proteins and genetic materials that are regarded as biocompatible and are being employed in the preparation of nanovaccines [42]. They are targeted against immunosuppressive conditions and cancer to improve the therapeutic index of both human and animal infections. The size of the exosomes usually varies from 50 to 100 nm and can induce an immune response at the targeted sites [43].
Proteosomes
Proteosomes are one of the most safe and biocompatible nanovaccines for human vaccination. They are hydrophilic in nature, with sizes ranging from 10 to 50 nm, and can easily interact with the immune system by presenting the antigen to T cells. They are in practice in cancer immunisation and nasal drug delivery systems [44].
Nanobeads
The development of nanobeads for nanovaccination has great potential owing to their size and high surface ratio. The antigen can be loaded onto the surface of nanobeads, which can be delivered at the targeted sites. They are reported to activate both humoral and cell-mediated immune responses. They are often used in cancer vaccinations. The size of the nanobeads can be prepared in accordance with the desired application [45].
Virosomes
It is composed of liposomes and viral proteins, which can easily penetrate through the cell and reach the target site. The hydrophobic property of the surface can ease the loading of the antigens. The size of the virosomes can vary from 20 to 50 nm in diameter and can be composed of glycoproteins from different viruses, such as influenza virus and herpes virus. In recent years, they have been used as a carrier molecule for desired antigens [46].
Bacterial spores
The bacterial spores are used in the nanovaccine formulation to transport antigens. The spores secreted by Bacillus subtilis can carry antigens on their surface, which can bind with proteins like Cot B and Cot C on the surface of the spores. They are considered to be cost-effective and highly reliable, especially for treating anthrax in humans and animals [47].
Possible target of nanovaccines against microbial pathogens
In recent studies, nanoparticle-based vaccines have shown great potential in combating wide range of microbial pathogens (Table 1). There is need to promote further studies to optimise the important parameters. In the context of tuberculosis, chitosan-based nanoparticles have shown potential for vaccine delivery at targeted site [48,49,50,51,52,53,54,55,56,57,58,59,60,61,62]. Similarly, in case of anthrax the poly(D, L-lactic-co-glycolic acid) nanospheres have been employed for vaccine development [49]. The use of lipo-peptide-based nanovaccines are being investigated to control the infection [50]. The medical benefit of gold nanoparticles is also evaluated for the development of nanovaccines against tetanus [51]. Chamydia infection has been targeted using vault nanoparticles which contains immunogenic proteins to target different sites [52]. In case of development of viral vaccines, use of liposomes and gold nanoparticles has been explored [53,54,55,56,57,58,59,60]. Similarly, in order to combat parasitic infections, α-helix self-assembling peptide nanoparticles have been studied as nanovaccines for toxoplasmosis [61]. Iron oxide nanoparticles have been explored as nanocarriers for malaria vaccines [62].
Vaccines for bacterial infections
Methicillin-resistant staphylococcus aureus (MRSA)
It is recognised as a prolific pathogenic bacterium and is considered one of the major causes of nosocomial infection [20]. The strains of MRSA are capable of having a propensity to develop resistance to a different range of antibiotics, including the new chemically derived synthetic variants of existing antibiotics [63]. The degree of the infection caused by MRSA can range from minor to severe bacteremia. Targeting the colonisation of MRSA and preventing the infection have been a major challenge due to the expansion of resistant strains. In recent years, the use of nanomaterials with bactericidal potential against MRSA has shown significant activity [64]. These nanomaterials can be used to develop nanovaccines by developing nanomaterials composed of target antigens like polysaccharides and toxoids. According to a study conducted by [65] Wang et al., a nanosponge-based vaccine was designed and developed that could target the membrane-disturbing mechanism caused by the pore-forming toxins, which are recognised as virulent proteins of most bacterial pathogens. The nanosponge is composed of hybrid nanomaterials coated with RBC membrane, which can absorb different pore-forming toxins. Conventional vaccines are often not feasible as infections caused by MRSA colonise different niches in the body, and these may have different virulence factors. Hence, solely capsular-based vaccines are not able to generate an immune response sufficient to provide complete protection. Nanovaccination can provide a new platform to develop a universal vaccine formulation composed of different immunogenic components bearing specific activity.
Vibrio cholera
The enteric infections caused by Vibrio cholerae are considered to have one of the leading global health implications [66]. According to the WHO, half of the diarrheal infections are caused by the Vibrio cholerae strain, especially in children. According to the WHO, half of the diarrheal infections are caused by the Vibrio cholerae strain, especially in low-resource and underdeveloped countries. If untreated, the infection leads to the death of the individual due to the enterotoxins produced by Vibrio cholerae. The conventional vaccines used in the control of enteric infection caused by Vibrio cholerae strains are based on the toxoid, attenuated form of whole cell and outer membrane vesicles [67]. But most of these vaccines require cold storage facilities, which are rare in remote areas of low-income developing countries, which are at high risk of these enteric infections. As an alternative strategy, a vaccine based on nanomaterials can be developed, which can provide relief in providing the vaccination to the targeted population with minimal healthcare facilities. Further, chitosan-based nanomaterials containing cholera toxin (Ctx) formulation was developed to entrap the antigen protein responsible for the cause of infections. The formulations were prescribed to BALB/c mouse groups in three different batches, such as oral, injection, and oral injection groups. The results displayed neutralisation of Ctx toxin in the immunised mice, and the study concluded with the fact that chitosan nanoparticles can improve immune responses and can be implemented for vaccine delivery and development [68].
Drug-resistant Escherichia coli (E. coli)
Escherichia coli is one of the most prevalent bacterial species that inhabits normal microbial flora and, in severe conditions, becomes an opportunistic pathogen [69]. They are reported to cause deleterious health effects such as urinary tract infections and sepsis in the blood. Zoonotic organisms are considered to be one of the major reservoirs [70]. The increase in drug-resistant E. coli strains has created a huge impact with a limited choice of drug treatment. There are different virulence factors, such as EspA, EspB, and EspD, which contribute to the resistance mechanisms. Hence, in order to control this expansion of drug-resistant E. coli strains, nanovaccines are being developed as alternatives. According to Khanifar et al., 2019, a nanovaccine against Enterohemorragic E. coli (EHEC) infections was developed by encapsulating eEIT onto chitosan nanoparticles The mice immunised orally with hybrid nanomaterials were capable of strong humoral and mucosal immune responses and reduced the infections caused by EHEC [71]. In a recent study, SinH, a gene-coding protein responsible for the bacteremia and urinary tract infections, was barcoded. In order to elicit the immune response, a recombinant SinH-based vaccine was developed and immunised a murine host [72]. The administration resulted in protection against various strains responsible for causing severe infections. The immunised cohorts were able to protect themselves with the production of high levels of serum IgG and urinary IgG and IgA. Such studies can be one of the bases for the development of nanovaccines and enhance the protection level with minimal dosages [73]. Studies have also revealed that administering hybrid nanomaterials orally to mice causes strong immunological reactions, including humoral (antibody) and mucosal immunity. As a result, the infections caused by EHEC are significantly reduced [74].
