Abstract
Background
Sofosbuvir/ledipasvir (SOF/LDV), a combination of antiviral drugs, has been recently repurposed for COVID-19 management, according to Food and Drug Administration approval. Paracetamol (PAR) identified as a first-line antipyretic for COVID-19 symptoms' management. The use of these three drugs together has significantly influenced the management of COVID-19 by providing symptomatic relief via inhibiting viral activity. A validated ultra-high performance liquid chromatographic (UHPLC) method has been introduced for the quantification of these repurposed drugs in COVID-19 treatment. This novel chromatographic method allows the simultaneous detection of SOF, LDV, and PAR in bulk. Additionally, the method has been applied to determine the levels of SOF and LDV in human plasma samples with PAR used as an internal standard.
Results
A new UHPLC method was developed, using a mobile phase with a combination of acetonitrile and 0.1% orthophosphoric acid in a proportion of 42:58 (v/v).Flow rate was set at 0.4 ml/min, and UV detection was adjusted at 254 nm. The concentration of SOF, LDV, and PAR were measured by their corresponding peak areas, and showed linear relationships between concentration and peak area within the ranges of (5–60) µg/ml for SOF, (2–22) µg/ml for LDV, and (1–22) µg/ml for PAR. The presented UHPLC method was used to quantify the amounts of SOF, LDV, and PAR in both bulk samples and human plasma samples being spiked with the mentioned analytes. The elution process was completed within 4 min, with retention times of 3.28 min for SOF, 2.28 min for LDV, and 1.70 min for PAR. The method showed high separation selectivity, with an injection volume of 1µl. The precision, accuracy and repeatability of the method were found to be within acceptable limits.
Conclusion
The recently developed method has been successfully validated in accordance with the guidelines set by the International Council for Harmonization (ICH). This validation process ensures that the method is suitable for routine quality control analysis, making it convenient for regular use.
Similar content being viewed by others
Background
In December 2019, the Chinese government informed the World Health Organization (WHO) about the occurrence of pneumonia cases in hospitalized patients with an unknown cause. Subsequently, these patients were identified as having COVID-19, caused by the SARS-CoV-2 virus. The WHO declared the outbreak of this coronavirus illness as a global pandemic in March 2020. By the end of June 2020, the total number of reported cases worldwide had exceeded 10 million, with a significant number of deaths [1].
In mild cases, individuals experienced symptoms such as fatigue, fever, and dry cough. However, severe infections led to the failure of the respiratory and renal systems [2]. To manage the emerging COVID-19, numerous studies have been conducted internationally, and several existing medications have been repurposed [3]. Some of these medications include compounds containing heterocyclic structures, which are widely used in the pharmaceutical industry [4]. One such medication is the combination of SOF/LDV, which has been repurposed for the treatment of COVID-19 [5, 6]. SOF/LDV is an FDA-approved combination used for treatment of hepatitis C virus (HCV) infection [7]. SOF works by suppressing the non structural protein 5B—RNA dependent RNA polymerase (NS5B–RdRp) enzyme, which is necessary for hepatitis C virus replication. LDV, on the other side, inhibits the non structural protein 5A(NS5A), a crucial protein required for the function of RdRp [8].
Many researches have been directed for the discovery of drugs capable of reversing the COVID-19 most severe and potentially fatal consequences, particularly hyper coagulation and cytokine storm [9]. Ibuprofen is a popular over-the-counter pain reliever. Recent research, however, have raised concerns regarding its possible hazardous impact with corona virus disease 2019, after French authorities announced in March 2020 the risk of negative effects of ibuprofen in COVID-19 patients via Angiotensin-converting enzyme 2(ACE2) regulation. As a result, PAR is preferred over ibuprofen for the treatment of COVID-19 symptoms [10, 11]. PAR, also known as N-(4-Hydroxyphenyl) acetamide exhibits antipyretic and analgesics actions and was recently identified as the first-line antipyretic in COVID-19 symptomatic management [12].
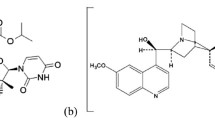
Sofosbuvir(SOF) also known as (S)-isopropyl-2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydro-pyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyl-tetrahydro-furan-2-yl)methoxy-phenoxy-phosphoryl) amino)propanoate, is a solid substance, off-white in color. It is non-hygroscopic crystals (Fig. 1a). Ledipasvir (LDV) which is methyl [(2S)-1-{(6S)-6-[5-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamatepropan-2-one (1:1), is another crystalline substance. It is slightly hygroscopic and forms crystals (Fig. 1b). Paracetamol (PAR),N-(4-hydroxyphenyl) acetamide, appears as white solid crystals [13] (Fig. 1c).
