Abstract
Parkinson's disease (PD) is a neurodegenerative disorder that is caused by degeneration of nerve cells in the part of the brain called the substantia nigra, which controls movement. Although there is some considerable evidence with conventional drugs for PD, treating patients becomes increasingly difficult due to their short- and long-term adverse effects and other restrictions. This dire circumstance emphasizes the need for an innovative, strong alternative treatment for PD. Plants and natural products are considered one of the most important sources of bioactive molecules against a wide range of health disorders. With mechanistic insights, this systematic review explains the efficacy of clinically proven natural products in managing PD. This review is based on comprehensive literature searches from PubMed, Science Direct, Scopus, and Google Scholar databases using the keywords- “plants or natural products in Parkinson's”, “plants or herbs used in Parkinson's treatment”, or keywords that are similar to those. Natural products that have been clinically proven for their anti-Parkinson effect have only been selected for this study, and the products are- Mucuna pruriens, Caffeine, Camellia sinensis or green tea leaves, and a traditional Chinese herbal called Jiawei-Liujunzi Tang. In comparison to currently available medications, we firmly feel that the mentioned clinically proven natural products would be more effective at treating PD while having fewer adverse effects. However, further study is required to confirm their exact mechanism of action.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Parkinson's disease (PD) is a chronic neurological disorder characterized by both motor and nonmotor indications (DeMaagd and Philip 2015; Jankovic and Tan 2020). PD is one of the most general causes of mental deterioration and it is marked by the steady dent of dopaminergic neurons in the substantia nigra (Iarkov et al. 2020). PD has many risk factors and genetic abnormalities. PD is linked to high cholesterol, carbon disulfide, herbicides, organic solvents, and pesticides. Brain damage, high caloric intake, obesity, and other variables have been related to PD (Zhou et al. 2008; Logroscino 2005; DeMaagd and Philip 2015). PD is characterized by a gradual loss of motor functions, bradykinesia, gait changes, and postural instability (Gelb et al. 1999; Dexter and Jenner 2013; Iarkov et al. 2020). Anxiety, sadness, dysphagia, hallucinations, hypophonia and cognitive dysfunction are among the other symptoms, as are hyposmia, decreased color vision, nociception and sleep disorders (Voon et al. 2011; Lindqvist et al. 2012; Iarkov et al. 2020). The number of people diagnosed with PD increased 2.4 times between 1990 and 2016, reaching an estimated 6.1 million worldwide in 2016 (Dorsey et al. 2018). As per the Parkinson's Disease Foundation reports, 1 million Americans have the disease.
PD is prevalent globally, including in Russia, Germany, USA, the UK, France, China, India, Pakistan and Bangladesh. Despite five decades of research, no effective cure for this illness has been discovered. Both pharmaceutical and nonpharmacological methods are used in the current course of treatment. Upon closer examination of treatments for neurological disorders, it is evident that many pharmacological drugs are commonly utilized for patients with such conditions. In addition, natural compounds are widely employed in treating various neurological disorders (Gyawali and Ibrahim 2014; Sohel et al. 2022a, b; Sohel, Sultana, et al. 2022; Sohel, Biswas, et al. 2022; Biswas et al. 2022; Mitra et al. 2023; Paul et al. 2021; Roni et al. 2024). These natural products, compounds from many natural sources, can heal almost all medical conditions, notably neural ones (Butler 2008). Traditional medicines encompass diverse sources, drawing from terrestrial flora, animal-derived products, aquatic species, and even the biochemical processes of microorganisms (Atanasov et al. 2021; Waltenberger et al. 2016). These natural medicinal products are particularly relevant in less developed countries, where people often self-medicate for diseases without the advice of a doctor and natural products have fewer potential adverse effects than conventional medications (Jami and Biswas 2023). Indian Ayurveda uses herbs, spices, and minerals to heal physiological illnesses (Roni et al. 2024). Charaka Samhitha, an old Ayurvedic text, recommends Sida cordifolia, Mucuna pruriens (MP), Withania somnifera, and Hyoscyamus niger for the treatment of PD. H. niger has been used to cure a wide range of diseases in traditional systems of medicine from various civilizations, including Indian, Byzantine, Roman, and Chinese (T Sengupta et al. 2016a, b). Passion flower, also known as Passiflora incarnata (Passifloraceae), Ginseng, the leaf extract of Ginco biloba, Valeriana officinalis, St. John's wort and Pycnog,enol (derived from French maritime pine bark) are just a few of the natural compounds that have shown promise in the treatment of PD and attention deficit hyperactivity disorder (ADHD) (Corona 2018). Albizia adianthifolia (Leguminosae) leaf aqueous extract has antioxidant capacity and can help regulate neurological anomalies caused by PD (Rabiei et al. 2019).