Vaccines against viral infections
Hepatitis
Infections caused by the hepatitis virus are often severe owing to the fact that they target immune-compromised patients and have various health implications [75]. Conventional vaccines are never feasible to target secondary infections. Hence, based on these considerations, the recent development of nanovaccines composed of nanoparticles conjugated with an attenuated form of a viral component can provide protection by producing antibodies [76]. These nanovaccines can offer a better cure against hepatitis infections without the use of any needle vaccination process by using nanoemulsions, which can be immunised via nasal vaccination [77]. According to Gregory et al. [78], modified nanoparticles of poly(D,L-lactic-co-glycolic acid) encapsulated with viral envelope protein would elicit an immune response by triggering the cells to secrete pro-inflammatory cytokines [78]. The nanoparticles, owing to their physicochemical properties, can also participate in the generation of a strong immune response against the encephalitis in the mouse model [79]. Furthermore, a chitosan nanoparticle-based nanovaccine formulation was designed and composed of hepatitis B surface antigen, which was coated with sodium alginate. The developed nanovaccine was administered to mice, which resulted in an enhanced immune response [80].
Influenza
Influenza viruses are one of the most common causes of respiratory illness [81]. The degree of infection is associated with the economic crisis in the healthcare sector owing to the fact that these pathogens undergo antigenic drift [82]. These drifts give rise to different variants of strains. Hence, there is great interest in developing universal novel vaccines for influenza infections [83]. To provide cross-strain protection, nanoparticle-based vaccines are being developed. The nanovaccines containing capsid and hemagglutinin proteins were developed to provide humoral and cellular responses by activating B-cells along with CD4 and CD8 T cells [84, 85]. The seasonal influenza vaccines provide minimal coverage against influenza strains causing pandemic infections. In a recent study, mRNA-based lipid nanoparticle-based vaccines were developed that encode the hemagglutinin antigens from all 20 different reported subtypes of the influenza A and B lineages. This multivalent vaccine could produce antibodies against all 20 encoded antigens. The study concluded with the fact that mRNA–lipid nanoparticle-based vaccines provided protection against multiple antigenically variant strains of influenza viruses [86].
Human immunodeficiency virus (HIV)
The burden posed by the epidemic of HIV drastically influences the socio-economic status of countries, especially the developing countries [87]. Several prevention measures have been implemented in the recent past, but it is still striving to be one of the most deadly infections to mankind based on the fact that it deteriorates the immune system, making it vulnerable to secondary infections [88]. According to the review proposed by Barouch [89], attempts at developing conventional vaccines have failed owing to myriad facts such as the lack of an appropriate animal host to conduct the research trials, extensive viral clade and sequence diversity, the fact that attenuated forms of viruses are unsafe for human trials, and the fact that antibody responses are type-specific [89]. Based on these facts, developing effective vaccines against HIV is still a large and difficult task. In recent attempts, the development of nanovaccines has gained momentum to design a nanomaterial-based vaccination. According to Vela Ramirez et al. [90], biofunctionalised polymeric nanoparticles with ligands were able to target the receptors on immune cells. These ligands bind to the specific receptors on immune cells and mimic the processes of immune response, such as activation of antigen-presenting cells, immunomodulation, and stabilisation of protein antigens. The study aimed to develop positively charged polyanhydride nanoparticles functionalised with carbohydrate, which were efficiently taken up by dendritic cells and activated CD40 and CD206 to secrete cytokines. The study provides a basis for developing a biofunctionalised nanoparticle-based vaccine against HIV [90].
Vaccines against fungal infections
Fungal infections are affecting human populations in a vast geographical area of the globe, and yet scanty reports are available on research pertaining to developing vaccines against these infections [91]. The scientific literature envisions major obstacles to developing potent vaccines against targeted fungal infections, for instance, biocompatible hosts, quality of vaccine formulations, stability, and immunogenicity. Some of the serious fungal infections, which are considered to be the major contribution of drug resistance, are briefly described in the following section, along with possible strategies to develop nanovaccines.
Candida
The incidence and prevalence of infections caused by Candida species have a devastating impact on the quality of living organisms across the globe. The drug-resistant species of Candida are reported to have components like capsules, resistant enzymes, and adhesins that mediate the degree of fungal infection [92]. The vaccines, which are reported to be under clinical trials against Candida infections, are based on the secretory aspartyl proteinase (SAP) enzyme, which is reported to provide protection by neutralising SAP antibodies [93]. Furthermore, the agglutinin-like sequence (Als3) vaccine is composed of alum as an adjuvant with the N-terminal portion of recombinant Als3 [94]. This vaccine provides protection by initiating Th1 and Th17 cells along with B cells. The use of nanoparticles as adjuvants can enhance stability, cellular uptake, and immunogenicity. Also, biodistribution at different sites with controlled release can also be possible with nanoparticles [95].
Aspergillus spp.
The infections caused by Aspergillus species have been mounting in recent years. It is reported that aspergillosis infections are one of the major causes of increased mortality in hospital-acquired infections [96]. Targeting the population for vaccination against aspergillosis is often difficult owing to the fact that it is associated with immune-compromised patients. They are often associated with candidiasis and weaken the immune system. Recently, a panfungal vaccine composed of β-glucan and a crude extract preparation of Aspergillus Asp. f3 could generate protection against A. fumigatus. Furthermore, protein-conjugated vaccines consisting of glucan can be effective against all fungi that contain glucan in their cell walls [97].
Cryptococcus spp.
Similar to Aspergillosis, developing a vaccine against Cryptococcus infection is a difficult task as it infects patients with absent or defective T-cell generation. Attempts have been made to develop vaccines using capsule components of C. neoformans glucuronoxylomannan (GMX), which induce an immune response. Also, engineered strains of C. neoformans were produced and injected into mice to secrete IFN-g cytokines in the absence of T cells [98]. In order to make the nanovaccine a successful candidate, myriad factors play pivotal roles. Some of these essential components are discussed in the following sections; one such priority includes the mode of administration. There are several ways of administering nanovaccines, including oral and injectable, which are the most frequent, as well as transdermal, transmucosal, ophthalmic, pulmonary, and implantation. Furthermore, other particles are being investigated, including PLGA, PGA, PCL, and PEO [99,100,101].