Upon literature review, many chromatographic techniques, including LC–MS/MS, were known to be used for the determination of SOF/LDV in combination [14,15,16], and RP-HPLC–DAD [17]. Also, dissolution studies applying RP-HPLC were performed [18,19,20]. On the other hand, several chromatographic techniques for determination of PAR were conducted, including TLC densitometry [21,22,23] and HPLC approaches [24,25,26]. Hereby, in this study the pharmacological relevance of combining SOF and LDV with PAR for the treatment of COVID-19 was the urge for the development of convenient method for the analysis of the repurposed drugs.
Material and methods
Instrumentation
Agilent technology 1290 infinity system was utilized for chromatographic separation. This system consisted of various components including a UV Diode array detector (G4212A), a Quaternary pump (G4204A), a thermostat (G330B), a thermostated column compartment (G1316C), an auto injector sampler (G4226A), and Open LAB ChemStation C.01.05 software (USA) for data analysis. For sonication purposes, a Branson Model 3510 Ultrasonic Cleaner from the UK was employed. A high-speed and refrigerated centrifuge (centrifuge sigma 3-30k, Germany) was used for centrifugation. An analytical balance (Italy, Sartorius CPA225D) was utilized for weighing samples accurately. A pH meter instrument (Jenway 3505, UK) was utilized for determining solutions pH. To obtain deionized water, pure lab flex (FLC00006641) was utilized in the laboratory.
Reagents and chemicals
Authentic standards of SOF & LDV (purity 99.5%) were supplied by Optimus in India, and PAR (purity 99.9%) was purchased from EL-Rewad Industrial Pharmaceutical Company (RPIC) in Cairo, Egypt. Methanol, acetonitrile and orthophosphoric acid, all HPLC grade, were obtained from (Sigma-Aldrich, Germany). Shabrawishi Blood Bank, Eldokki, Cairo, Egypt, provided fresh frozen plasma.
Standard and working solutions
Standard stock solutions were prepared by accurately weighing and transferring 0.1 g of SOF, LDV, and PAR to separate 100 ml volumetric flasks and completing the volume with methanol. Stock solutions of 1000 µg/ml concentration were separately produced. UHPLC working solutions(100 µg/ml),were developed by individual transfer of 10 ml of SOF, LDV and PAR standard stock solutions (1000 µg/ml) to 100 ml volumetric flasks and completing the volume with methanol.
Procedures and chromatographic conditions
Various concentrations of SOF, LDV, and PAR, ranging from 5 to 60 µg/ml, 2 to 22 µg/ml, and 1 to 22 µg/ml), respectively, were prepared by transferring different volumes from a working solution of 100 µg/ml into 10 ml volumetric flasks and then completed with methanol. An auto sampler was utilized to inject 1 µl of each sample. To achieve chromatographic separation, an Agilent Infinity Lab 101 Poroshell 120 EC-C18 (3 × 150 mm 1.9-Micron) column from the USA was employed. The mobile phase consisted of a mixture of acetonitrile and 0.1% orthophosphoric acid in a ratio of 42:58 (v/v), with a flow rate of 0.4 ml/min. UV detection was set at 254 nm using a diode array detector (DAD).
Drug spiked plasma method
Six non-zero drug spiked human plasma calibration standards were prepared with concentration range of 5–35 μg/ml and 4–20 μg/ml for SOF and LDV, respectively. Preparation was completed by adding 50 μl of known working solution of SOF(50–350 μg/ml) and 50 μl of known working solution of LDV (40–200 μg/ml) to 350 μl of drug free human plasma. PAR was used as internal standard by adding 50 μl of 100 μg/ml PAR working solution.
For drug extraction, 500 μl of all drugs spiked calibration plasma standards were mixed with 500 μl of acetonitrile for protein precipitation. The solutions were then vortexed for 10 min then centrifuged at 3000 rpm for 15 min and supernatants were transferred to vials for UHPLC analysis.
Results
Linearity
Linear correlations were established while plotting the peak area against the concentrations of each of: SOF, LDV, and PAR within their concentration ranges of 5–60.0 µg/ml, 2–22 µg/ml, and 1–22 µg/ml, respectively. The obtained results are presented in Table 1 and Fig. 2. It's worth noting that oral doses of the mentioned drugs was reported to show maximum plasma concentrations (Cmax) of 567 ng/ml and 323 ng/ml for sofosbuvir and ledipasvir, respectively. While after oral administration of acetaminophen, Cmaxis 12.3 μg/ml.
