Abstract
Background
Luffa cylindrica (L.) is an annual climbing plant that produces fibrous fruit and can also be used as a vegetable in northern parts of India. Various studies have been carried out on the plant and found to have anti-inflammatory, antifungal, analgesic, anti-myocardial, anti-hyper triglyceride, immunostimulant, anti-allergic, and other properties. The ethanolic extract of the Luffa cylindrica (L.) fruit has not yet been subjected to LC–MS analysis for several bioactive chemicals that target neurological diseases. Oxidative stress is an inevitable situation in AD mechanisms and is a key bridge connecting various AD pathways.
Results
Luffa cylindrica contains various phytochemicals and showed highest alkaloid content of 21.39 ± 1.47 mg of AE/g. A total of 80 compounds were identified in the ethanolic extract from LC–MS analysis. The bioactive compounds were screened for eligibility by Lipinski's rule of five for docking with receptors responsible for causing oxidative stress-associated Alzheimer's disease. Perlolyrine was chosen to perform in-silico docking. An in vitro activity of cholinesterase showed highest inhibition at 500 µg/ml. In-silico docking of perlolyrine showed better binding affinity and score. Results revealed that out of 10 docked receptors, amyloid beta showed the highest binding affinity with an energy of − 46.1 kcal/mol showing promising drug for Alzheimer's disease.
Conclusion
Based on current findings, the study reports the presence of a promising, bioactive compound (perlolyrine) and in turn provides an optimistic note in exploring its biological activity in vivo with oxidative stress-related Alzheimer's disease.
Similar content being viewed by others
Background
Luffa cylindrica (L.) is an annual climbing plant that produces fibrous fruit and can also be used as a vegetable in northern parts of India. It belongs to the Cucurbitaceae family and is also called as loofa, bath sponge, sponge gourd, etc., and contains smooth and cylindrical-shaped fruits. The medicinal plant Luffa cylindrica has been used extensively to cure various ailments. Family Cucurbitaceae is economically beneficial as it is a significant food source and has pharmacological properties that include anticancer, antiulcer, anti-diabetic, analgesic, and nephroprotective [1]. In Bangladesh, Luffa cylindrica (L.) and Luffa acutangula are widely used as vegetables, meals, and folkloric medicines to treat various illnesses [2]. The plant is traditionally used to treat a variety of conditions, including asthma, intestinal worms, sinusitis, chronic bronchitis pain, carbuncles, abscesses, inflammation, heat rashes, bowel or bladder hemorrhage, hemorrhoids, jaundice, menorrhagia, hematuria, leprosy, splenopathy, and as an antiseptic, emmenagogue, and carminative [3, 4]. Luffa cylindrica (L.) fruit has been used extensively to clear phlegm along with antipyretic effect. Its fruit is also used to treat hyperglycemia [5, 6], cataract [7], anti-emetic, anti-inflammatory [8], and antimicrobial effect. [9] Its leaves are also studied extensively on cancer [10], hepatoprotective [11], and anthelminthic [12] activities. Similarly, its seed also has the potential in treating asthma-related effects [13], anti-fungal [14], abortifacient [15], and anti-HIV activity [16]. Various studies have been carried out on the plant and found to have anti-inflammatory, antifungal, analgesic, anti-myocardial, anti-hyper triglyceride, immunostimulant, anti-allergic, and other properties [2, 17]. The components that make up plant antioxidants include terpenoids, polyphenolic chemicals, ascorbic acid, and tocopherols, which serve a variety of essential roles in both plants and humans [18]. Oxidative damage contributes significantly to the aging process and the etiology of various illnesses, including atherosclerosis, diabetes, cataracts, bronchial asthma, Alzheimer’s disease, cancer, and rheumatoid arthritis. Chemical methods such as radical scavenging assays (DPPH, ABTS, hydroxyl assays), lipid peroxidation assays (carotene-linoleate model systems, thiobarbituric acid-reactive substances), and reduction power assays (FRAP, CUPRAC), as well as enzyme-based assays, can be used to investigate the antioxidant potential of plants [19]. These antioxidants can safeguard plant cells from reactive oxygen species damage, which frequently causes biochemical and physiological lesions, metabolic dysfunction, and ultimately cell death [20]. The endothelin receptor (ER) has a well-established significance in metabolic and cardiovascular problems because of its role and function in Ca2+ handling, protein synthesis/folding, and secretory pathway control. Because ER redox state strongly affects protein folding, when disulfide bond production is regulated in response to ER stress, luminal oxidative stress rises, and ER function falls [21]. Chronic oxidative stress conditions speed up the neurodegenerative process by increasing the calcium influx into the cells caused by reactive oxygen species and altering the mitochondria by lowering succinate dehydrogenase (SDH) activity [22]. Additionally, it can disrupt ATP receptors by augmenting phosphorylation pathways and decreasing membrane depolarization. In conclusion, ozone exposure leads to oxidative stress, which stimulates the purinergic receptor and GSK-3 response, activating the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) to produce the pro-inflammatory IL-1 and lowering IL-10, both of which give rise to cell death [23]. Luffa plants are a significant source of anti-retroviral medications since several species have ribosome-inactivating proteins (MAP30, luffin A & B) and anti-HIV activity [24]. In a study, ethanol extract proved the presence of various phytochemicals and antioxidant activity. It also demonstrated the beneficial effects of plant-based therapy on free radical scavenging effects [25].
The most common cause of dementia and cognitive decline in older people (over age 65) is Alzheimer's disease (AD) worldwide. Pathophysiological alterations include the build-up of toxic amyloid beta plaques, neurofibrillary tangles of hyperphosphorylated tau protein, and neurodegeneration carried by unregulated microglial activation in the brain, which secretes neurotoxins and inflammatory cytokines [26]. Population-based European studies show that the age-standardized prevalence of dementia and AD is 6.4 percent and 4.4 percent among older adults of age 65. Vascular risk factors in middle-aged and older persons strongly contribute to the start and development of dementia and AD [27]. The ethanolic extract of the Luffa cylindrica (L.) fruit has not yet been subjected to LC–MS analysis for several bioactive chemicals that target neurological diseases. Cholinesterase inhibitors have been the best track record of effectiveness for several AD features. Newer potential therapeutic agents are still in the early stages of clinical research, and alternative therapy modalities have not undergone as much testing or demonstrated as much efficacy [28]. Traditional cholinesterase inhibitors are found to have cholinergic actions as well as activity against several AD molecular targets in several in vitro and in vivo investigations. However, their effects in slowing AD progression are still minimal [29]. Oxidative stress is an inevitable situation in AD mechanisms and is a key bridge connecting various AD pathways. Even though the majority of antioxidants have potent benefits in animal research, the outcomes of trials in people are insufficient [30]. For this instance, various oxidative stress receptors associated with Alzheimer’s disease can be docked with identified compounds and understand the physiology to prevent the disease.