Administration of nanovaccines
There are several ways of administering nanovaccines, including oral and injectable, which are the most frequent, as well as transdermal, transmucosal, ophthalmic, pulmonary, and implantation (Fig. 2). These modes are one of the established routes of administration [102]. Intradermal administration involves injecting the vaccine into the epidermis, or outer layer of skin, where it is absorbed slowly, producing a sustained effect [103]. This method is often combined with adjuvants, such as oil emulsions or saponins, to enhance the immune response [104]. There are various scientific studies that support the importance of the intradermal vaccination process against viral infections like influenza and hepatitis B. One notable advantage of this mode of vaccination is the dose-sparing effect, where small vaccine doses can easily enhance the immune responses. The use of microneedles and patches as modes of delivery can be convenient and self-administrative [100]. In the case of nanovaccines, the intradermal will be handy for prolonging the duration of the immune response for effective treatment against targeted pathogens [12]. Intramuscular injection delivers the vaccine directly to muscle tissue, but this method may produce a modest immune response [32]. The intramuscular mode of vaccination is one of the established routes for administering vaccines. It offers great advantages in terms of generating immunogenicity, vaccine delivery, ease of administration, and many more. In this type of process, it stimulates the immune cells present in the muscle, which leads to robust and effective immune responses [105]. In a recent study, the nanovaccine delivered intramuscularly generated strong humoral and cellular immune responses in mice, making it a good option for single-dose antigen delivery via the intramuscular route [30]. The study concluded the importance of nanovaccines against Hepatitis B treatments that require repeated administration and booster doses in conventional mode administration [106]. Subcutaneous injection, or injecting the vaccine just under the skin, can be enhanced by using PEGylated liposomes, which help stabilise the vaccine and increase its uptake in the lymph nodes [107]. The subcutaneous mode of vaccine administration offers several advantages in stimulating the immune response, as this is one of the most convenient modes and the vaccines are delivered to a site that is rich in immune cells, which results in the production of antibodies in higher concentrations. This mode serves as one of the reservoirs and allows the sustained release of vaccines. A large number of vaccines are widely used in the practice of using this mode of administration process [105], oral administration requires a high concentration of the vaccine due to the dilution that occurs in the gastrointestinal tract [108]. Oral administration offers advantages such as ease of administration, stimulation of mucosal immune responses, enhanced stability, and the potential for wider acceptance with a needle-free vaccination process. In some cases, the vaccines are more stable in their oral formulation [109]. Nasal administration can be done using a needle-free method, such as a nasal spray, but this method can be challenging in terms of accurately delivering the vaccine to the entire mucosal area of the nose [110]. Mucosal administration has been investigated as a route for administering nanovaccines, and it has been demonstrated to shield mucosal surfaces against most diseases. Administration through the mucosa, targeted antigen delivery, enhanced antigen presentation, and prolonged antigen release are only a few of the nanovaccine techniques that have been shown to improve the immunogenicity of antigens [109]. Topical administration involves using patches to deliver the vaccine through the skin, which has the advantage of being non-invasive and producing localised effects with fewer side effects [111]. This mode of administration has advantages wherein the immune system can mount a response by accelerating antibody production: it is also a needling-free process of administration. There are various types of vaccines that hold promise via this mode of administration, for instance, influenza, measles, polio, etc. [112]. However, this method may be limited in terms of size and the number of uses. Administering a vaccine through the vaginal route has several benefits, including a high level of permeability, a large surface area, and the ability to bypass the first pass of metabolism [113]. The vaginal route also has a rich blood supply, making it a good option for vaccination, especially against pathogens that can infect this area [114]. The vaginal route is considered advantageous as it may induce local mucosal immunity, providing protection at the site of potential pathogen entry. Furthermore, this mode of administration might increase vaccine accessibility for women in regions with limited access to health care and may offer an alternative to traditional needle-based vaccinations. The vaginal route of vaccine administration involves delivering vaccines directly into the vagina for the purpose of eliciting an immune response in the female reproductive tract. This method is being explored for specific vaccines, such as those targeting sexually transmitted infections like the human papillomavirus and herpes simplex virus [115]. A vaccine for the human papillomavirus was administered using a mucoadhesive delivery system, which improved the production of antibodies against the virus [116]. The mucoadhesive delivery systems allow the adherence of vaccine formulations to mucosal surfaces. This type of route has a prolonged immune response and is target-specific; studies show the stimulation of both local and systemic immune responses. It is used to broaden immunisation strategies; for instance, it prevents the entry of pathogens through mucosal surfaces [115]. The vaccine, which was composed of virus-like particles, induced a local and systemic immune response and helped control the entry of associated pathogens. Vaccines can be administered through the mucous membrane lining the eye as a way to protect against infections [117]. However, this method has some challenges, such as the high rate at which tears are produced, the risk of the vaccine being absorbed into the body, and the limited ability of the cornea to drain and metabolise the vaccine [118]. Despite these limitations, eye drops have been used to vaccinate against influenza H1N1, and vaccination against herpes simplex virus type 1 was tried on mice using an ocular approach that included iron nanoparticles, glutamic acid, and a DNA vaccine for herpes stromal keratitis to generate protection [119]. Overall, researchers are seeking ways to improve the effectiveness of vaccine delivery and enhance the immune system's response to specific antigens.
Physicochemical properties of nanoparticles for nanovaccines
In recent decades, the physicochemical properties of nanoparticles have been studied to evaluate their applicative properties [120]. In developing nanovaccines, these properties play an important role in attenuating the activity. The size of the nanoparticles constitutes one of the important aspects of nanovaccines. The studies have demonstrated the role of the diameter of the nanovaccines and their immunogenicity [121]. Nanovaccines with smaller particle sizes may readily pass through epithelia and other biological barriers, but particles with diameters ranging from 20 to 50 nm are more likely to drain to lymphatic arteries and collect in lymph nodes [122]. Similarly, the shape of the nanovaccine influences the immune response; a spherical shape tends to deliver the most effective immune response in comparison with other shapes of nanoparticles [123]. Studies report that shape of nanoparticles affects the reciprocity between nanoparticles and immune cells. According to Kumar et al. Ovalbumin, with a sphere-shaped small particle diameter of 193 nm, induced a primary immune reaction as compared to the rod-shaped particle of 1530 nm [124]. The hydrophobicity of the nanoparticles can influence the immune response in several ways. The hydrophobic nanoparticles may be more efficiently taken up by certain immune cells, for instance, the memory cells in the Peyer's patches, which can facilitate their interaction with immune cells and potentially enhance the immune response [125]. Studies have also proven the hydrophobicity of the nanoparticles can affect the cell membranes, which in turn triggers an immune response [126]. In contrast, hydrophilic nanoparticles may be less efficiently taken up by immune cells and may cause less damage to cell membranes, leading to a weaker immune response [127]. The surface charge of a nanoparticle can influence its ability to interact with cells and the immune response. The negatively charged nanoparticles may be more efficiently taken up by macrophages, while positively charged nanoparticles may be more efficiently taken up by dendritic cells [128]. It has been demonstrated that the positive surface charge of nanoparticles is one of the factors influencing their concentration in the blood. When compared to nanoparticles with neutral or negative surface charges, it causes haemolysis and platelet aggregation [129]. The surface charge of a nanoparticle can also affect its ability to interact with extracellular matrix proteins and other components of the microenvironment, which can influence its uptake and distribution within the body. Studies suggest that the positively charged nanoparticle causes an inflammatory reaction and activates complementary systems [130]. When studies were conducted on negatively charged gold nanoparticles, they were capable of inducing a higher immunological response. It has also been found that nanoparticles with positive surface charges accumulate in the bloodstream. It induces haemolysis and platelet aggregation compared to the neutral or negative surface charge of nanoparticles [131].
Perspective and conclusion
The extensive research on nanotechnology has harnessed potential in various sectors. Nanoparticle-based diagnostic tools and novel formulation are under the clinical trails for the approvals. The nanoparticle-based vaccines have generated immense potential owing to the facts that these nanobased vaccines can elicit the immune response with minimal dosages [132]. The nanovaccines exhibits potent antigenicity with prolonged retention process and targeted site to induce cell-mediated and antibody-mediated immune responses. This characteristic minimises the usage of booster dosages. Hence development of nanovaccines hold promise for both prophylactic and therapeutic applications. However, in order to attenuate commercialised process, nanovaccines should addresses the issues like the toxicity studies, scalability, stability and meet the regulatory guidelines [19]. In case of antimicrobial resistance, the use of nanovaccines can offer promising strategies to neutralise the pathogenic micro-organisms and their toxic elements. The nanovaccines can stimulate immune response against particular pathogens and suppress their colonisation within the body [133]. The conventional vaccination for antimicrobial resistance has been hampered by various limitations, for instance, weak immunogenicity, targeting the normal commensal members, durability, and economic feasibility [134]. Hence, recent scientific advancements in nanotechnology and improvements in vaccine technology have resulted in the development of nanovaccines that can act efficiently and reduce the burden of antimicrobial resistance [135]. The size and functional conjugating chemistry of nanomaterials can benefit the development of novel multi-nanovaccines that can target a pool of antimicrobial-resistant organisms by generating an immune response based on the conserved virulence factors of pathogens, which are not found in normal bacteria [136]. This can aid in eradicating the pathogenic micro-organisms which have gained resistant to last resort antimicrobial agents by exerting the counter selection [137]. Ideally, these nanovaccines can minimise the colonisation of the pathogens by targeting the site of pathogen transmission, which is not always possible with conventional vaccines. In cases like MRSA strains and the lineage of S. aureus, developing conventional vaccination is difficult due to multiple virulent factors and the lack of an ideal animal model to test the efficacy of vaccines [138]. Also, a diverse class of polysaccharides, which forms virulent factors in drug resistance, is difficult to target. Furthermore, developing vaccine against tuberculosis and viruses like HIV, is yet to gain the success to reach the mass eradication. These facts make nanovaccines which can be developed based on the multi-virulent factors. The nanovaccines can also aid in controlling drug resistance among farm animals, which are reported to be one of the major reservoirs of antimicrobial resistance and can transmit easily. The nanovaccines, owing to their unique and extraordinary properties, can also add synergistic effects along with antibiotics or with the conserved components of pathogens, which can elicit the immune response by targeting the efflux pump cassette [133, 139]. This can help in eradicating the health care-associated infections that reflect the burden posed by antimicrobial resistance. The nanovaccination can eventually reduce the transmission of resistant organisms and minimise the use of antibiotics in the targeted population. Further, research policies, clinical trials, and funding resources are likely to play an important role in the successful development of nanovaccines and their commercialisation to reach the masses and the targeted population [140].