Therefore, the significance and possibilities of natural products for treating PD are boundless. Quite a few natural compounds produced from plants have the potential to be employed as notably essential medications to treat PD. This study aimed to shed light on how clinically proven natural products help manage PD treatment.
2 Methodology
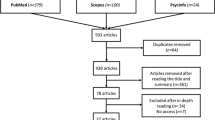
This review has been written based on a systematic search strategy and meta-analyses (PRISMA) guidelines (Moher et al. 2009). SciVerse Scopus® (Elsevier Properties S. A, USA), Web of Science® (Thomson Reuters, USA), and PubMed® (U.S. National Library of Medicine, USA) are the general databases we have used. Common search keywords are PD, natural products in PD management, clinically proven natural products in PD, and so on. A total of 101 non-duplicate articles were identified in the initial phase, and 81 relevant articles were selected after the initial screening. Finally, after 16 exclusions, only 65 more relevant articles have been selected. Non-English articles have been kept out of search. Only clinical and relevant scientific articles were considered inclusion criteria for this study. The complete manuscripts of the relevant articles, including the title, abstract, and concluding remarks, have been thoroughly read to verify the expedience criterion.
3 Natural products in the treatment of Parkinson's disease
There are several clinically proven natural products for managing Parkinson’s disease.
3.1 Mucuna pruriens
MP is a leguminous plant of Fabaceae family, rich in natural levodopa (L-DOPA). In contrast to synthetic levodopa, it is more potent and better tolerated in treating patients with PD. It grows in both tropical and subtropical environments. Levodopa, the most crucial medication for a PD patient, MP seed contains plenty of it (Hirsch and Hunot 2009; Joglar et al. 2009; Khan et al. 2013; Hald and Lotharius 2005).
Previous studies have described that long-term neuroinflammation plays a significant role in the loss of neurons in PD (Hirsch and Hunot 2009; Joglar et al. 2009; Khan et al. 2013; Hald and Lotharius 2005). Inflammation during neurodegeneration is poorly understood. After dopaminergic neurons are injured, glial cells release proinflammatory mediators such as cytokines/chemokines, COX-2, and inducible nitric oxide synthase (Hald and Lotharius 2005). Moreover, through oxidative stress-mediated neurodegeneration, nuclear transcription factor-κB (NF-κB) induces TNF-α and IL-1β production, which contributes to PD pathogenesis (Adepoju and Odubena 2009)These cytokines and enzymes might kill neurons by cytotoxicity. Researchers have speculated that herbs with anti-oxidants and anti-inflammatory properties are valuable for human health. So, MP is a great medicinal herb with significant therapeutic capacities for PD.