This work aims to provide a comprehensive view of the phytochemical screening of L. cylindrica fruit, identify various bioactive compounds from LC–MS analysis, in vitro cholinesterase activity, and perform in-silico docking of different receptors targeting Alzheimer's disease-associated oxidative stress.
Methods
Collection and identification of plant material
The entire fruit of Luffa cylindrica (L.) was collected in and around Uttar Pradesh from June to August 2021. The plant specimen, preserved in the Department of Pharmacognosy, was authenticated by Dr. P. Jayaraman, Director of the Plant Anatomy Research Center in Tambaram, Chennai (Registration No. PARC/2021/4528).
Processing and preservation of plant material
Luffa cylindrica (L.) unripe fruit was washed well in running tap water and then rinsed in distilled water. The unripe fruits were cut into small pieces and dried in the shade for two weeks to achieve total dryness. A mechanical grinder was used to ground the dried unripe fruits to a fine powder. For later usage, the powdered substance was stored in airtight containers.
Reagents and chemicals
All the organic solvents and acids used for these experiments were of analytical or HPLC grade and were procured from SISCO laboratories.
Preparation of ethanolic extract (Soxhlet extraction method)
Chemical constituent screening in medicinal plants is critical for obtaining helpful information for further research and in determining pharmacological effects. The dry powder of Luffa cylindrica (L.) fruit was defatted using petroleum ether and extracted with 2000 ml of ethanol using Soxhlet at 45 °C for 3 days [31]. Whatman filter paper (Grade 1- particle retention 11 µm) was used to filter it, and the filtrate was concentrated in a water bath and then stored in an airtight sterile container for further research. Table 1 shows the obtained residue weights and extractive values of the extraction.
Phytochemical screening
Preliminary phytochemical results were carried out in order to identify the presence or absence of certain phytochemicals. The tests performed utilizing ethanolic extract were subjected to a variety of qualitative tests to identify the present phytoconstituents [25]. Table 2 lists the preliminary phytochemical screening results of ethanolic extract of Luffa cylindrica (L.) fruit. The ethanolic extract was subjected to variety of qualitative tests to identify the present phytoconstituents.
Determination of the total phenolics content
The total phenolic content of the Luffa cylindrica (L.) fruit's ethanolic extracts was determined using the spectrophotometric technique [32]. In a nutshell, 2.5 mL of the Folin-Ciocalteu's reagent (10 percent deionized water) and 4 mL of a NaHCO3 solution (7.5 percent w/v) were combined with 0.5 mg of plant extract. The reaction mixture was then incubated for 30 min at room temperature with intermittent shaking for color development. The absorbance of the resulting reaction mixtures (blue color) was measured at 765 nm using a spectrophotometer (Jasco V-730). Gallic acid equivalent (mg of GAE/g dry weight) was used to express the overall amount of phenolics in the investigated samples.
Determination of the total flavonoids content
The total flavonoid content of the Luffa cylindrica (L.) fruit's ethanolic extracts was determined [33] using the spectrophotometric technique [21]. The extract was produced at the same concentration of 1 mg/mL for analysis. In a nutshell, 0.5 mg of the extract was combined with 0.1 mL of a 10% AlCl3 solution, 0.1 mL of a 1 mol/L potassium acetate solution, and 4.3 mL of distilled water. The reaction mixture was then incubated at room temperature for 30 min. The spectrophotometer measured the absorbance at 510 nm (Jasco V-730). The standard calibration curve (10–100 mg/mL) was created using quercetin (Sigma-Aldrich). The quercetin equivalent (mg QE/g dry weight) was used to express the overall flavonoid content of the samples under investigation.
Determination of the total alkaloids content
The total alkaloid content of the Luffa cylindrica (L.) fruit’s ethanolic extracts was determined by spectroscopic technique [34]. In a nutshell, 0.5 mg of Luffa cylindrica (L.) fruit extract was diluted in 20 mL of ethanol solution (1:1) and filtered. The resulting mixture was poured into several funnels for separation. H2SO4 was blended with the filtrates at a 60% ratio (5 ml). After the combination had been left for 5 min, 5 ml of 0.5 percent formaldehyde was added, and the mixture was then left for 3 h. The spectrophotometer measured the absorbance at 565 nm (Jasco V-730). The standard calibration curve (10–100 mg/mL) was created using the atropine standard solutions, which were manufactured similarly. The atropine equivalent (mg AE/g dry weight) was used to reflect the overall amount of alkaloids in the examined samples.
Determination of the total saponins content
The total saponin content of the Luffa cylindrica (L.) fruit's ethanolic extracts was determined by vanillin method using the spectrophotometric technique [35]. In a nutshell, 0.5 mg of Luffa cylindrica (L.) fruit extract was combined with 0.5 mL of an 8 percent vanillin solution and 5 mL of an H2SO4 solution (72%). The resulting liquids were carefully combined before being set aside to chill on ice. They were then incubated in a water bath at 60 °C for 15 min. On the ice, the mixtures were cooled once again, and the spectrophotometer measured their absorbance at 560 nm (Jasco V-730). Oleanolic acid (10–100 mg/mL) was utilized as a reference for creating the calibration curve. Oleanolic acid equivalent (mg OA/g dry weight) was used to express the overall amount of saponins.
Determination of the total triterpenoid content
The total triterpenoid content of the Luffa cylindrica (L.) fruit's ethanolic extracts was determined by using the spectrophotometric technique [36]. Briefly, 2 mL of H2SO4 and 0.5 mg of plant extract in acetic anhydride were combined in a microplate before being incubated for 10 min at room temperature. A calibration curve was created based on ginsenoside Re (10–100 mg/ml) as a standard. At 350 nm, the sample's absorbance was measured compared to a blank sample made up of acetic anhydride-infused plant extract. Triterpenoid concentration was measured in milligrams of ginsenoside Re equivalents (GRE)/g of plant extract, and samples were conducted in triplicate. All the determination content is mentioned in Table 3.
