Abstract
Background
As per current treatment guidelines, artemether-lumefantrine and efavirenz-based antiretroviral therapy are recommended drugs for falciparum malaria and human immunodeficiency virus (HIV) infections, respectively. A liquid chromatography-ultraviolet detection method for simultaneous quantification of lumefantrine and efavirenz was developed and validated for efficacy and pharmacokinetic clinical studies. Lumefantrine and efavirenz were separated using a 100 × 4.6 mm × 3 µm Fortis C18 chromatographic column, and a multistep gradient mobile phase. Calibration curves were obtained with a series of standard solutions containing known concentrations of the chemical reference of both analytes prepared concomitantly in drug-free plasma. The assay was validated within the calibration ranges of 78.125–20,000 ng/mL for lumefantrine and 187.15–24,000 ng/mL for efavirenz. Stability assessment was carried out with or without heating the quality control sample to 58 °C for 45 min. The method was employed to measure the plasma concentrations of lumefantrine and efavirenz in a study conducted among malaria-HIV co-infected patients.
Result
Lumefantrine and efavirenz were well separated from each other and from the biological matrix. The method demonstrated a good recovery of 72.64% for lumefantrine and 117.17% for efavirenz. The intra- and inter-day accuracy presented as 95.36–105.14% for lumefantrine and 104.11–115% for efavirenz and precision ranged from 1.15 to 6.45% for lumefantrine and 0.43 to 13.12 for efavirenz, were within ± 15% at the three quality control levels. The analytes from both quality control lots and samples collected from HIV-malaria co-infected individuals were found to be stable post-deactivation of infectious virus by heating to 58 °C for 45 min.
Conclusion
The assay is accurate, precise and shown to simultaneously measure the lumefantrine and EFV in human plasma.
Similar content being viewed by others
Background
Malaria-HIV co-infection remains a major public health concern in malaria-endemic areas. This is more remarkable among pregnant women and paediatrics. At present, artemisinin-based combination therapy (ACT) is recommended by the World Health Organisation (WHO) as a first-line treatment for Plasmodium falciparum malaria with artemether plus lumefantrine being the most preferred ACT in sub-Saharan African (SSA) settings [1, 2]. For HIV treatment, the use of efavirenz (EFV)-based antiretroviral therapy (ART) regimen is now mainly reserved for the management of HIV during pregnancy, mostly in SSA settings. However, it is upheld as the first option in settings where dolutegravir-based ART is not available [3].
Despite all the beneficial therapeutic outcomes attributed to the ACT regimen, there have been worries about their current therapeutic efficacy such that the current malaria report strongly recommends therapeutic efficacy studies (TES) in malaria-endemic settings [2]. The determination of the plasma exposure of antimalarial drugs and their active metabolites is usually integrated into the TES [4], particularly when the plasma exposure is considered a surrogate marker for therapeutic efficacy [4,5,6]. Thus, the availability of a simple and sensitive bioanalytical technique that has been pre-qualified, in line with the approved validation criteria, is central to the measurement of drugs in biological matrices and for conducting TES successfully.
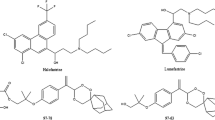
Chromatographic methods are the major analytical techniques for the quantification of drugs in biological matrices. High-performance liquid chromatography with UV detection (HPLC–UV) or a more sophisticated liquid chromatography in tandem with a mass spectrometer (LC/MS/MS) has been employed to measure the various components of EFV-based ART and ACTs in some biological matrices [7,8,9,10,11,12,13]. Lumefantrine and EFV (Fig. 1), are lipophilic molecules and sensitive to UV detectors. As a result, they have been successfully quantified from plasma and dried blood spots using HPLC–UV. However, most of these methods are cumbersome or involve an expensive sample pre-treatment step.
Besides, in situations where ACT and ART are co-administered, interfering peaks, either wholly or partially co-eluted, might arise from different components of the ACT or ART. This presents the need for a simple, specific, selective, and sensitive bioanalytical assay to measure the plasma concentration of lumefantrine in the presence of ART or for simultaneous determination of both analytes. Thus, this work aimed to develop and validate an HPLC–UV method for the quantification of lumefantrine and EFV in individuals with ongoing EFV-based ART.