Availability of data and materials
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
Abbreviations
- WHO:
-
World Health Organization
- T cells:
-
T lymphocytes
- nm:
-
Nanometres
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- Ctx:
-
Cholera toxin
- BALB/c:
-
Bagg Albino mice strain
- E. coli :
-
Escherichia coli
- EHEC:
-
Enterohemorrhagic Escherichia coli
- eEIT:
-
Encapsulated Esp A, Intimin, Tir (Esp: E. coli-secreted protein)
- B cells:
-
B Lymphocytes
- CD4:
-
Cluster of differentiation type 4
- CD8:
-
Cluster of differentiation type 8
- HIV:
-
Human immunodeficiency virus
- CD40:
-
Cluster of differentiation type 40
- CD206:
-
Cluster of differentiation type 206
- SAP:
-
Secretory aspartyl proteinase
- Als3:
-
Agglutinin-like sequence
- Th1:
-
T helper1 cells
- Th17:
-
T helper17 cells
- GMX:
-
Glucuronoxylomannan
- PLGA:
-
Polylactic-co-glycolic acid
- PGA:
-
Polyglycolic acid
- PCL:
-
Polycaprolactone
- PEO:
-
Polyethylene oxides
- PEG:
-
Polyethylene glycol
- H1N1:
-
Hemagglutinin1 neuraminidases1
- DNA:
-
Deoxyribonucleic acid
- S. aureus :
-
Staphylococcus aureus
References
Chinemerem-Nwobodo D, Ugwu MC, Oliseloke Anie C, Al-Ouqaili MT, Chinedu Ikem J, Victor Chigozie U (2022) Antibiotic resistance: the challenges and some emerging strategies for tackling a global menace. J Clin Lab Anal 36:1–10. https://doi.org/10.1002/jcla.24655
Dadgostar P (2019) Antimicrobial resistance: implications and costs. Infect Drug Resist 12:3903–3910. https://doi.org/10.2147/idr.s234610
Chu EW, Karr JR (2017) Environmental impact: concept, consequences, measurement ref mod. Life Sci. https://doi.org/10.1016/b978-0-12-809633-8.02380-3
Mills, (2020) The health systems of low- and middle-income countries. Public Health. https://doi.org/10.1093/obo/9780199756797-0199
Mobarki N, Almerabi B, Hattan A (2019) Antibiotic resistance crisis. Int J Med Develop Countries https://doi.org/10.24911/ijmdc.51-1549060699.
Podolsky SH (2018) The evolving response to antibiotic resistance (1945–2018). Palgrave Commun. https://doi.org/10.1057/s41599-018-0181-x
Llor C, Bjerrum L (2014) Antimicrobial resistance: risk associated with antibiotic overuse and initiatives to reduce the problem. Ther Adv Drug Saf 5(6):229–241. https://doi.org/10.1177/2042098614554919
Manyi-Loh C, Mamphweli S, Meyer E, Okoh A (2018) Antibiotic use in agriculture and its consequential resistance in environmental sources: potential public health implications. Molecules 23(4):795. https://doi.org/10.3390/molecules23040795
Prestinaci F, Pezzotti P, Pantosti A (2015) Antimicrobial resistance: a global multifaceted phenomenon. Pathog Glob Health 109:309–318. https://doi.org/10.1179/2047773215y.0000000030
Sim S, Wong N (2021) Nanotechnology and its use in imaging and drug delivery (review). Biomed Rep. https://doi.org/10.3892/br.2021.1418
Bezbaruah R, Chavda VP, Nongrang L, Alom S, Deka K, Kalita T (2022) Nanoparticle-based delivery systems for vaccines. Vaccines 10:1946. https://doi.org/10.3390/vaccines10111946
Pati R, Shevtsov M, Sonawane A (2018) Nanoparticle vaccines against infectious diseases. Front Immunol. https://doi.org/10.3389/fimmu.2018.02224
Cha SH (2012) The history of vaccination and current vaccination policies in Korea. Clin Exp Vaccine Res 1:3. https://doi.org/10.7774/cevr.2012.1.1.3
Rodrigues AF, Soares HR, Guerreiro MR, Alves PM, Coroadinha AS (2015) Viral vaccines and their manufacturing cell substrates: new trends and designs in modern vaccinology. Biotechnol J 10:1329–1344. https://doi.org/10.1002/biot.201400387
Pulendran B, Ahmed R (2011) Immunological mechanisms of vaccination. Nat Immunol 12:509–517. https://doi.org/10.1038/ni.2039
Marshall JS, Warrington R, Watson W, Kim HL (2018) An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol. https://doi.org/10.1186/s13223-018-0278-1
Maina TW, Grego EA, Boggiatto PM, Sacco RE, Narasimhan B, McGill JL (2020) Applications of nanovaccines for disease prevention in cattle. Front Bioeng Biotechnol. https://doi.org/10.3389/fbioe.2020.608050
Hess KL, Medintz IL, Jewell CM (2019) Designing inorganic nanomaterials for vaccines and immunotherapies. Nano Today 27:73–98. https://doi.org/10.1016/j.nantod.2019.04.005
Azharuddin M, Zhu GH, Sengupta A, Hinkula J, Slater NKH, Patra HK (2022) Nano Toolbox in immune modulation and nanovaccines. Trends Biotechnol 40:1195–1212. https://doi.org/10.1016/j.tibtech.2022.03.011
Bhardwaj P, Bhatia E, Sharma S, Ahamad N, Banerjee R (2020) Advancements in prophylactic and therapeutic nanovaccines. Acta Biomater 108:1–21. https://doi.org/10.1016/j.actbio.2020.03.020
Kim CG, Kye YC, Yun CH (2019) The role of nanovaccine in cross-presentation of antigen-presenting cells for the activation of CD8+ T cell responses. Pharmaceutics 11:612. https://doi.org/10.3390/pharmaceutics11110612
Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R (2020) Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20:101–124. https://doi.org/10.1038/s41573-020-0090-8
Heng WT, Yew JS, Poh CL (2022) Nanovaccines against viral infectious diseases. Pharmaceutics 14:2554. https://doi.org/10.3390/pharmaceutics14122554
Tenchov R, Bird R, Curtze AE, Zhou Q (2021) Lipid nanoparticles─from liposomes to mrna vaccine delivery, a landscape of research diversity and Advancement. ACS Nano 15:16982–17015. https://doi.org/10.1021/acsnano.1c04996
Singh, (2020) Eliciting B cell immunity against infectious diseases using nanovaccines. Nat Nanotechnol 16:16–24. https://doi.org/10.1038/s41565-020-00790-3
Bagheri-Josheghani S, Bakhshi B, Najar-peerayeh S (2022) The influence of nanoparticle on vaccine responses against bacterial infection. J Nanotechnol. https://doi.org/10.1155/2022/6856982
Chen S, Yang L, Ou X, Li JY, Zi CT, Wang H (2022) A new polysaccharide platform constructs self-adjuvant nanovaccines to enhance immune responses. J Nanobiotechnol. https://doi.org/10.1186/s12951-022-01533-3
Irvine DJ, Aung A, Silva M (2020) Controlling timing and location in vaccines. Adv Drug Deliv Rev 158:91–115. https://doi.org/10.1016/j.addr.2020.06.019