Identification and quantification analysis of constituents by LC–MS analysis
LC–MS (liquid chromatography–mass spectroscopy) analysis was carried out using Column (Hypersil GOLD C18 100 × 2.1 mm-3 MICRON); 30min_+ESI_01112021_MSMS.m method and detected with Q-TOF analyzer for the ethanolic extract of Luffa cylindrica (L.). MS Q-TOF (G6550A) component was deployed with 200 Ms Abs threshold and ion mode as Dual AJS ESI. Acquisition mode for AutoMS2 was set to Ms Min range to 120 (m/z) and max of 1100 m/z. Source parameters were set with gas temp (2500C); gas flow (13 l/min); nebulizer (35 psig). Injection model with needle wash of 5 µL volume was set to 3-secs wash time. Solvent composition was 0.1% formic acid in water (95%) for Channel A and 90% Acetonitrile + 10% Water + 0.1% Formic acid (5%) for channel B. Flow rate was kept constant to 0.300 mL/min, 1200 bar constant pressure, and 40 °C temperature. Nonetheless, to maximize the number of the monitored metabolites ions, MS analysis was carried out in a negative ionization mode. The resulting total ion chromatogram is a time vs. area plot that displays each constituent’s overall response based on the abundance of its molecular ions. The identification of constituents by LC–MS analysis is mentioned in Table 4.
Cholinesterase activity
Ellman’s approach was used to determine AChE inhibition. In a 1-mL cuvette, 640 L of Tris–HCl buffer with a pH of 8 and 0.12 U of AChE enzyme were introduced to the reaction mixture [37]. This reaction mixture was incubated for five minutes at room temperature. 100 mL of DTNB (Ellman’s reagent of 7.5 mM) and 100 mL of acetylcholinesterase (AchEI of 1 mM) were combined, and a spectrophotometer was used to measure the reading at 405 nm. Triplicates of each experiment were performed. The percentage of inhibition was used to characterize the anti-cholinesterase activity and is mentioned in Tables 5 and 6.
In-silico docking analysis
Ligand and receptor preparations
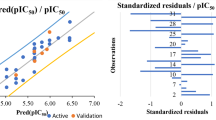
Based on the Lipinski rule of five, the ADMET analysis [38] was performed for all the phytocompounds chosen from the LC–MS data fractions. Using the software ChemDraw Ultra 12.0, the structure of perlolyrine was made and converted to.pdb format using Molegro molecular viewer. The docking computation and techniques to replicate the pocket binding residues were validated using co-crystal ligands. Autodock v4.0 (version) software was employed to open macromolecule and ligand to initiate docking and mentioned in Table 7. In the current investigation, we started the docking analysis with the standard settings such as ligand preparation and saving in.pdbqt format. Grid was prepared for both ligand and macromolecule to cover the area and run for autogrid 4.0. Further docking was initiated to set all the parameters required and saved as a Lamarckian genetic algorithm to further run in Autodock v4.0. All molecules were deleted and analyzed for clusterings which were further confirmed as rank by energy. The RMSD table was used to cluster all the transformations, and the most advantageous binding postures were chosen based on their low free energies and low inhibition constants. The targets chosen for this investigation were based on earlier studies, and protein data bank was used to retrieve all the 3D crystal structures in PDB format. One of the best molecules discovered using LC–MS analysis is perlolyrine. Perlolyrine showed improved idiopathic pulmonary fibrosis [39], osteoarthritis [40], and vascular dementia [41], respectively.
Purification and refinement of proteins and ligands
All the proteins utilized in the study were purified using Biovia Discovery studio to remove unwanted ligands, water molecules, and other contaminants. For better interactions, polar hydrogens were added to the protein during the preparation process, followed by Kollman calculations and determining Gasteiger charges. The PubChem database was used to visualize the 2D and 3D structures of perlolyrine and confirm with our structure (PubChem CID: 160179).
Root-mean-square validation of docked structures
Autodock v4.0 predicted 9 different docking sites for the studied ligand. All of them had different RMSD values. However, we have considered only those sites which gave RMSD value 0 for the betterment of our understanding.
In-silico toxicity predictions
Based on a proven Lipinski concept, compounds' potential drug-likeness was anticipated [42]. All pharmacologically significant substances changed their structures to their canonical, streamlined molecular-input line-entry scheme (SMILE). To identify organ toxicities and toxicological endpoints, obtained SMILE was entered to OSIRIS Property explorer [43]. A drug score measure is used to select molecules as drug candidates. The likelihood of a molecule being regarded as a drug candidate increases with the drug score value as mentioned in Table 8 [44].
Statistical analysis
Each experiment was repeated in triplicate, and data are presented as mean ± standard deviation (SD).
Results
Total phenolic content
Total phenolic content was determined to be 21.42 mg of GAE/g, respectively, when expressed in terms of gallic acid equivalent.
Total flavonoids content
The total flavonoid content expressed in terms of quercetin equivalent was found to be 19.57 ± 2.39 mg of QE/g, respectively.
Total alkaloids content
The total alkaloid content expressed in terms of atropine equivalent was found to be 20.39 ± 1.47 mg of AE/g, respectively.
Total saponin content
The total saponin content expressed in terms of oleanolic acid equivalent was found to be 14.39 ± 2.36 mg of OA/g, respectively.
Total triterpenoid content
The total triterpenoid content expressed in terms of ginsenoside Re equivalent was found to be 9.48 ± 1.64 mg of GRE/g, respectively (Fig. 1).
Liquid chromatography–mass spectrometer (LC–MS) analysis
The outcomes showed the presence of numerous medicinally significant substances, including Mahaleboside (Coumarin glycosides), Chlorogenic acid (Polyphenols), Crotanecine (Pyrrolizidine alkaloids), Perlolyrine (Harmala alkaloids), Phrymarolin I (Benzodioxoles), Diplodiatoxin (Gamma keto acids), Dihydrocapsaicin (Capsaicinoid), Morindone (Anthraquinone), Corchorifatty acid (Linoleic acid), and Polyporusterone B (triterpene carboxylic acid), which were all tentatively identified using database by comparing their molecular fragmentation patterns. The results of the LCMS analysis of the fruit's ethanolic extract, Luffa cylindrica, are shown in Table 4 and Fig. 2.