Regarding drug spiked human plasma, linear relationships were observed by plotting the ratio of the peak area for each analyte (SOF and LDV) to the peak area of the PAR internal standard (10 µg/ml) against the concentrations of SOF (ranging from 5 to 35 µg/ml) and LDV (ranging from 4 to 20 µg/ml). These findings are depicted in Fig. 3.
Accuracy
According to ICH recommendations [27], proposed methods' accuracy was assessed by analyzing different concentrations of SOF (15, 25and 40 μg/ml), LDV (8, 15and 18 μg/ml) and PAR (2, 6and18μg/ml). Standard deviation and mean recoveries were determined to be around acceptable parameters, with high accuracy (Table 2). For drug spiked human plasma method, different concentrations of drug spiked human plasma standard SOF (8, 18 and 25 µg/ml) and LDV (7, 14 and 18 µg/ml) were studied (Table 3).
Precision
Precision of the suggested method was valid for both bulk and drug spiked human plasma samples, considering intra-day and inter-day variations. In the case of bulk analysis, intra-day precision was assessed by analyzing three different concentrations of SOF (10, 30, and 60 µg/ml), LDV (5, 10, and 14 µg/ml), and PAR (4, 10, and 22 µg/ml) using the UHPLC method on the same day. Similarly, the same concentrations were analyzed on three different days to evaluate inter-daily precision, as shown in (Table 1).
For the drug spiked human plasma analysis, the precision of the suggested method was examined intra-daily and inter-daily using three different concentrations (low, medium, and high). Intra-day precision was evaluated by analyzing three different concentrations of drug spiked plasma, including SOF (10, 20, and 35 µg/ml) and LDV (6, 10, and 20 µg/ml), on the same day using the UHPLC method with PAR as the internal standard (at a concentration of 10 µg/ml). The same concentrations were analyzed on three different days to assess inter-daily precision.
Specificity
To evaluate the specificity of the proposed techniques, laboratory-made combinations of SOF, LDV, and PAR were prepared at different concentrations and ratios. These combinations were tested to ensure that there was no interference observed in the presence of each other, as depicted in Fig. 4. Additionally, the techniques were assessed for any interference with plasma content, as shown in Fig. 5. The results showed no significant interferences, confirming the specificity of the method.
Furthermore, the mean recoveries obtained from the analysis were found to be acceptable, indicating the accuracy of the suggested method. Additionally, the method demonstrated good resolution, as demonstrated in Table 4.
Robustness
Robustness of the suggested UHPLC method was assessed by examining the impact of small variations in flow rate (0.35 and 0.45 ml/min), the ratio of the mobile phase (40:60 and 44:56 v/v acetonitrile: 0.1 orthophosphoric acid), and temperature (30 ± 2 °C). These variations were evaluated to determine their effect on the peak areas. The results obtained demonstrated that the peak areas exhibited low %RSD (Relative standard deviation) values, indicating the robustness of the method. These findings are presented in Table 5.
System suitability
The system suitability parameters, such as retention time (min), capacity factor (k’), selectivity (α), resolution (Rs), tailing factor (T), number of theoretical plates (N), and height equivalent to theoretical plates (HETP), were examined and assessed in accordance with the guidelines of US Pharmacopeia [28] (Table 6).
Discussion
Extensive experimentation was conducted to determine the optimal chromatographic conditions for the separation of the analyte mixture. Various parameters, such as column type, mobile phase polarity, pH, and organic solvent ratio, were investigated.
Initially, using a ZORBAX CN column (4.6 × 250 mm, 5 µm) with a mobile phase consisting of acetonitrile and phosphate buffer in a 50:50 (v/v) ratio, adjusted to a pH of 5.8 using orthophosphoric acid, failed to achieve satisfactory separation. This resulted in overlapping peaks. Even after trying different mobile phase ratios, separation could not be achieved. However, when the column was switched to a Eurospher 100–5 C18 column (250 × 4.6 mm) and a mobile phase ratio of 60:40 (v/v) was employed, separation was achieved. However, a forked peak for PAR was observed.
Finally, the best separation results were obtained when an Agilent Infinity lab Poroshell 120 EC-C18 column (3 × 150 mm, 1.9 µm) was used applying a mobile phase consisting of acetonitrile and 0.1% orthophosphoric acid in a 40:60 (v/v) ratio. This system yielded sharp peaks for all the analyzed drugs. To further enhance peak sharpness and reduce retention time, the mobile phase ratio was adjusted to 42:58 (v/v) of acetonitrile and 0.1% orthophosphoric acid.