Cai T, Liu H, Zhang S, Hu J, Zhang L (2021) Delivery of nanovaccine towards lymphoid organs: recent strategies in enhancing cancer immunotherapy. J Nanobiotechnol 19:2021. https://doi.org/10.1186/s12951-021-01146-2
Roth GA, Picece VC, Ou BS, Luo W, Pulendran B, Appel EA (2021) Designing spatial and temporal control of vaccine responses. Nat Rev Mater 7:174–195. https://doi.org/10.1038/s41578-021-00372-2
Liao Z, Huang J, Lo PC, Lovell JF, Jin H, Yang K (2022) Self-adjuvanting cancer nanovaccines. J Nanobiotechnol. https://doi.org/10.1186/s12951-022-01545-z
Pollard AJ, Bijker EM (2020) A guide to vaccinology: from basic principles to new developments. Nat Rev Immunol 21:83–100. https://doi.org/10.1038/s41577-020-00479-7
Amico CD, Fontana F, Cheng R, Santos HA (2021) Development of vaccine formulations: past, present, and future. Drug Deliv Transl Res 11:353–372. https://doi.org/10.1007/s13346-021-00924-7
Bardhan N (2022) Nanomaterials in diagnostics, imaging and delivery: applications from covid-19 to cancer. MRS Communications 12:1119–1139. https://doi.org/10.1557/s43579-022-00257-7
Baig N, Kammakakam I, Falath W (2021) Nanomaterials: a review of synthesis methods, properties, recent progress, and challenges. Mater Adv 2:1821–1871. https://doi.org/10.1039/d0ma00807a
Lee S, Jun BH (2019) Silver nanoparticles: synthesis and application for nanomedicine. Int J Mol Sci 20:865. https://doi.org/10.3390/ijms20040865
Shao K, Singha S, Clemente-Casares X, Tsai S, Yang Y, Santamaria P (2014) Nanoparticle-based immunotherapy for cancer. ACS Nano 9:16–30. https://doi.org/10.1021/nn5062029
Reed SG, Orr MT, Fox CB (2013) Key roles of adjuvants in modern vaccines. Nat Med 19:1597–1608. https://doi.org/10.1038/nm.3409
Wagner J, Gößl D, Ustyanovska N, Xiong M, Hauser D, Zhuzhgova O (2021) Mesoporous silica nanoparticles as ph-responsive carrier for the immune-activating drug resiquimod enhance the local immune response in mice. ACS Nano 15:4450–4466. https://doi.org/10.1021/acsnano.0c08384
Bozzuto G, Molinari A (2015) Liposomes as nanomedical devices. Int J of Nanomed. https://doi.org/10.2147/ijn.s68861
Wang N, Chen M, Wang T (2019) Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release 303:130–150. https://doi.org/10.1016/j.jconrel.2019.04.025
Asadi K, Gholami A (2021) Virosome-based nanovaccines; a promising bioinspiration and biomimetic approach for preventing viral diseases: a review. Int J Biol Macromol 182:648–658. https://doi.org/10.1016/j.ijbiomac.2021.04.005
Dai J, Su Y, Zhong S, Cong L, Liu B, Yang J (2020) Exosomes: key players in cancer and potential therapeutic strategy. Signal Transduct Target Ther. https://doi.org/10.1038/s41392-020-00261-0
Chaudhary N, Weissman D, Whitehead KA (2021) MRNA vaccines for infectious diseases: principles, delivery and clinical translation. Nat Rev Drug Discov 20:817–838. https://doi.org/10.1038/s41573-021-00283-5
Panda AK (2012) Nanotechnology in vaccine development. Proc Nat Acad Sci India Sec B: Biol Sci 82:13–27. https://doi.org/10.1007/s40011-012-0073-6
Wallis J, Shenton DP, Carlisle RC (2019) Novel approaches for the design, delivery and administration of vaccine technologies. Clin Exp Immunol 196:189–204. https://doi.org/10.1111/cei.13287
Tavares Batista M, Souza RD, Paccez JD, Luiz WB, Ferreira EL, Cavalcante RC (2014) Gut adhesive bacillus subtilis spores as a platform for mucosal delivery of antigens. Infect Immun 82:1414–1423. https://doi.org/10.1128/iai.01255-13
Das I, Padhi A, Mukherjee S, Dash DP, Kar S, Sonawane, (2017) A Biocompatible chitosan nanoparticles as an efficient delivery vehicle for Mycobacterium tuberculosis lipids to induce potent cytokines and antibody response through activation of gammadelta T cells in mice. Nanotechnol 28:323
Manish M, Rahi A, Kaur M, Bhatnagar R, Singh SA (2013) single-dose PLGA encapsulated protective antigen domain 4 nanoformulation protects mice against Bacillus anthracis spore challenge. PLoS ONE 8(e61885):315
Schulze K, Ebensen T, Chandrudu S, Skwarczynski M, Toth I, Olive C, Guzman CA (2017) Bivalent mucosal peptide vaccines administered using the LCP carrier system stimulate protective immune responses against Streptococcus pyogenes infection. Nanomed 13(2463–2474):502
Barhate G, Gautam M, Gairola S, Jadhav S, Pokharkar V (2014) Enhanced mucosal immune responses against tetanus toxoid using novel delivery system comprised of chitosan-functionalized gold nanoparticles and botanical adjuvant: characterization, immunogenicity, and stability assessment. J Pharmaceut Sci 103(3448–3456):505
Jiang J, Liu G, Kickhoefer VA, Rome LH, Li LX, McSorley SJ, Kelly KA (2017) A protective vaccine against chlamydia genital infection using vault nanoparticles without an added adjuvant. Vaccines 5:141
Beeh KM, Kanniess F, Wagner F, Schilder C, Naudts I, Hammann-Haenni A, Willers J, Stocker H, Mueller P, Bachmann MF, Renner WA (2013) The novel TLR-9 agonist QbG10 shows clinical efficacy in persistent allergic asthma. J Allergy Clin Immunol 131(866–874):418
Tao W, Ziemer KS, Gill HS (2014) Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomed 9(237–251):99
Xu L, Liu Y, Chen Z, Li W, Liu Y, Wang L, Liu Y, Wu X, Ji Y, Zhao Y, Ma L, Shao Y, Chen C (2012) Surface-engineered gold nanorods: promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett 12(2003–2012):496
Dacoba TG, Omange RW, Li H, Crecente-Campo J, Luo M, Alonso MJ (2019) Polysaccharide nanoparticles can efficiently modulate the immune response against an HIV peptide antigen. ACS Nano 13(4947–4959):407
Thomas C, Rawat A, Hope-Weeks L, Ahsan F (2011) Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol Pharmaceut 8(405–415):316
Liu G, Song L, Beasley DW, Putnak R, Parent J, Misczak J, Li H, Reiserova L, Liu X, Tian H, Liu W, Labonte D, Duan L, Kim Y, Travalent L, Wigington D, Weaver B, Tussey L (2015) Immunogenicity and efficacy of flagellin-envelope fusion dengue vaccines in mice and monkeys. Clin Vaccine Immunol CVI 22(516–525):499
Chen YS, Hung YC, Lin WH, Huang GS (2010) Assessment of gold nanoparticles as a size-dependent vaccine carrier for enhancing the antibody response against synthetic foot-and-mouth disease virus peptide. Nanotechnol 21:93