Liquid chromatography–mass spectrometer (LC–MS) chromatogram of Luffa cylindrica extract
All the compounds were subjected for screening based on Lipinski’s rule of five, and perlolyrine compound (Fig. 3, Fig. 4) was able to cross blood–brain barrier which was observed through online server tool SwissADME (http://www.swissadme.ch/). Perlolyrine compound was later subjected for in-silico molecular docking to see potential score of various receptors involving Alzheimer’s disease-associated oxidative stress (Fig. 5).
Cholinesterases are specialized carboxylic ester hydrolases that break down esters of choline, and its activity with absorbance is mentioned in Figs. 6 and 7.
Molecular docking analysis
According to the LC–MS analysis, Luffa cylindrica (L.) extract included 80 bioactive compounds. Table 4 lists the top compounds with peak areas. These phytochemicals' effects on oxidative stress-related target proteins for Alzheimer's disease were examined. To clarify the binding affinities to the target proteins, phytoligand docking studies were conducted using the Autodock v4.0 software. In general, the Lipinski rule of five was used to select the 80 phytocompounds that were discovered. All ten selected targets' corresponding protein structures were docked against perlolyrine; the best-screened ligand found using ADMET screening based on various criteria, including solubility, toxicity, absorption, molecular weight, and excretion. The best-docked complexes were then filtered based on their binding energy. Using Biovia discovery studio, the interaction between the ligand and the target protein was illustrated. Using Biovia discovery studio, distinct bonds between the ligand and the target protein were also displayed in 2D interaction diagrams. The binding conformations for perlolyrine ([5-(9H-pyrido[3,4-b] indol-1-yl) furan-2-yl] methanol) had the highest binding energy values for amyloid beta protein.
The perlolyrine docking outcomes with target proteins were rated based on binding energies. Perlolyrine docking results with proteins like catalase, glutathione peroxidase, superoxide dismutase, Cox-2, AchE, BuchE, A, GSK-3, tau kinase, and keap-1 indicated that the ligand has a stronger affinity for amyloid beta, which is shown in Fig. 8 and is a critical regulator in Alzheimer’s disease. Figure 8 shows the docked structure depicting ligand interactions with essential amino acids through hydrogen bonds and Vander Waal forces. Table 7 shows the identified compound's affinity for proteins associated with Alzheimer's disease.
Amyloid beta (1IYT) with a binding energy of − 46.1 kcal/mol showed interactions with essential amino acids, viz., ASN27, GLY29, GLY25, LYS28, SER26. Cox-2 (1PXX) with a binding energy of − 7.66 kcal/mol showed interactions with essential amino acids, viz., THR-A206, ALA202, TRP-A387, LEU-A391, ALA-A199, HIS-A388, PHE-A210, HIS-A386, HIS-A207. Butyrylcholine esterase (6IOC) with a binding energy of − 7.59 kcal/mol showed interactions with essential amino acids, viz., ASP-A170, GLY-A169, PRO-A171, GLU-A132, PRO-A133. Tau kinase (1J1C) with a binding energy of − 7.59 kcal/mol showed interactions with essential amino acids, viz., GLN-A151, SER-A147, LEU-A252, THR-A152, GLY-A253, ALA-A143, LEU-A153, TYR-A146. Keap-1 (2FLU) with a binding energy of − 7.26 kcal/mol showed interactions with essential amino acids, viz., GLY-X367, VAL-X606, ALA-X366, ARG-X415. SOD (1CB4) with a binding energy of − 6.77 kcal/mol showed interactions with essential amino acids, viz., LEU-X557, GLY-X367, VAL-X606, ALA-X556, ARG-X415. Catalase (2CAG) with a binding energy of − 6.54 kcal/mol showed interactions with essential amino acids, viz., LYS-A85, LEU-A132, VAL-A110, CYS-A199, LEU-A188, ALA-A83, VAL-A70. Acetylcholine esterase (3LII) with a binding energy of − 6.38 kcal/mol showed interactions with essential amino acids, viz., TYR-B341, SER-B293, TRP-B286. GSK-3β (1UV5) with a binding energy of − 6.14 kcal/mol showed interactions with essential amino acids, viz., LEU-A188, ILE-A109, VAL-A110, LEU-A198, CYS-A199, ASP-A200, LEU-A132, ALA-A83, VAL-A135. Glutathione peroxidase (2P31) with a binding energy of − 5.75 kcal/mol showed interactions with essential amino acids, viz., HIS-B77, ARG-B177, PRO-B151, HIS-B78, VAL-B176, PRO-B76.
In-silico toxicity predictions
The toxicity of the compound was identified using SMILES in OSIRIS property explorer and identified as safe other than mutagenic response as displayed in Table 8.
Discussion
Luffa cylindrica (L.) is used in the food and cosmetic industries since it is a rich source of bioactive chemicals. A study observed that FT-IR analysis and physicochemical characterization of luffa seed oil showed higher content of unsaturated hydrocarbon and esters, enabling the oil to be suitable for the production of paint, pharmaceutical substances, and cosmetics [45]. Studies have shown that the fruit and seeds of the luffa plant contain phytochemicals that are good for human health. Gourd vegetables, especially bitter and bottle gourds, are preventative against gastric ulcers, bacterial and viral infections, arthritis, diabetes, hypertension, and cardiac conditions [46]. A study documented that vegetable peels have beneficial activities and antioxidant potential and promoted the utilization of agricultural wastes to achieve nutritional benefit at zero cost and maintain proper health [47]. Bioactive substances, particularly phytochemicals and antioxidants, are necessary for large-scale and significant bioactivities. Their presence necessitates adequate validation and verification of bioactive metabolites. According to this analysis, the fruits of L. cylindrica contained a reliable amount of phenolics, flavonoids, terpenoids, and alkaloids. As secondary metabolites are now shown to have multiple functions, a fresh wave of genetic and pharmacological studies has further blurred these boundaries [48]. The hydroxyl radical scavengers -carbolines and tetrahydrocarbolines have activity comparable to indole melatonin, a potent scavenger of hydroxyl radicals and antioxidants [49].