Methods
Materials
Lumefantrine reference standard was purchased from Sigma-Aldrich (Gillingham, UK), efavirenz reference standard was generously donated by the Department of Chemistry, University of Liverpool, Liverpool. Eppendorf® tubes (2 mL), 100 µL and 1000 µL extended micropipette tips, 7-mL screw-capped borosilicate glass tubes, 5 mL borosilicate glass tubes, 300 µL auto-sampler vials, 2 mL autosampler vials and caps for the autosampler vials were sourced from Thermo-Fisher Scientific Ltd (Loughborough, UK). Methanol and acetonitrile (HPLC grade) were obtained from Honeywell® Chromasolv™ Fisher Scientific Ltd (Loughborough, UK). Analytical grade n-hexane, tert-butyl methyl ether (TBME), ammonium formate and formic acid were purchased from Sigma-Aldrich Ltd (Gillingham, UK). EDTA tubes, Cryovial tubes and artemether-lumefantrine tablets (Coartem® 80/480 mg) were sourced from retail pharmacies. HPLC-grade deionized water (16.8 Ω) was generated from an Elga Option 4 water purifier and a Purelab Classic UVF Elga LabWater (High Wycombe, UK).
Equipment
The list of equipment includes a vortex mixer from Stuart® (Staffordshire, UK), a Savant Speedvac® system SPD 111 V centrifugal concentrator, Sorvall™ Legend™ benchtop microcentrifuge from Thermo Scientific, and a Dionex high-performance liquid chromatography system (Sunnyvale, USA).
HPLC-UV system and chromatographic conditions
The HPLC–UV was fitted with a quaternary pump and a diode array UV/Vis detector ranging from 190 to 900 nm. Liquid chromatography separation was achieved on a 100 × 4.6 mm × 3 µm Fortis C18 column (Fortis Technologies Ltd, Cheshire, United Kingdom) with a Fortis guard column. The mobile phase A consisted of 10 mM NH4COOH at pH 6.38 while mobile phase B was acetonitrile with formic acid (0.1%). The gradient program consisted of linear segments as; 0–1 min: 50% B to condition the column; 1–3 min: 50–90% B steep gradient; 3–8 min: 90% B; 8–8.1 min: 90–50% B and 8.1–9.5 min: 50% B at a constant flow rate of 1 mL/min.
Preparation of 1000 µg/mL lumefantrine stock solution
10 mg of lumefantrine reference standard was weighed into a 10 mL volumetric flask and dissolved in 5 mL methanol and 100 µL of 0.1% formic acid. The mixture was made up to the 10 mL mark with methanol, sonicated for 30 min, and stored at − 20 °C.
Preparation of 1000 µg/mL efavirenz stock solution
10 mg of EFV reference standard was weighed into a 10 mL volumetric flask and dissolved in 5 mL of methanol. The mixture was made up to the 10 mL mark with methanol, sonicated for 30 min and stored at − 20 °C.
Preparation of calibration and quality control standards
Drug-free plasma, previously collected from healthy individuals and stored for research purposes, was thawed at 37 °C and a 40,000 ng/mL solution of lumefantrine in plasma was prepared by spiking blank plasma with an appropriate volume of the 1000 µg/mL lumefantrine stock solution. Nine calibration standards ranging from 78.125 ng/mL to 20,000 ng/mL were prepared by serial dilutions. Similarly, aliquots of the 40,000 ng/mL lumefantrine solution in plasma were taken to obtain 15,000 ng/mL, 2000 ng/mL and 400 ng/mL as high-quality control (HQC), medium quality control (MQC) and low-quality control (LQC) standards respectively.
Sample pre-treatment
The extraction procedure was carried out by liquid–liquid extraction procedure using a mixture (80:20) of n-hexane and TBME. The plasma samples were thawed at room temperature, and subsequently heat-inactivated at 58 °C for 45 min, to inactivate the virus in the clinical samples. Drug-free plasma, spiked with lumefantrine stock solution for calibration and QC standard sets, were also subjected to heating at 58 °C for 45 min.
To 200 µL plasma, in a 7 mL screw-capped glass tube, was added a 500 µL mixture of acetonitrile and 0.5 M acetic acid (50:50). The mixture was vortex-mixed briefly and a 2.5 mL mixture of n-hexane and TBME (80:20) was added, vortex-mixed again for 20 s, and agitated using a mechanical shaker for 45 min to mix the content thoroughly. Thereafter, the sample was centrifuged at 4000 × g for 10 min. The organic phase was transferred into a 5 mL (13 mm × 75 mm) borosilicate glass tube and evaporated under vacuum in a concentrator at room temperature. The sample was reconstituted with a 200 µL mixture of 20% mobile phase A and 80% mobile phase B and vortex-mixed for 15 s. A 150 µL aliquot was transferred into the HPLC auto-sampler vial and 50 µL was injected into the HPLC column.