Zhao K, Chen G, Shi XM, Gao TT, Li W, Zhao Y, Zhang FQ, Wu J, Cui X, Wang YF (2012) Preparation and efficacy of a live newcastle disease virus vaccine encapsulated in chitosan nanoparticles. PLoS ONE 7(e53314):504
El Bissati K, Zhou Y, Paulillo SM, Raman SK, Karch CP, Roberts CW, Lanar DE, Reed S, Fox C, Carter D, Alexander J, Sette A, Sidney J, Lorenzi H, Begeman IJ, Burkhard P, McLeod R (2017) Protein nanovaccine confers robust immunity against toxoplasma. NPJ Vaccines 2(24):150
Pusic K, Aguilar Z, McLoughlin J, Kobuch S, Xu H, Tsang M, Wang A, Hui G (2013) Iron oxide nanoparticles as a clinically acceptable delivery platform for a recombinant blood-stage human malaria vaccine. FASEB J Off Publ Fed Am Soc Exp Biol 27(1153–1166):301
Nandhini P, Kumar P, Mickymaray S, Alothaim AS, Somasundaram J, Rajan M (2022) Recent developments in methicillin-resistant staphylococcus aureus (MRSA) treatment: a review. Antibiot 11:606. https://doi.org/10.3390/antibiotics11050606
Aires-de-Sousa M (2017) Methicillin-resistant Staphylococcus aureus among animals: current overview. Clin Microbiol Infect 23:373–380. https://doi.org/10.1016/j.cmi.2016.11.002
Wang F, Gao W, Thamphiwatana S, Luk BT, Angsantikul P, Zhang Q (2015) Hydrogel retaining toxin-absorbing nanosponges for local treatment of methicillin-resistant Staphylococcus aureus infection. Adv Mater 27:3437–3443. https://doi.org/10.1002/adma.201501071
Hsiao A, Zhu J (2020) Pathogenicity and virulence regulation of vibrio cholerae at the interface of host-gut microbiome interactions. Virulence 11:1582–1599. https://doi.org/10.1080/21505594.2020.1845039
Sedaghat M, Siadat SD, Mirabzadeh E, Keramati M, Vaziri F, Shafiei M (2019) Evaluation of antibody responses to outer membrane vesicles (OMVS) and killed whole cell of Vibrio cholerae o1 el tor in Immunized Mice. Iran J Microbiol https://doi.org/10.18502/ijm.v11i3.1317.
Tabrizi NM, Amani J, Ebrahimzadeh M, Nazarian S, Kazemi R, Almasian P (2018) Preparation and evaluation of chitosan nanoparticles containing CtxB antigen against Vibrio cholera. Microb Pathog 1(124):170–177. https://doi.org/10.1016/j.micpath.2018.08.037
Foster-Nyarko E, Pallen MJ (2022) The microbial ecology of Escherichia coli in the vertebrate gut. FEMS Microbiol Rev. https://doi.org/10.1093/femsre/fuac008
da Silva GJ, Mendonça N (2012) Association between antimicrobial resistance and virulence in Escherichia coli. Virulence 3:18–28. https://doi.org/10.4161/viru.3.1.18382
Khanifar J, Hosseini RH, Kazemi R, Ramandi MF, Amani J, Salmanian AH (2019) Prevention of EHEC infection by chitosan nano-structure coupled with synthetic recombinant antigen. J Microbiol 157:100–107. https://doi.org/10.1016/j.mimet.2019.01.002
Xing Y, Clark JR, Chang JD, Chirman DM, Green S, Zulk JJ, Jelinski J, Maresso PKA, AW, (2023) Broad protective vaccination against systemic Escherichia coli with autotransporter antigens. Plos Pathog. https://doi.org/10.1371/journal.ppat.1011082
Fan J, Jin S, Gilmartin L, Toth I, Hussein WM, Stephenson RJ (2022) Advances in infectious disease vaccine adjuvants. Vaccines 10(7):1120. https://doi.org/10.3390/vaccines10071120
Abianeh HS, Nazarian S, Sadeghi D, Razgi AS, Samarin MZ (2023) PLGA nanoparticles containing intimin-flagellin fusion protein for E. coli O157:H7. Nano-Vacc J Immunol Meth. https://doi.org/10.1016/j.jim.2023.113517
Giacchetta PC, Cavalieri R, Domnich A, Waure C (2021) The burden of seasonal influenza in Italy: a systematic review of influenza-related complications, hospitalizations, and mortality. Influenza Other Respir Viruses 16:351–365. https://doi.org/10.1111/irv.12925
Mohamed NA, Abou-Saleh H, Mohamed HA, Al-Ghouti MA, Crovella S, Zupin, (2022) Think like a virus: toward improving nanovaccine development against SARS-COV-2. Viruses 14:1553. https://doi.org/10.3390/v14071553
Kim C, Kim JD, Seo SU (2022) Nanoparticle and virus-like particle vaccine approaches against SARS-COV-2. J Microbiol 60:335–346. https://doi.org/10.1007/s12275-022-1608-z
Gregory AE, Titball R, Williamson D (2013) Vaccine delivery using nanoparticles. Front Cell Infect Microbiol. https://doi.org/10.3389/fcimb.2013.00013
Demento SL, Cui W, Criscione JM, Stern E, Tulipan J, Kaech SM (2012) Role of sustained antigen release from nanoparticle vaccines in shaping the T cell memory phenotype. Biomater 33:4957–4964. https://doi.org/10.1016/j.biomaterials.2012.03.041
Nguyen NYT, Grelling N, Wetteland CL, Rosario R, Liu H (2018) Antimicrobial activities and mechanisms of magnesium oxide nanoparticles (nmgo) against pathogenic bacteria, yeasts, and biofilms. Sci Rep. https://doi.org/10.1038/s41598-018-34567-5
Monto AS, Sellwood C (2012) History and epidemiological features of pandemic influenza. Pandemic Influenza. https://doi.org/10.1079/9781845938567.0040
Parums D V (2021) Editorial: Covid-19 and multisystem inflammatory syndrome in children (mis-C). Med Sci Monit https://doi.org/10.12659/msm.933369
Hill EM, Tildesley MJ, House T (2017) Evidence for history-dependence of influenza pandemic emergence. Sci Rep. https://doi.org/10.1038/srep43623
Wang Y, Deng L, Kang SM, Wang BZ (2017) Universal influenza vaccines: from viruses to nanoparticles. Exp Rev Vaccines 17:967–976. https://doi.org/10.1080/14760584.2018.1541408
Ding P, Jin Q, Chen X, Yang S, Guo J, Xing G (2019) nanovaccine confers dual protection against influenza A virus and porcine circovirus type 2. Int J Nanomed 14:7533–7548. https://doi.org/10.2147/ijn.s218057
Freyn AW, Ramos da Silva J, Rosado VC, Bliss CM, Pine M, Mui BL, Tam YK, Madden TD, de Souza Ferreira LC, Weissman D, Krammer F, Coughlan L, Palese P, Pardi N, Nachbagauer R (2020) A multi-targeting, nucleoside-modified mrna influenza virus vaccine provides broad protection in mice. Mol Ther 28(7):1569–1584. https://doi.org/10.1016/j.ymthe.2020.04.018
Kumar V, Singh J (2019) A prospective study on impact of early initiation of antiretroviral therapy in human immunodeficiency virus-positive adults on immunological status and adverse events. J Glob Infect Dis 11:73. https://doi.org/10.4103/jgid.jgid_160_18