3.1.1 Mechanism of action of Mucuna pruriens
In comparison to L-DOPA, MP is more protective of nerve cells and possesses pain-relieving, anti-inflammatory, anti-neoplastic, and anti-microbial characteristics (Logroscino 2005). It is among the most crucial aspects of MP that benefit those with PD. In addition, other critical active components, including betulinic acid and ursolic acid, also play a cardioprotective effect. Alkaloids, phenolic compounds, tannins, and flavonoids are just a few of the bioactive components that are abundant in MP (Duke 2002). MP's aqueous seed extract has also been discovered to contain a variety of phytochemicals, including proanthocyanidin, tannin, gallic acid, quercetin, and phytic acid. The existence of these active substances could halt neuroinflammation. According to various pieces of research, the neuroprotective activity of MP against Parkinson’s was examined in several PD models that are toxin-induced, including 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), Paraquat, Rotenone and 6-hydroxydopamine (6-OHDA). In a study with mouse models of PD caused by MPTP, Rai et al. have revealed the mechanism of action of MP. By preventing NF-κB from translocating into the nucleus and by suppressing the production of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNF-α) and enzymes like iNOS, scientists have demonstrated that MP prevents MPTP-induced neuroinflammation (Rai et al. 2017). Dopamine transporter (DAT) is essential for 1-methyl-4-phenylpyridinium (MPP +) absorption. DAT sustains damage during MPP + uptake, which is seen in the immunofluorescence labeling of DAT. Through inflammatory pathways, MP induces the gradual degeneration of nigrostriatal neurons after entering dopaminergic neurons. After that, MPTP intoxication decreased the expression of the anti-apoptotic protein pAkt1. By increasing the expression of DAT and pAKT1 and decreasing the production of proinflammatory cytokines by limiting the nuclear translocation of NF-κB, MP demonstrates its powerful anti-inflammatory effect (Dexter and Jenner 2013).
MP has strong anti-PD action since L-DOPA makes up much of the substance. The effectiveness of various sections of the MP has been studied in many sick states with notable results however the main concern is the pharmacokinetic effects on various organs. A sizable sample size will be required for clinical trials to verify MP's anti-PD capability. Several community-based studies are required to assess the effectiveness of various MP components in treating PD and other disorders. Additionally, the anti-PD capability of several bioactive MP components was required to show any underlying mechanisms of action. A comparison of MP's potential between in vitro and in vivo activity of different cell lines should be conducted. Whether there are any side effects associated with MP on various regions of the brain should also be explored. According to recent studies, MP provides a potent herbal medication that could be utilized to treat neurological illnesses. The detailed mechanism of action behind MP’s anti-Parkinsonian activity is described in Fig. 1.
The effect of MP, a plant used in PD neuroprotection, is shown schematically. Rotenone attaches to the DAT receptor found on dopaminergic neurons in response to external stimuli like MPP + and other toxins like 6-OHDA, activating the PI3K/AKT, Ras/MAPK, and PL-C pathways. Rotenone, MPP + , and 6-OHDA bind to DAT, ultimately activating JNK and NF-B signaling. Along with releasing Nrf2 from the Nrf2-KEAP-1 complex, these triggers also activate ARE (Rai et al. 2020)
3.2 Caffeine
One of the most extensively used psychoactive drugs is caffeine, and it is probably the world's most commonly taken pharmacologically active agent (Chawla and Suleman 2011; Nawrot et al. 2003). Tea, cocoa beverages, coffee, candy bars, and soft drinks are just a few of the foods that contain caffeine. Known also as 1,3,7-trimethylxanthine, caffeine shares structural similarities with uric acid. With an imino nitrogen at position 9 and a heterocyclic structure composed of pyrimidinedione and imidazole rings, caffeine possesses the properties of a weak acid. Three methyl groups are also present at positions 1, 3, and 7 (Schepici et al., n.d.). Few metabolic reaction pathways, such as demethylation and oxidation pathways, are used for its metabolism (Chawla and Suleman 2011). The liver is where caffeine metabolism usually occurs. Caffeine is pretty much entirely metabolized in adults from the primary metabolite paraxanthine intermediate to 1-methylxanthine and 1-methyluric acid (Nawrot et al. 2003; Guerreiro et al. 2008). Caffeine which has been shown to increase vigilance, mood, attention, and arousal (Fredholm et al. 1999), could also be neuroprotective (Ritchie et al. 2007) Fig. 2.