Conclusions
The suggested chromatographic method was utilized for the simultaneous quantification of SOF, LDV, and PAR, which are commonly used together as a repurposed combination for COVID-19 management. The method has been demonstrated to be accurate, precise, specific, and robust. It also features a short run time, ensuring its economic, simple, and fast operation. Consequently, the method can be effectively employed for routine quality control analysis in the pharmaceutical industry. Moreover, the proposed method was successfully applied to drug spiked human plasma analysis and underwent validation in accordance with the guidelines set by the ICH of technical requirements for pharmaceuticals for human use.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- UHPLC:
-
Ultra-high performance liquid chromatography
- SOF:
-
Sofosbuvir
- LDV:
-
Ledipasvir
- FDA:
-
Food and Drug Administration
- PAR:
-
Paracetamol
- ICH:
-
International Conference on Harmonization
- WHO:
-
Food and Drug Administration
- HCV:
-
Hepatitis C virus
- NS5B:
-
Nonstructural protein 5B
- RdRp:
-
RNA dependent RNA polymerase
- NS5A:
-
Nonstructural protein 5A
- ACE2:
-
Angiotensin-converting enzyme 2
References
Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC (2020) Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. JAMA 324(8):782–793. https://doi.org/10.1001/jama.2020.12839
Scavone C, Brusco S, Bertini M, Sportiello L, Rafaniello C, Zoccoli A, Berrino L, Racagni G, Rossi F, Capuano A (2020) Current pharmacological treatments for COVID-19: what’s next? Br J Pharmacol 177(21):4813–4824. https://doi.org/10.1111/bph.15072
Barghash RF, Fawzy IM, Chandrasekar V, Singh AV, Katha U, Mandour AA (2021) In silico modeling as a perspective in developing potential vaccine candidates and therapeutics for COVID-19. Coatings 11(11):1273. https://doi.org/10.3390/coatings11111273
Mandour AA, Nabil N, Zaazaa HE et al (2020) Review on analytical studies of some pharmaceutical compounds containing heterocyclic rings: brinzolamide, timolol maleate, flumethasonepivalate, and clioquinol. Future J Pharm Sci 6:52. https://doi.org/10.1186/s43094-020-00068-4
Medhat MA, El-Kassas M, Karam-Allah H, Al Shafie A, Abd-Elsalam S, Moustafa E et al (2022) Sofosbuvir/ledipasvir in combination or nitazoxanide alone are safe and efficient treatments for COVID-19 infection: a randomized controlled trial for repurposing antivirals. Arab J Gastroenterol 23(3):165–171. https://doi.org/10.1016/j.ajg.2022.04.005
Elgohary MA, Hasan EM, Ibrahim AA et al (2022) Efficacy of Sofosbuvir plus Ledipasvir in Egyptian patients with COVID-19 compared to standard treatment: a randomized controlled trial. J Med Life 15(3):350–358. https://doi.org/10.25122/jml-2021-0175
Schinazi RF, Shi J, Whitaker T (2015) Sofosbuvir (Sovaldi): the first-in-class HCV NS5B nucleotide polymerase inhibitor. In: Li JJ, Johnson DS (eds) Innovative drug synthesis. https://doi.org/10.1002/9781118819951.ch4
Zuccaro V, Lombardi A, Asperges E, Sacchi P, Bruno R (2020) PK/PD and antiviral activity of anti-HCV therapy: is there still a role in the choice of treatment? Expert Opin Drug Metab Toxicol 16(2):97–101. https://doi.org/10.1080/17425255.2020.1721459
Poggiali E, Bastoni D, Ioannilli E, Vercelli A, Magnacavallo A (2020) Deep vein thrombosis and pulmonary embolism: two complications of COVID-19 pneumonia? Eur J Case Rep Internal Med. https://doi.org/10.12890/2020_001646
Micallef J, Soeiro T, Jonville-Béra AP (2020) COVID-19 and NSAIDs: primum non nocere. Therapie S0040–5957:30142–30146. https://doi.org/10.1016/j.therap.2020.07.008
Micallef J, Soeiro T, Jonville-Béra AP (2020) Non-steroidalanti-inflammatory drugs, pharmacology, and COVID-19 infection. Therapie 75:355–362. https://doi.org/10.1016/j.therap.2020.05.003
Casalino G, Monaco G, Di Sarro PP, David A, Scialdone A (2020) Coronavirus disease 2019 presenting with conjunctivitis as the first symptom. Eye (Lond) 34(7):1235–1236. https://doi.org/10.1038/s41433-020-0909-x
European Medicines Agency (2014) http://www.ema.europa.eu, Procedure No. EMEA/H/C/003850/0000, 25 September 2014