Assay validation
All bioanalytical assays employed in this study were validated in line with the FDA guidelines [14]. The following parameters were assessed: linearity, recovery, specificity, accuracy, precision, including inter-and intra-day variabilities.
Calibration and sensitivity
Calibration curves for the analytes were generated by plotting the instrument response (peak area) versus standard concentrations extracted from the spiked plasma samples as previously described. The calibration sets for EFV and lumefantrine were prepared in plasma from standard concentrations ranging from 187.15 ng/mL to 24,000 ng/mL, and 78.125 ng/mL to 20,000 ng/mL respectively. The relationship between peak areas versus concentration of analytes was generated with a linear regression equation using an auto-integrator setting on the HPLC. Linearity was assessed by three independent preparations of the standard curves. Each set of calibration curve data consisted of an extracted blank, a zero sample and eight to nine non-zero samples. For the calibration set to be accepted, at least 75% of non-zero standards were not deviated by more than 15% of nominal concentrations.
The lowest limit of quantification (LLOQ) was established as the lowest analyte concentration that can be measured with acceptable accuracy and precision within a 20% deviation from the nominal values. The selectivity of the assay was determined by comparing the degree of interference from the matrix (due to potential interfering substances including endogenous matrix components, metabolites and decomposition products). This was assessed via a comparison of extracted blank samples with the lowest point of the standard curve.
Accuracy and precision
The method's accuracy defines the closeness of mean test results to the analyte's actual value (concentration). This was done in five replicate analyses of QC samples at three QC levels. A measure of accuracy was achieved by using the deviation of the mean from the nominal value and the mean value is expected to be within 15% of the nominal value except at LLOQ, where it should not deviate by more than 20% (FDA, 2018) [14]. The precision was determined at QC levels by measuring the closeness of individual measures of an analyte when the procedure is applied repeatedly to multiple aliquots of a single volume of the biological matrix. Both accuracy and precision were assessed for intra- and inter-day variability.
Analysis for recovery and stability
Recovery experiment was performed based on the equation stated below;
Recovery experiments were performed by comparing the results for the extracted samples at three quality control (QC) concentrations-LQC, MQC and HQC levels with non-extracted spiked solutions at corresponding concentrations which were taken to represent 100% recovery. Short-term stability of lumefantrine and EFV were evaluated by leaving extracted QC samples on the benchtop (room temperature) and in the auto-sampler (4 °C) for 24 h. The samples were re-injected after 24 h of storage. Stability following the heat-inactivation process was assessed by comparing it with the data from another QC set without heating at 58 °C for 45 min.
Application of validated bioanalytical methods to clinical samples
The assay developed was employed to measure both efavirenz and lumefantrine in plasma samples obtained from the patients recruited for a drug-drug interaction study [15], which involved sixty-nine study participants which included thirty-four non-pregnant and thirty-five pregnant women, were recruited into pharmacokinetic clinical study.
The assays were performed in batches of a maximum of 85 samples. Each run included a set of calibration standards, QC samples (at LQC, MQC and HQC) in triplicates, blank (mobile phase), a chemical mix, three extracted blank plasma and clinical samples. The decision to accept or reject a run was determined based on the closeness of the result from QCs sets to the nominal values. At least two-thirds (67% or four out of six) of the QCs sets were expected to fall within the range of 85 to 115% of the nominal values (variation of 15%) except for LQC in which 80–120% (variation of 20%) is allowed. Similarly, at least 50% of QCs at each level were expected to be within 15% of their nominal concentrations (FDA, 2018).
Results
Chromatographic separation
Shown in Fig. 2a–d are representative chromatograms produced following the injection of extracted blank plasma, blank plasma spiked with 10,000 ng/mL each of EFV and lumefantrine, clinical sample with UV detection at 335 nm and clinical sample with UV detection at 254 nm, respectively, into the HPLC. EFV and lumefantrine were well separated, with retention times of 3.9 min and 5.2 min, respectively, without interference from endogenous substances.
Linearity, LOD and LLOQ
Figure 3 shows the calibration curves generated for EFV and lumefantrine. External calibration standards were suitable to generate linear plots between the peak area and concentrations of EFV and LUM standards. The lower limits of quantification (LLOQ) were 187.5 ng/mL for EFV, 78.125 ng/mL for lumefantrine, while the corresponding limits of detection (LOD) were 50 ng/mL and 30 ng/mL for EFV and lumefantrine, respectively.