Several studies suggest various pharmacological actions, such as antioxidant, antibacterial, anti-inflammatory, and neuroprotective effects, present in harmala alkaloids [50]. Proximate analysis is a quantitative analysis performed to confirm the amount of identified phytochemicals to understand their role in the treatment of disease. Proximate analysis of various processing methods was seen in luffa gourds and confirmed the presence of several variables, including dry matter, crude protein, crude fiber ash, etc. [51, 52]. Alkaloids are renowned for their ability to scavenge free radicals, inhibit hydrolytic and oxidative enzymes, and have anti-inflammatory properties [53]. Alkaloids have been studied for their neuroprotective properties for a long time. They have disclosed their neuroprotective properties and can be useful for curing cognitive impairments and associated diseases such as Alzheimer’s [54, 55]. Alkaloids are essential in the pharmaceutical and cosmetic industries. They have anti-parasitic properties, anti-plasmodial, anti-corrosive, anti-oxidative, antibacterial, and insecticidal properties [56]. Among the most common diseases of the twenty-first century, dementia and depression are becoming more significant as the world's population ages [57].
The most recent therapeutic approach, which takes into account the primary pathobiochemical changes in the system caused by Alzheimer's disease, is an anti-amyloid therapy intended to slow down the progression of the disease [58]. Cucurbitan species are reported highly on inflammation, cancer, and genotoxic activities [59,60,61]. Antineoplastic and anti-inflammatory action is the pharmacological activities that have received the greatest attention in the Cucurbitaceae family, where anti-inflammatory, antioxidant, and immunomodulatory potential has been studied recently. Mechanisms of the inhibition of COX-2, NOS, oxidative stress, pro-inflammatory cytokines, and modulation of acquired immunity proteins are highly focused and can also be studied in neurodegenerative diseases like Alzheimer's disease, which also works on a similar mechanism [62].
Acetylcholine is one of the molecules responsible for communication between nerve cells and the brain. Alzheimer's disease symptoms begin when its level is lowered. The drugs known as acetylcholinesterase inhibitors stop the body from breaking down acetylcholine. Alzheimer's line of research lies on anticholinesterase and antioxidant mechanisms as it targets multiple pathways of dementia [63]. Acetylcholinesterase inhibitors (AChEIs) are primarily used to inhibit AChE since there is enough evidence of a deficit in cholinergic neurotransmission in AD to support therapy intended to repair this deficiency. AChE inhibitors also have antioxidant properties and control amyloid plaque development. Tacrine, donepezil, and rivastigmine are a few AChE inhibitors that have already received FDA approval. The majority of these inhibitors, however, are connected to limited availability and acute toxicity [64].
The extract of Luffa cylindrica (L.) showed inhibitory activity on the AchE enzyme. Similar reports have been reported by Patel SB et al. (2021) in Luffa echinata [1]. Liquid chromatography–high-resolution mass spectrometry (LC-HRMS) has become a robust analytical method for massive target screening. Its use can be expanded to assess the possibility of natural product poisoning in clinical situations [65]. An ultra-performance liquid chromatography–quadrupole-time-of-flight-mass spectrometry has been employed in several studies to characterize the potential components [66]. Additionally, LC–MS analysis revealed the presence of compounds like Mahaleboside (Coumarin glycosides), Chlorogenic acid (Polyphenols), Crotanecine (Pyrrolizidine alkaloids), Perlolyrine (Harmala alkaloids), Phrymarolin I (Benzodioxoles), Diplodiatoxin (Gamma keto acids), Dihydrocapsaicin (Capsaicinoid), Morindone (Anthraquinone), Corchorifatty acid (Linoleic acid), and Polyporusterone B (triterpene carboxylic acid) were known to possess anticancer, depression, hypertension, anti-inflammatory activities [67,68,69,70].
Compounds detected in high-resolution LC–MS analysis could help exhibit potent bioactivities in L. cylindrica. To find potential Alzheimer targeting compounds, affinity ultrafiltration LC–MS/MS was employed to screen and identify target compounds from the extract. A total of five AChE ligands were identified by LC–MS/MS and confirmed the activity using molecular docking and in vitro acetylcholinesterase data [71]. Similarly, we employed HR-LCMS to screen extract and identify the potential components present and to confirm their activity by performing molecular docking and in vitro acetylcholinesterase activity. We performed LC–MS/MS-based molecular networking to ascertain their chemical composition and quantify existing metabolites using a pre-generated plant extract library. This provided a novel method for metabolite authentication and profiling and enabled the discovery of new, allegedly undiscovered metabolites for biological separation and evaluation in the future [72]. We identified various bioactive compounds, among which perlolyrine molecule showed less toxicity in comparison with other compounds by OSIRIS property explorer, and it had the potential to cross the blood–brain barrier. We planned for in-silico molecular docking of perlolyrine with several receptors, which are closely associated with Alzheimer’s disease and oxidative stress, a pathological hallmark of Alzheimer’s disease.