Okasha H (2020) Risk factors and key principles for prevention of surgical site infections. Surg Infect Some Facts. https://doi.org/10.5772/intechopen.85284
Barouch DH (2008) Challenges in the development of an HIV-1 vaccine. N n at 455:613–619. https://doi.org/10.1038/nature07352
Vela Ramirez JE, Roychoudhury R, Habte HH, Cho MW, Pohl NLB, Narasimhan B (2014) Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells. J Biomater Sci Polym Ed 25:1387–1406. https://doi.org/10.1080/09205063.2014.940243
Spellberg B (2011) Vaccines for invasive fungal infections. F1000 Med Rep. https://doi.org/10.3410/m3-13
Arendrup MC, Patterson TF (2017) Multidrug-resistant candida: Epidemiology, molecular mechanisms, and treatment. J Infect Dis. https://doi.org/10.1093/infdis/jix131
Hoyer LL, Cota E (2016) Candida albicans agglutinin-like sequence (ALS) family vignettes: a review of ALS protein structure and function. Front Microbiol. https://doi.org/10.3389/fmicb.2016.00280
Gaffen S, Bechara R (2019) Faculty opinions recommendation of the NDV-3A vaccine protects mice from multidrug resistant Candida auris infection, Faculty Opinions – post-publication peer review of the biomedical literature https://doi.org/10.3410/f.736348689.793563849
Garg DHK (2020) Nanoparticles as adjuvants in vaccine delivery. Crit Rev Ther Drug Carrier Syst 37:183–204. https://doi.org/10.1615/critrevtherdrugcarriersyst.2020033273
Kauffman CA, Nicolasora NP (2009) Epidemiology of invasive pulmonary aspergillosis. From Diagnosis to Prevention, Aspergillosis. https://doi.org/10.1007/978-90-481-2408-4_20
Oliveira LV, Wang R, Specht CA, Levitz SM (2021) Vaccines for human fungal diseases: close but still a long way to go. Npj Vaccines. https://doi.org/10.1038/s41541-021-00294-8
Iyer KR, Revie NM, Fu C, Robbins N, Cowen LE (2021) Treatment strategies for cryptococcal infection: challenges, advances and future outlook. Nat Rev Microbiol 19:454–466. https://doi.org/10.1038/s41579-021-00511-0
Han J, Zhao D, Li D, Wang X, Jin Z, Zhao K (2018) Polymer-based nanomaterials and applications for vaccines and drugs. Polym 10:31. https://doi.org/10.3390/polym10010031
Hettinga J, Carlisle R (2020) Vaccination into the dermal compartment: techniques, challenges, and prospects. Vaccines 8:534. https://doi.org/10.3390/vaccines8030534
Zhu M, Wang R, Nie G (2014) Applications of nanomaterials as vaccine adjuvants. Hum Vaccin Immunother 10:2761–2774. https://doi.org/10.4161/hv.29589
Cordeiro AS, Patil-Sen Y, Shivkumar M, Patel R, Khedr A, Elsawy MA (2021) Nanovaccine delivery approaches and advanced delivery systems for the prevention of viral infections: from development to clinical application. Pharmaceutics 13:2091. https://doi.org/10.3390/pharmaceutics13122091
Jeong WY, Kwon M, Choi HE, Kim KS (2021) Recent advances in transdermal drug delivery systems: a review. Biomater Res. https://doi.org/10.1186/s40824-021-00226-6
Wang P (2021) Natural and synthetic saponins as vaccine adjuvants. Vaccines 9:222. https://doi.org/10.3390/vaccines9030222
Rosenbaum P, Tchitchek N, Joly C, Rodriguez Pozo A, Stimmer L, Langlois S, Hocini H, Gosse L, Pejoski D, Cosma A, Beignon AS, Dereuddre-Bosquet N, Levy Y, Le Grand R, Martinon F (2021) Vaccine inoculation route modulates early immunity and consequently antigen-specific immune response. Front Immunol. https://doi.org/10.3389/fimmu.2021.645210
Zhao H, Wang H, Hu Y, Xu D, Yin C, Han Q, Zhang J (2021) Chitosan nanovaccines as efficient carrier adjuvant system for IL-12 with enhanced protection against HBV. Int J Nanomed 16:4913–4928. https://doi.org/10.2147/ijn.s317113
Nijen MK, Twilhaar L, Czentner CF, Nostrum V, Storm G, den Haan JM (2021) Mimicking pathogens to augment the potency of liposomal cancer vaccines. Pharmaceutics 13:954. https://doi.org/10.3390/pharmaceutics13070954
Frizzell H, Woodrow KA (2020) Biomaterial approaches for understanding and overcoming immunological barriers to effective oral vaccinations. Adv Funct Mater 30:1907170. https://doi.org/10.1002/adfm.201907170
Hameed SA, Paul S, Dellosa GK, Jaraquemada D, Bello MB (2022) Towards the future exploration of mucosal mrna vaccines against emerging viral diseases; lessons from existing next-generation mucosal vaccine strategies. Npj Vacc. https://doi.org/10.1038/s41541-022-00485-x
Tai J, Han M, Lee D, Park IH, Lee SH, Kim TH (2022) Different methods and formulations of drugs and vaccines for nasal administration. Pharmaceutics 14:1073. https://doi.org/10.3390/pharmaceutics14051073
Yadav HKS, Dibi M, Mohammad A, Srouji AE (2018) Nanovaccines formulation and applications- a review. J Drug Deliv Sci Technol 44:380–387. https://doi.org/10.1016/j.jddst.2018.01.015
Pielenhofer J, Sohl J, Windbergs M, Langguth P, Radsak MP (2020) Current progress in particle-based systems for transdermal vaccine delivery. Front Immunol. https://doi.org/10.3389/fimmu.2020.00266
Mangla B, Javed S, Sultan MH, Ahsan W, Aggarwal G, Kohli K (2022) Nanocarriers-assisted needle-free vaccine delivery through oral and intranasal transmucosal routes: a novel therapeutic conduit. Front Pharmacol. https://doi.org/10.3389/fphar.2021.757761
Acarturk F (2009) Mucoadhesive vaginal drug delivery systems. Recent Pat Drug Deliv Formul 3:193–205. https://doi.org/10.2174/187221109789105658
Anggraeni R, Ana ID, Wihadmadyatami H (2022) Development of mucosal vaccine delivery: an overview on the mucosal vaccines and their adjuvants. Clinic Exp Vacc Res 11(3):235. https://doi.org/10.7774/cevr.2022.11.3.235
Park JS, Oh YK, Kang MJ, Kim CK (2003) Enhanced mucosal and systemic immune responses following intravaginal immunization with human papillomavirus 16 L1 virus-like particle vaccine in thermosensitive mucoadhesive delivery systems. J Med Virol 70:633–641. https://doi.org/10.1002/jmv.10442
Nooraei S, Bahrulolum H, Hoseini ZS, Katalani C, Hajizade A, Easton AJ (2021) Virus-like particles: preparation, immunogenicity and their roles as nanovaccines and drug nanocarriers. J Nanobiotechnol. https://doi.org/10.1186/s12951-021-00806-7