Accuracy and precision
The accuracy and precision for each analyte are presented accordingly in Table 1. The carryover effect was less than 15% for both analytes.
Recovery and stability
Recoveries for EFV and lumefantrine are also presented in Table 1. When plasma samples were heated to 58 °C for 45 min, as part of sample pre-treatment, the analytes were found to be stable within an acceptable range. Heated QC samples were stable with a loss of 6.7–9.4%. The percentage deviations from non-heated QC sampled were within an acceptable level of 15%. Additionally, processed QC and clinical samples were stable overnight in the autosampler (4 °C). For QC samples, the percentage deviations from the nominal concentrations were within 15% (4.5–8.9%) when left overnight.
Clinical sample analysis
The validated method was employed for the determination of plasma concentrations of EFV and lumefantrine in 69 and 453 samples, respectively, from pregnant and non-pregnant women with malaria-HIV co-infection. The median (interquartile range) of plasma concentrations of EFV among pregnant and non-pregnant women were 1821 (1300–2609) ng/mL and 2763 (2018–5673) ng/mL, respectively. Median (IQR) plasma concentrations of lumefantrine on Day 7 were 279.2 (119.9–605.5) ng/mL and 212.05 (133.4–400.1) ng/mL in the pregnant and non-pregnant women, respectively.
Discussion
The present HPLC–UV assay represents a simple, robust and sensitive method for the quantification of lumefantrine and EFV in plasma. The method described here is comparatively simple, sensitive, cost-effective, rugged, reliable and repeatable when compared to similar HPLC–UV or more sophisticated LC–MS methods [9,10,11, 16,17,18,19,20]. Previously reported methods often involved a sample pre-treatment step in order to optimize sensitivity and recovery of lumefantrine from plasma. The present study combined both protein precipitation and liquid–liquid extraction for sample clean-up as against protein precipitation plus solid-phase extraction (SPE) employed by Huang et al.[9]. Thus, the present method, in its own right, might be less expensive when compared to the use of either SPE or LC–MS techniques.
Besides, the present assay involves the use of a relatively small sample volume (200 µL) which may further enhance its suitability for clinical studies. To the best of our knowledge, only three LC–MS methods, previously adopted for clinical studies, utilised a much lower sample volume, ranging from 25 to 100 µL [16, 20, 21]. However, limitations concerning availability or access to LC–MS might discourage the use of those methods in resource-limited settings like SSA. For ethical reasons, the volume of the blood sample taken for clinical studies may be curtailed to a safe limit among paediatrics and pregnant women [22,23,24]. These populations are known to be at the highest risk for falciparum malaria in malaria-endemic settings which might warrant the setting-up of efficacy and pharmacokinetic clinical studies among the vulnerable population. A 200 µL plasma sample volume is quite affordable and could be obtained within the safe limits of blood sample volumes in paediatric and gestational health research.
During the extraction procedure, the samples underwent a heat-inactivation procedure, at 58 °C for 45 min, to inactivate any HIV. The study subjects included people living with HIV (PLWH) and falciparum malaria positivity. Therefore, the plasma samples collected were suspected to contain HIV, which necessitated the need for heat-inactivation before subjecting the samples to bioanalytical assay [25]. In the present study, the QC samples showed that both lumefantrine and EFV were sufficiently stable with or without heat inactivation. The stability result has doused the apprehension of how to inactivate the virus without affecting the integrity of the analytes. The average recoveries of lumefantrine and EFV were sufficiently reproducible and met the set criteria in the FDA guidelines (FDA, 2018) within the range of 80–120% [14].
The retention time of the analytes and run time for the present assay are short compared to previously described HPLC–UV methods for individual quantification of either efavirenz or lumefantrine [9, 25]. The chromatographic condition using a gradient with a high content of organic modifier, acetonitrile, appeared to favour a timely elution of lumefantrine at 5.14 min. We considered 0.1% formic acid to establish an acidic condition to curtail peak tailing. The peaks of EFV and lumefantrine were symmetrical with a narrow width suggesting an accurate measurement of the peak area for each analyte and reliability of the peak area for external calibration. The present method is noted for the simplicity of its extraction protocol, chromatographic settings, satisfactory yields, and high sensitivity and is tailor-made to measure EFV and lumefantrine in human plasma from malaria-HIV co-infected patients.