Conclusion
The current therapeutic strategies against AD include acetylcholinesterase inhibitor, anti-tau, N-methyl-D-aspartate antagonist, etc., However, core pathogenesis of AD lies in oxidative stress mechanism and efficient compound with less toxicity is the need. Moreover, many researchers are focusing on oxidative stress-related mechanism in order to prevent extension of disease. This study's investigation of the extracts revealed that Luffa cylindrica (L.) extracts act on many target proteins of Alzheimer's disease and have sufficient AchE activity. Furthermore, this extract showed the highest concentrations of various phytochemical components (alkaloids, phenolics, flavonoids, and saponins). Numerous investigations have confirmed that these phytochemical elements give the extract its bioactivity. The LCMS profile identified prominent metabolites that were reported to be good antioxidants and reported various other biological activities. The compound perlolyrine could be of interest against oxidative stress-related diseases like Alzheimer's and other pharmacological benefits. Based on current findings, the study reports the presence of a promising bioactive compound (perlolyrine) and, in turn, provides an optimistic note in exploring its biological activity (in vivo) in oxidative stress-related Alzheimer’s disease.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- ABTS:
-
2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid)
- FRAP:
-
Ferric-reducing antioxidant power assay
- CUPRAC:
-
Cupric ion-reducing antioxidant capacity
- ER:
-
Endothelin receptor
- SDH:
-
Succinate dehydrogenase
- ATP:
-
Adenosine triphosphate
- GSK-3:
-
Glycogen synthase kinase-3 beta
- NF-kB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- Keap:
-
Kelch-like ECH-associated protein
- MAP30:
-
Mitogen-activated protein
- AD:
-
Alzheimer’s disease
- LC–MS:
-
Liquid chromatography–mass spectroscopy
- QE/g:
-
Quercetin equivalent per gram
- AE/g:
-
Atropine equivalent per gram
- OA/g:
-
Oleanolic acid equivalent per gram
- GRE/g:
-
Ginsenoside Re equivalents per gram
- Q-TOF:
-
Quadrupole time-of-flight analyzer
- DTNB:
-
5,5′-Dithiobis-(2-nitrobenzoic acid)
- AchEI:
-
Acetylcholinesterase inhibitor
- ADMET:
-
Absorption, distribution, metabolism, excretion, toxicity
- RMSD:
-
Root-mean-square deviation
- SMILE:
-
Streamlined molecular-input line-entry scheme
- BBB:
-
Blood–brain barrier
References
Patel SB, Ghane SG (2021) Phyto-constituents profiling of Luffa echinata and in vitro assessment of antioxidant, anti-diabetic, anticancer and anti-acetylcholine esterase activities. Saudi J Biol Sci 28:3835–3846. https://doi.org/10.1016/j.sjbs.2021.03.050
Bulbul IJ, Zulfiker AHM, Hamid K et al (2011) Comparative study of in vitro antioxidant, antibacterial and cytotoxic activity of two Bangladeshi medicinal plants—Luffa cylindrica L. and Luffa acutangula. Pharmacogn J 3:59–66. https://doi.org/10.5530/pj.2011.23.9
Esmail A-S (2019) Constituents and pharmacology of Luffa cylindrical—a review. IOSR J Pharm 9:68–79
Azeez M, Bello O, Adedeji A (2013) Traditional and medicinal uses of Luffa cylindrica: a review. J Med Plants Stud 1:102–111
Hazra M, Kundusen S, Bhattacharya S et al (2011) Evaluation of hypoglycemic and antihyperglycemic effects of Luffa cylindrica fruit extract in rats. J Adv Pharm Educ Res 2:138–146
Shukla V, Nanjappaiah HM, Patil VP, Hugar S (2015) Protective effect of Luffa cylindrica fruit extracts on alloxan intoxicated diabetic rats. RGUHS J Pharm Sci 5:9–15
Dubey S, Saha S, Kaithwas G, Saraf S (2015) Effect of standardized fruit extract of Luffa cylindrica on oxidative stress markers in hydrogen peroxide induced cataract. Indian J Pharmacol 47:644. https://doi.org/10.4103/0253-7613.169586
Kanwal W, Syed W, Salman A, Mohtasheem HM (2013) Anti-emetic and anti-inflammatory activity of fruit peel of Luffa cylindrica (L.) Roem. Asian J Nat Appl Sci 2:75–80
Devi GS, Muthu A, Dharmarajan SK et al (2009) Studies on the antibacterial and antifungal activities of the ethanolic extracts of Luffa cylindrica (Linn) fruit. Int J Drug Dev Res 1
Abdel-Salam IM, Ashmawy AM, Hilal AM et al (2018) Chemical composition of aqueous ethanol extract of Luffa cylindrica leaves and its effect on representation of caspase-8, caspase-3, and the proliferation marker Ki67 in intrinsic molecular subtypes of breast cancer in vitro. Chem Biodivers 15:e1800045. https://doi.org/10.1002/cbdv.201800045
Sharma NK, Priyanka JK et al (2014) Hepatoprotective activity of Luffa cylindrica (L.) M. J. Roem. leaf extract in paracetamol intoxicated rats. Indian J Nat Prod resurces 5:143–148
Tripathi A, Tandon M, Chandekar A et al (2016) In vitro antioxidant and anthelmintic activity on Luffa cylindrica leaf extracts. J Herbs Spices Med Plants 22:348–355. https://doi.org/10.1080/10496475.2016.1224211
Muthumani P, Meera R, Mary S et al (2010) Phytochemical screening and anti inflammatory, bronchodilator and antimicrobial activities of the seeds of Luffa cylindrica. Phytochem Screen Anti inflammatory Bronchodilator Antimicrob Act Seeds Luffa Cylind 1:11–22
Parkash A, Ng TB, Tso WW (2002) Isolation and characterization of luffacylin, a ribosome inactivating peptide with anti-fungal activity from sponge gourd (Luffa cylindrica) seeds. Peptides 23:1019–1024. https://doi.org/10.1016/S0196-9781(02)00045-1
Ng TB, Wong RN, Yeung HW (1992) Two proteins with ribosome-inactivating, cytotoxic and abortifacient activities from seeds of Luffa cylindrica roem (Cucurbitaceae). Biochem Int 27:197–207
Ng Y-M, Yang Y, Sze K-H et al (2011) Structural characterization and anti-HIV-1 activities of arginine/glutamate-rich polypeptide Luffin P1 from the seeds of sponge gourd (Luffa cylindrica). J Struct Biol 174:164–172. https://doi.org/10.1016/j.jsb.2010.12.007
Partap S, Kumar A, Sharma K, Jha KK (2012) Luffa Cylindrica : An important medicinal plant. J Nat Prod Plant Resour 2012:127–134
Graßmann J (2005) Terpenoids as plant antioxidants. Vitam Horm. https://doi.org/10.1016/S0083-6729(05)72015-X
Michalak M (2022) Plant-derived antioxidants: significance in skin health and the ageing process. Int J Mol Sci 23:585. https://doi.org/10.3390/ijms23020585