Seo KY, Han SJ, Cha HR, Seo SU, Song JH, Chung SH (2010) Eye mucosa: an efficient vaccine delivery route for inducing protective immunity. The J Immunol 185:3610–3619. https://doi.org/10.4049/jimmunol.1000680
Kim ED, Han SJ, Byun YH, Yoon SC, Choi KS, Seong BL (2015) Inactivated eyedrop influenza vaccine adjuvanted with poly(i:C) is safe and effective for inducing protective systemic and mucosal immunity. PLoS ONE. https://doi.org/10.1371/journal.pone.0137608
Joudeh N, Linke D (2022) Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J Nanobiotechnol. https://doi.org/10.1186/s12951-022-01477-8
Luo M, Samandi LZ, Wang Z, Chen ZJ, Gao J (2017) Synthetic nanovaccines for immunotherapy. J Control Release 263:200–210. https://doi.org/10.1016/j.jconrel.2017.03.033
Cai T, Liu H, Zhang S, Hu J, Zhang L (2021) Delivery of nanovaccine towards lymphoid organs: recent strategies in enhancing cancer immunotherapy. J Nanobiotechnol. https://doi.org/10.1186/s12951-021-01146-2
Yin Q, Wang Y, Xiang Y (2022) Nanovaccines: merits, and diverse roles in boosting antitumor immune responses. Human Vacc Immunother 10(1080/21645515):2119020
Kelly HG, Kent SJ, Wheatley AK (2019) Immunological basis for enhanced immunity of nanoparticle vaccines. Exp Rev Vaccines 18:269–280. https://doi.org/10.1080/14760584.2019.1578216
Li M, Kaminskas LM, Marasini N (2021) Recent advances in nano/microparticle-based oral vaccines. J Pharmaceut Invest 51:425–438. https://doi.org/10.1007/s40005-021-00537-9
Liu Y, Hardie J, Zhang X, Rotello VM (2017) Effects of engineered nanoparticles on the innate immune system. Sem Immunol 34:25–32. https://doi.org/10.1016/j.smim.2017.09.011
Sabourian P, Yazdani G, Ashraf SS, Frounchi M, Mashayekhan S, Kiani S (2020) Effect of physico-chemical properties of nanoparticles on their intracellular uptake. Int J Mol Sci 21:8019. https://doi.org/10.3390/ijms21218019
González-García LE, MacGregor MN, Visalakshan RM, Lazarian A, Cavallaro AA, Morsbach S (2022) Nanoparticles surface chemistry influence on protein corona composition and inflammatory responses. Nanomater 12:682. https://doi.org/10.3390/nano12040682
Cruz GG, Rodríguez-Fragoso P, Reyes-Esparza J, Rodríguez-López A, Gómez-Cansino R, Rodriguez-Fragoso L (2018) Interaction of nanoparticles with blood components and associated pathophysiological effects unraveling the safety profile of nanoscale particles and materials. From Biomed Environ Appl. https://doi.org/10.5772/intechopen.69386
Chen L, Glass JJ, De Rose R, Sperling C, Kent SJ, Houston ZH (2018) Influence of charge on hemocompatibility and immunoreactivity of polymeric nanoparticles. ACS Appl Bio Mater 1:756–767. https://doi.org/10.1021/acsabm.8b00220
Sengupta A, Azharuddin M, Al-Otaibi N, Hinkula J (2022) Efficacy and immune response elicited by gold nanoparticle- based nanovaccines against infectious diseases. Vaccines 10:505. https://doi.org/10.3390/vaccines10040505
Feng C, Li Y, Ferdows BE, Patel DN, Ouyang J, Tang Z, Kong N, Chen E, Tao W (2022) Emerging vaccine nanotechnology: from defense against infection to sniping cancer. Acta Pharmaceutica Sinica B 12(5):2206–2223. https://doi.org/10.1016/j.apsb.2021.12.021
Celis-Giraldo CT, López-Abán J, Muro A, Patarroyo MA, Manzano-Román R (2021) Nanovaccines against animal pathogens: the latest findings. Vaccines 9:988. https://doi.org/10.3390/vaccines9090988
Micoli F, Bagnoli F, Rappuoli R, Serruto D (2021) The role of vaccines in combatting antimicrobial resistance. Nat Rev Microbiol 19:287–302. https://doi.org/10.1038/s41579-020-00506-3
Fries CN, Curvino EJ, Chen JL, Permar SR, Fouda GG, Collier JH (2020) Advances in nanomaterial vaccine strategies to address infectious diseases impacting global health. Nat Nanotechnol 16:1–14. https://doi.org/10.1038/s41565-020-0739-9
Makabenta JM, Nabawy A, Li CH, Schmidt-Malan S, Patel R, Rotello VM (2020) Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat Rev Microbiol 19:23–36. https://doi.org/10.1038/s41579-020-0420-1
Alqahtani FA, Almustafa HI, Alshehri RS, Alanazi SO, Khalifa AY (2022) Combating antibiotic resistance in bacteria: the development of novel therapeutic strategies. J Pure Appl Microbiol 16:2201–2224. https://doi.org/10.22207/jpam.16.4.01
Ahmad-Mansour N, Loubet P, Pouget C, Dunyach-Remy C, Sotto A, Lavigne JP (2021) Staphylococcus aureus toxins: an update on their pathogenic properties and potential treatments. Toxins 13:677. https://doi.org/10.3390/toxins13100677
Malonis RJ, Lai JR, Vergnolle O (2019) Peptide-based vaccines: current progress and future challenges. Chem Rev 120:3210–3229. https://doi.org/10.1021/acs.chemrev.9b00472
Baker S, Volova T, Prudnikova SV, Shumilova AA, Perianova OV, Zharkov SM, Kuzmin A, Olga K, Bogdan K, Shidlovskiy IP, Potkina ZK, Khohlova OY, Lobova TI (2018) Bio-hybridization of nanobactericides with cellulose films for effective treatment against members of ESKAPE multi-drug-resistant pathogens. Appl Nanosci 8(5):1101–1110. https://doi.org/10.1007/s13204-018-0717-9
Acknowledgements
All authors acknowledge the Karnataka State Open University for providing the facility.
Funding
No funding was received for the present study.
Author information
Authors and Affiliations
Contributions
SYB contributed to the conceptualisation of study, NRS wrote the original draft, RHK and MNP curated the data, CNS collected the articles from the databases, MK collected the resources, AP contributed to writing part, SS corrected the draft, and RSC contributed to conceptualisation.
Corresponding author
Ethics declarations
Ethical approval and consent to participation
The present work does not involve any animal studies nor clinical trials; hence, no ethical approval is required. All authors have agreed for their participation.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Manju, K., Raj, S.N., Ranjini, H.K. et al. Nanovaccines to combat drug resistance: the next-generation immunisation. Futur J Pharm Sci 9, 64 (2023). https://doi.org/10.1186/s43094-023-00515-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00515-y