As a partner drug to artemether, lumefantrine has become a cornerstone in the treatment of uncomplicated falciparum malaria [26]. It has been shown to effectively eradicate the remnant of P. falciparum following the activity of a short-lived artemisinin component and has also been shown to offer a post-treatment prophylactic effect. Measurement of plasma concentration of lumefantrine on day 7 (Cday7) after the first dose of artemether-lumefantrine can be useful in a clinical setting as a surrogate marker for therapeutic efficacy purposes. In addition, when EFV is co-administered at steady-state with artemether-lumefantrine, the concentration of EFV 12 h post-dose can be determined with the present method. It is noted that the Cday7 among the study population was below the cut-off point, of 280 ng/mL, for the therapeutic efficacy of artemether-lumefantrine. This has been ascribed to drug-drug interaction perpetrated by EFV.
Conclusion
Herein, we described an HPLC–UV method that allows accurate and reproducible quantification of EFV and lumefantrine in plasma. The extraction efficiency was good; sensitivity as per the low limit of quantification makes this an acceptable assay for use in therapeutic efficacy and pharmacokinetic clinical studies. This method has a short run time and will enable measurements of the plasma levels of EFV at steady-state, and lumefantrine during TES from a large number of samples.
Availability of data and materials
Data are available upon reasonable request to the corresponding author.
Abbreviations
- EFV:
-
Efavirenz
- ART:
-
Antiretroviral therapy
- LC-UV:
-
Liquid chromatography-ultraviolet detection
- ACT:
-
Artemisinin-based combination therapy
- WHO:
-
World Health Organisation
- SSA:
-
Sub-Saharan African
- TES:
-
Therapeutic efficacy studies
- HPLC–UV:
-
High-performance liquid chromatography with UV detection
- LC/MS/MS:
-
Liquid chromatography in tandem with a Mass Spectrometer
- TBME:
-
Tert-butyl methyl ether
- QC:
-
Quality control
- LQC:
-
Low quality control
- MQC:
-
Medium quality control
- HQC:
-
High quality control
- LOD:
-
Limit of detection
- LOQ:
-
Limit of quantification
- SPE:
-
Solid-phase extraction
- PLWH:
-
People living with HIV
References
Organization WH (2022) Guidelines for Malaria: WHO Guidelines for Malaria. In: vol. WHO/UCN/GMP/2022.01.Rev2. World Health Organization, Geneva
WHO (2022) World Malaria Report 2022. World Health Organisation, Geneva
WHO (2016) Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, 2nd edn. World Health Organisation, Switzerland
WHO (2009) Methods for surveillance of antimalarial drug efficacy
WHO (2010) Methods and techniques for assessing exposure to antimalarial drugs in clinical field studies
WHO (2008) Methods and techniques for clinical trials on antimalarial drug efficacy genotyping to identify parasite populations. World Health Organization, Geneva
Khalil IF, Abildrup U, Alifrangis LH, Maiga D, Alifrangis M, Hoegberg L, Vestergaard LS, Persson OP, Nyagonde N, Lemnge MM et al (2011) Measurement of lumefantrine and its metabolite in plasma by high-performance liquid chromatography with ultraviolet detection. J Pharm Biomed Anal 54(1):168–172
Wiesner L, Govender K, Meredith S, Norman J, Smith P (2011) A liquid-liquid LC/MS/MS assay for the determination of artemether and DHA in malaria patient samples. J Pharm Biomed Anal 55:373–378
Huang L, Lizak PS, Jayewardene AL, Marzan F et al (2010) A modified method for determination of lumefantrine in human plasma by HPLC-UV and combination of protein precipitation and solid-phase extraction: application to a pharmacokinetic study. Anal Chem Insights 5:15–23
Khuda F, Iqbal Z, Shaz Y, Ahmmad L, Nasir F, Zada A, Amanullah K, Shahbaz N (2014) Method development and validation for simultaneous determination of lumefantrine and its major metabolite, desbutyl lumefantrine in human plasma using RP-HPLC/UV detection. J Chromatogr B 944:114–122
César IC, de Aquino Ribeiro JA, de Souza TL, Bellorio KB, de Abreu FC, Moreira JM, Chellini PR, Pianetti GA (2011) Liquid chromatography–tandem mass spectrometry for the simultaneous quantitation of artemether and lumefantrine in human plasma: application for a pharmacokinetic study. J Pharm Biomed Anal 54(1):114–120