Hajam YA, Rani R, Ganie SY et al (2022) Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells 11:552. https://doi.org/10.3390/cells11030552
Forrester SJ, Kikuchi DS, Hernandes MS et al (2018) Reactive oxygen species in metabolic and inflammatory signaling. Circ Res 122:877–902. https://doi.org/10.1161/CIRCRESAHA.117.311401
Rodríguez-Martínez E, Martínez F, Espinosa-García MT et al (2013) Mitochondrial dysfunction in the hippocampus of rats caused by chronic oxidative stress. Neuroscience 252:384–395. https://doi.org/10.1016/j.neuroscience.2013.08.018
Velázquez-Pérez R, Rodríguez-Martínez E, Valdés-Fuentes M et al (2021) Oxidative stress caused by ozone exposure induces changes in P2X7 receptors, neuroinflammation, and neurodegeneration in the rat hippocampus. Oxid Med Cell Longev 2021:1–12. https://doi.org/10.1155/2021/3790477
Yadav S, Batra J (2015) Mechanism of anti-HIV activity of ribosome inactivating protein, saporin. Protein Pept Lett 22:497–503. https://doi.org/10.2174/0929866522666150428120701
As S, Vellapandian C (2022) Phytochemical studies, antioxidant potential, and identification of bioactive compounds using GC–MS of the ethanolic extract of Luffa cylindrica (L.) fruit. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-022-03961-1
Tahami Monfared AA, Byrnes MJ, White LA, Zhang Q (2022) Alzheimer’s disease: epidemiology and clinical progression. Neurol Ther 11:553–569. https://doi.org/10.1007/s40120-022-00338-8
Qiu C, Kivipelto M, von Strauss E (2009) Epidemiology of Alzheimer’s disease: occurrence, determinants, and strategies toward intervention. Dialogues Clin Neurosci 11:111–128. https://doi.org/10.31887/DCNS.2009.11.2/cqiu
Schneider LS (2000) A critical review of cholinesterase inhibitors as a treatment modality in Alzheimer’s disease. Dialogues Clin Neurosci 2:111–128. https://doi.org/10.31887/DCNS.2000.2.2/lschneider
Moreira NCDS, Lima JEBDF, Marchiori MF et al (2022) Neuroprotective effects of cholinesterase inhibitors: current scenario in therapies for Alzheimer’s disease and future perspectives. J Alzheimer’s Dis Rep 6:177–193. https://doi.org/10.3233/ADR-210061
Bai R, Guo J, Ye X-Y et al (2022) Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev 77:101619. https://doi.org/10.1016/j.arr.2022.101619
Patel SB, Attar UA, Sakate DM, Ghane SG (2020) Efficient extraction of cucurbitacins from Diplocyclos palmatus (L.) C. Jeffrey: optimization using response surface methodology, extraction methods and study of some important bioactivities. Sci Rep 10:2109. https://doi.org/10.1038/s41598-020-58924-5
Alhakmani F, Kumar S, Khan SA (2013) Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera. Asian Pac J Trop Biomed 3:623–627. https://doi.org/10.1016/S2221-1691(13)60126-4
Bouchelaghem S, Das S, Naorem RS et al (2022) Evaluation of total phenolic and flavonoid contents, antibacterial and antibiofilm activities of hungarian propolis ethanolic extract against Staphylococcus aureus. Molecules 27:574. https://doi.org/10.3390/molecules27020574
Naz S, Alam S, Ahmed W et al (2022) Therapeutic potential of selected medicinal plant extracts against multi-drug resistant Salmonella enterica serovar Typhi. Saudi J Biol Sci 29:941–954. https://doi.org/10.1016/j.sjbs.2021.10.008
Sharifi-Rad M, Pohl P, Epifano F et al (2022) Teucrium polium (L.): phytochemical screening and biological activities at different phenological stages. Molecules 27:1561. https://doi.org/10.3390/molecules27051561
Chen M, He X, Sun H et al (2022) Phytochemical analysis, UPLC-ESI-Orbitrap-MS analysis, biological activity, and toxicity of extracts from Tripleurospermum limosum (Maxim.) Pobed. Arab J Chem 15:103797. https://doi.org/10.1016/j.arabjc.2022.103797
Sreedhar V, Reddenna L, Rajavardhana T et al (2022) Phytochemical composition of Nigella sativa extract as potential source for inhibiting β-amyloid aggregation: significance to Alzheimer’s disease. J Pharm Sci Res 14:689–696
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:42717. https://doi.org/10.1038/srep42717
Wu X, Li W, Luo Z, Chen Y (2022) The molecular mechanism of Ligusticum wallichii for improving idiopathic pulmonary fibrosis. Medicine 101:e28787. https://doi.org/10.1097/MD.0000000000028787
Liu D, Ran S, Yang S et al (2022) Network pharmacology research of Chuanxiong Rhizoma-Acori Tatarinowii Rhizoma in the treatment of vascular dementia. Aging Commun 4:6. https://doi.org/10.53388/AGING202204006
Xiang C, Liao Y, Chen Z et al (2022) Network pharmacology and molecular docking to elucidate the potential mechanism of Ligusticum Chuanxiong against osteoarthritis. Front Pharmacol. https://doi.org/10.3389/fphar.2022.854215
Lipinski CA, Lombardo F, Dominy BW, Feeney PJ (1997) Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 23:3–25. https://doi.org/10.1016/S0169-409X(96)00423-1
Sahilu R, Eswaramoorthy R, Mulugeta E, Dekebo A (2022) Synthesis, DFT analysis, dyeing potential and evaluation of antibacterial activities of Azo dye derivatives combined with in-silico molecular docking and ADMET predictions. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.133279
Periwal V, Bassler S, Andrejev S et al (2022) Bioactivity assessment of natural compounds using machine learning models trained on target similarity between drugs. PLoS Comput Biol 18:e1010029. https://doi.org/10.1371/journal.pcbi.1010029
Nwosu-Obieogu K, Dzarma GW, Ugwuodo CB et al (2022) Luffa seed oil extraction: response surface and neuro-fuzzy modelling performance evaluation and optimization. Process Integr Optim Sustain 6:175–188. https://doi.org/10.1007/s41660-021-00210-6
Hadi N, Tiwari P, Singh RB et al (2022) Beneficial effects of gourds in health and diseases. In: Functional foods and nutraceuticals in metabolic and non-communicable diseases. Elsevier, Amsterdam, pp 61–77
Sadef Y, Javed T, Javed R et al (2022) Nutritional status, antioxidant activity and total phenolic content of different fruits and vegetables’ peels. PLoS ONE 17:e0265566. https://doi.org/10.1371/journal.pone.0265566
Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy. Plant Physiol 184:39–52. https://doi.org/10.1104/pp.20.00433