Rezk MR, Bendas ER, Basalious EB, Karim IA (2016) Quantification of sofosbuvir and ledipasvir in human plasma by UPLC-MS/MS method: application to fasting and fed bioequivalence studies. J Chromatogr B Anal Technol Biomed Life Sci 1028:63–70. https://doi.org/10.1016/j.jchromb.2016.06.004
Pan C, Chen Y, Chen W, Zhou G, Jin L, Zheng Y, Lin W, Pan Z (2016) Simultaneous determination of ledipasvir, sofosbuvir and its metabolite in rat plasma by UPLC-MS/MS and its application to a pharmacokinetic study. J Chromatogr B Anal Technol Biomed Life Sci 1008:255–259. https://doi.org/10.1016/j.jchromb.2015.11.056
Elkady EF, Aboelwafa AA (2016) a rapid and optimized LC-MS/MS method for the simultaneous extraction and determination of sofosbuvir and ledipasvir in human plasma. J AOAC Int 99(5):1252–1259. https://doi.org/10.5740/jaoacint.16-0021
Farid NF, Abdelwahab NS (2017) Chromatographic analysis of ledipasvir and sofosbuvir: new treatment for chronic hepatitis C infection with application to human plasma. J Liquid Chromatogr Relat Technol 40(7):327–332. https://doi.org/10.1080/10826076.2017.1298526
ZamanB SF, Hassan W (2016) RP-HPLC method for simultaneous determination of sofosbuvir and ledipasvir in tablet dosage form and its application to in vitro dissolution studies. Chromatographia 79(23–24):1605–1613
HassounaME AMM, Mohamed MA (2017) Assay and dissolution methods development and validation for simultaneous determination of sofosbuvir and ledipasvir by RP-HPLC method in tablet dosage forms. J Forensic Sci Crim Inves 1(3):555–562
Rote AP, Alhat J, Kulkarni AA (2017) Development and validation of RP-HPLC method for the simultaneous estimation of ledipasvir and sofosbuvir in bulk and pharmaceutical dosage form. Int J Pharm Sci Drug Res 9(6):291–298
Abdellatef HE, Ayad MM, Soliman SM, Youssef NF (2007) Spectrophotometric and spectrodensitometric determination of paracetamol and drotaverineHCl in combination. Spectrochimica Acta Part A 66(4–5):1147–1151. https://doi.org/10.1016/j.saa.2006.05.028
Pyka A, Budzisz M, Dołowy M (2013) Validation thin layer chromatography for the determination of acetaminophen in tablets and comparison with a pharmacopeial method. BioMed Res Int. https://doi.org/10.1155/2013/545703
Abdelaleem EA, Naguib IA, Hassan ES, Ali NW (2015) HPTLC and RP-HPLC methods for simultaneous determination of paracetamol and pamabrom in presence of their potential impurities. J Pharm Biomed Anal 114:22–27. https://doi.org/10.1016/j.jpba.2015.04.043
Pappula N, Chintala P (2014) Development and validation of RP-HPLC method for the simultaneous estimation of paracetamol and flupirtine maleate in pharmaceutical dosage form. Indian J Pharma Edu Res 48:34–39
Patra S (2015) Development and validation of a novel RP-HPLC method for simultaneous determination of paracetamol, phenylephrine hydrochloride, caffeine, cetirizine and nimesulide in tablet formulation. Arab J Chem 8(4):591–598
Narwade SS (2014) Qualitative and quantitative analysis of paracetamol in different drug samples by HPLC technique. J Appl Chem 7(8):46–49
International Conference on Harmonization Q2 (2005) Validation of analytical procedures: text and methodology. Geneva, Switzerland.
Pharmacopeia, U.S. 28th revision (2004) United States Pharmacopeial Convention. Inc, Rockville, MD
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
SG: Conceptualization, Methodology, Analysis, Validation, Visualization, Writing original draft. GGM: Supervision, Visualization & editing. SAS: Supervision, Visualization & editing. MIE: Methodology, Supervision, Visualization, & editing. AAM: Methodology, Validation, Supervision, Visualization, & editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gamal, S., Mohamed, G.G., Salih, S.A. et al. Rapid and validated UHPLC method for simultaneous determination of sofosbuvir, ledipasvir and paracetamol as commonly repurposed drugs for COVID-19 treatment: application in spiked human plasma. Futur J Pharm Sci 9, 92 (2023). https://doi.org/10.1186/s43094-023-00548-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00548-3