Hoffman JT, Rossi SS, Espina-Quinto R, Letendre S, Capparelli EV (2013) Determination of efavirenz in human dried blood spots by reversed-phase high-performance liquid chromatography with UV detection. Ther Drug Monit 35(2):203–208
Curley P, Siccardi M, Moss DM, Owen A (2016) Development and validation of an LC–MS/MS assay for the quantification of efavirenz in different biological matrices. Bioanalysis 8(20):2125–2134
FDA (2018) Bioanalytical method validation guidance for industry. Centre for Drug Evaluation and Research (CDER), US Food and Drug Administration
Adegbola AJ, Abutaima R, Olagunju AE, Ijarotimi OO, Siccardi M, Owen A, Soyinka JO, Bolaji OO (2018) Effect of pregnancy on the pharmacokinetic interaction between efavirenz and lumefantrine in HIV-malaria coinfection. Antimicrobial Agent Chemother 62(10):e01252-e11218
Huang L, Li X, Marzan F, Lizak PS, Aweeka FT (2012) Determination of lumefantrine in small-volume human plasma by LC–MS/MS: using a deuterated lumefantrine to overcome matrix effect and ionization saturation. Bioanalysis 4(2):157–166
Pingale SG, Mangaonkar KV (2013) Quantification of lumefantrine in human plasma using LC-MS/MS and its application to a bioequivalence study. J Pharm 2013:437697
Govender K, Gibhard L, Du Plessis L, Wiesner L (2015) Development and validation of an LC-MS/MS method for the quantitation of lumefantrine in mouse whole blood and plasma. J Chromatogr B Anal Technol Biomed Life Sci 985:6–13
Hodel EM, Zanolari B, Mercier T, Biollaz J, Keiser J, Olliaro P, Genton B, Decosterd LA (2009) A single LC–tandem mass spectrometry method for the simultaneous determination of 14 antimalarial drugs and their metabolites in human plasma. J Chromatogr B 877(10):867–886
Sethi P, Dua VK, Jain R (2011) A LC-MS/MS method for the determination of lumefantrine and its metabolite desbutyl-lumefantrine in plasma from patients infected with plasmodium falciparum malaria. J Liq Chromatogr Relat Technol 34(20):2674–2688
Munjal V, Paliwal N, Chaursia BK, Varshney B, Ahmed T, Paliwal J (2010) LC-tandem mass spectrometry method for quantification of lumefantrine in human plasma and its application to bioequivalence study. Chromatographia 71(5):505–510
Howie SR (2011) Blood sample volumes in child health research: review of safe limits. Bull World Health Organ 89:46–53
Barker CI, Standing JF, Kelly LE, Faught LH, Needham AC, Rieder MJ, de Wildt SN, Offringa M (2018) Pharmacokinetic studies in children: recommendations for practice and research. Arch Dis Child 103(7):695–702
Blehar MC, Spong C, Grady C, Goldkind SF, Sahin L, Clayton JA (2013) Enrolling pregnant women: issues in clinical research. Womens Health Issues 23(1):e39–e45
Charbe N, Baldelli S, Cozzi V, Castoldi S, Cattaneo D, Clementi E (2016) Development of an HPLC-UV assay method for the simultaneous quantification of nine antiretroviral agents in the plasma of HIV-infected patients. J Pharm Anal 6(6):396–403
WHO (2020) World malaria report 2020: 20 years of global progress and challenges. World Health Organisation, Geneva
Acknowledgements
The authors appreciate the study participants and the members of the staff at the various clinics that assisted during patient recruitment. The authors are grateful for the support and training received from the Infection Pharmacology Group at the University of Liverpool.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AJA, JOS and OOB designed the study. RMG and AJA performed all laboratory analysis. AJA, RMG prepared the manuscript, and AJA, JOS and OOB revised the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was obtained from the Health Research and Ethics Committee (HREC) of the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife (Protocol Number-ERC/2016/03/11). The prospective participants were provided with adequate information about the study and written informed consent was obtained from all the study participants eligible for participation.
Consent for publication
Not applicable.
Competing interests
The authors have declared no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Adegbola, A.J., Ogboye, R.M., Soyinka, J.O. et al. A simple high-performance liquid chromatographic assay for concurrent quantification of lumefantrine and efavirenz in human plasma from malaria–HIV co-infected individuals. Futur J Pharm Sci 9, 59 (2023). https://doi.org/10.1186/s43094-023-00508-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00508-x