Herraiz T, Galisteo J (2015) Hydroxyl radical reactions and the radical scavenging activity of β-carboline alkaloids. Food Chem 172:640–649. https://doi.org/10.1016/j.foodchem.2014.09.091
Hussain G, Rasul A, Anwar H et al (2018) Role of plant derived alkaloids and their mechanism in neurodegenerative disorders. Int J Biol Sci 14:341–357. https://doi.org/10.7150/ijbs.23247
Ajuru MG, Kpekot KA, Robinson GE, Amutadi MC (2022) Proximate and phytochemical analysis of the leaves of Justicia carnea Lindi. and Justicia secunda Vahl and its taxonomic implications. J Biomed Biosens 2:1–12
Onigemoa M, Dairo FA, Oso Y, Onigemo H (2022) Proximate composition and phytonutrients of heat treated loofah gourd Luffa cylindrica (M J Roem) seeds. Int J Sci Eng Res 13:770–775
Atanassova M, Georgieva S, Ivancheva K (2011) Total phenolic and total flavonoid contents, antioxidant capacity and biological contaminants in medicinal herbs. J Univ Chem Technol Metall 46:81–88
Oboh G, Adedayo BC, Adetola MB et al (2022) Characterization and neuroprotective properties of alkaloid extract of Vernonia amygdalina Delile in experimental models of Alzheimer’s disease. Drug Chem Toxicol 45:731–740. https://doi.org/10.1080/01480545.2020.1773845
Apaza Ticona L, Hervás Povo B, Serban AM et al (2022) Alkaloids of Gnaphalium polycaulon with Anti-Alzheimer’s Activity. Rev Bras Farmacogn 32:122–126. https://doi.org/10.1007/s43450-021-00229-3
Kurek J (2019) Introductory chapter: alkaloids—their importance in nature and for human life. In: Alkaloids—their importance in nature and human life. IntechOpen, London
Gründer G (2022) Widespread diseases of the twenty-first century. how do we want to live? Springer Berlin Heidelberg, Berlin, pp 59–66
Vorobev SV, Emelin AY, Yanishevskij SN (2022) The evolution of ideas about the treatment of Alzheimer’s disease: from the past to the present day. Russ Neurol J 27:5–15. https://doi.org/10.30629/2658-7947-2022-27-1-5-15
Arroyo-Sandoval JA, Marin-Bravo MJ, Arroyo-Acevedo JL et al (2022) Pharmacobotany, phytochemical analysis and anti-inflammatory effect of the ethanolic extract of Luffa operculata. Pharmacogn J 14:622–628. https://doi.org/10.5530/pj.2022.14.80
Attar UA, Ghane SG, Chavan NS, Shiragave PD (2022) Simultaneous detection of anticancer compounds (Cucurbitacin I, B and E) and some pharmacological properties of Indian Blastania species. S Afr J Bot 147:871–881. https://doi.org/10.1016/j.sajb.2022.03.019
Galucio NCDR, Moysés DDA, Pina JRS et al (2022) Antiproliferative, genotoxic activities and quantification of extracts and cucurbitacin B obtained from Luffa operculata (L.) Cogn. Arab J Chem 15:103589. https://doi.org/10.1016/j.arabjc.2021.103589
Silvestre GFG, de Lucena RP, da Silva AH (2022) Cucurbitacins and the immune system: update in research on anti- inflammatory, antioxidant, and immunomodulatory mechanisms. Curr Med Chem 29:3774–3789. https://doi.org/10.2174/0929867329666220107153253
Kamli MR, Sharaf AAM, Sabir JSM, Rather IA (2022) Phytochemical screening of Rosmarinus officinalis L. as a potential anticholinesterase and antioxidant-medicinal plant for cognitive decline disorders. Plants 11:514. https://doi.org/10.3390/plants11040514
Sharma K (2019) Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol Med Rep. https://doi.org/10.3892/mmr.2019.10374
Luo YR, Goodnough R, Yun C et al (2022) Establishment of a high-resolution liquid chromatography-mass spectrometry spectral library for screening toxic natural products. J Anal Toxicol 46:303–321. https://doi.org/10.1093/jat/bkab015
Yang Z, Li L, Chen C et al (2022) Chemical composition and antibacterial activity of 12 medicinal plant ethyl acetate extracts using LC–MS feature-based molecular networking. Phytochem Anal 33:473–489. https://doi.org/10.1002/pca.3103
Nwafor E-O, Lu P, Zhang Y et al (2022) Chlorogenic acid: potential source of natural drugs for the therapeutics of fibrosis and cancer. Transl Oncol 15:101294. https://doi.org/10.1016/j.tranon.2021.101294
Murlanova K, Cohen N, Pinkus A et al (2022) Antidepressant-like effects of a chlorogenic acid- and cynarine-enriched fraction from Dittrichia viscosa root extract. Sci Rep 12:3647. https://doi.org/10.1038/s41598-022-04840-9
Wang X, Peng M, He C (2022) The antihypertensive effects of Eucommia ulmoides leaf water/ethanol extracts are chlorogenic acid dependent. J Funct Foods 94:105129. https://doi.org/10.1016/j.jff.2022.105129
Xu W, Luo T, Chai J et al (2022) Chlorogenic acid alleviates the inflammatory stress of LPS-Induced BV2 cell via interacting with TLR4-mediated downstream pathway. Comput Math Methods Med 2022:1–6. https://doi.org/10.1155/2022/6282167
Li J, Yang G, Shi W et al (2022) Anti-Alzheimer’s disease active components screened out and identified from Hedyotis diffusa combining bioaffinity ultrafiltration LC-MS with acetylcholinesterase. J Ethnopharmacol 296:115460. https://doi.org/10.1016/j.jep.2022.115460
Jouaneh TMM, Motta N, Wu C et al (2022) Analysis of botanicals and botanical supplements by LC-MS/MS-based molecular networking: approaches for annotating plant metabolites and authentication. Fitoterapia 159:105200. https://doi.org/10.1016/j.fitote.2022.105200
Acknowledgements
The authors would like to acknowledge DST and SAIF/CRNTS, IIT Bombay, for providing LC-MS analytical facility.
Funding
This study received no funding to carry out the research work.
Author information
Authors and Affiliations
Contributions
ASS helped in conceptualization, data curation, formal analysis, writing—original draft, writing—review & editing. CV was involved in conceptualization, data curation, formal analysis, writing—review & editing. All the authors critically reviewed the manuscript for intellectual content. All authors approved the final version of the manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Singh, S.A., Vellapandian, C. The promising guide to LC–MS analysis and cholinesterase activity of Luffa cylindrica (L.) fruit using in vitro and in-silico analyses. Futur J Pharm Sci 9, 33 (2023). https://doi.org/10.1186/s43094-023-00478-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00478-0