Three to four 8-oz cups of brewed coffee (400 mg per day) or five glasses of caffeinated soft drinks or tea tend to be a moderate quantity (110–345 mg per day) of caffeine appears to be neutral to perhaps good for health for most people (Gonzalez and Ramirez-Mares 2014). According to epidemiological research, drinking coffee may help with fat reduction (due to higher metabolic rate, energy balance, lipolysis, and thermogenic activities) and may reduce your chance of acquiring several cancers (including endometrial, liver, prostate, and colorectal) (Gonzalez and Ramirez-Mares 2014; O’Keefe et al. 2013; Cano-Marquina et al. 2013). Previous research has demonstrated that moderate caffeine consumption protects against cardiovascular disease. Products with caffeine in them are frequently sought after for their behavioral effects. Due to the benefits of low to moderate consumption levels, such as enhancements in mood, energy, alertness, and vigor, certain people may be gently encouraged to drink coffee. Caffeine use was connected to a lower risk of acquiring PD in various major prospective epidemiological studies that have already been undertaken (Ross et al. 2000; Ascherio et al. 2001; J.-F. Chen et al. 2001). Some evidence for anti-PD activity was also found in some retrospective study reports and articles (Benedetti et al. 2000; Anti Kalda et al. 2006a, b). Prospective research on caffeine intake and the risk of PD clearly demonstrated the neuroprotective effect of caffeine against the exacerbation of PD. The findings of this study suggest that moderate amounts of caffeine may have a preventive impact on PD risk (Ascherio et al. 2001).
Altman et al. established a 6-week dose-escalation open-label trial with caffeine to show dosage tolerance and examine possible motor and nonmotor advantages. Caffeine was begun at 200 mg per day and gradually escalated to 1,000 mg with 25 participants with PD in the study. Twenty of the twenty-five participants tolerated 200 mg, seventeen tolerated 400 mg, seven tolerated 800 mg, and three tolerated 1,000 mg. They found that taking 400 mg twice a day improved motor symptoms and somnolence in PD patients and also discovered early indications that caffeine may help with some motor and nonmotor elements of PD, but this has to be validated in longer-term, placebo-controlled clinical studies (Altman et al. 2011).
In another open-label 6-week randomized controlled trial of caffeine conducted by Postuma et al. comprising 61 PD patients, the motor advantage of caffeine was identified. PD patients who had daytime somnolence were given caffeine (100 mg twice daily for three weeks or 200 mg twice daily for three weeks) and a placebo-matched dose for three weeks. Of 61 patients, 31 were randomly allocated to the caffeine group and 31 to the placebo group. According to the findings of this randomized controlled experiment, caffeine provided a marginal improvement in excessive somnolence in PD patients but enhanced objective motor capabilities in PD patients. These putative motor benefits point to the need for a longer-term caffeine investigation (Postuma et al. 2012). Therefore, several clinical trials showed that caffeine reduced other PD symptoms and enhanced objective motor deficits in PD (Ren and Chen 2020).
3.2.1 Mechanism of action of caffeine
Increased oxidative stress, which specifically affects the brain of PD patients by inhibiting the endogenous antioxidant system and promoting lipid peroxidation, is one of the processes through which PD affects the neural system (Jo et al. 2019). Many medical research, as well as epidemiological evidence, has encouraged the speculation that adenosine A2A receptors (A2AR) could be a novel nondopaminergic treatment target for PD (Kalda et al. 2006a, b). The activation of A2AR in the substantia nigra and striatum of PD patients causes general neuroinflammation and PD symptoms by increasing oxidative stress and lowering dopamine levels (Dias, Junn, and Mouradian 2013). Caffeine has been found in animal models of PD to attenuate neurotoxicity (J.-F. Chen et al. 2001). Caffeine and other A2AR antagonists have also been demonstrated to improve motor function in animal models of PD by blocking A2AR and preventing excitotoxicity (Ikram et al. 2020.; Schwarzschild et al. 2002.). Caffeine has also been found to protect neurons against dopaminergic damage (Ascherio et al. 2001). Caffeine may have antioxidant properties, which may help to reduce oxidative stress, which is one of the main causes of PD (Rijk et al. 1997.; Joghataie et al. 2004).
Nrf2, also known as nuclear factor erythroid 2-related factor 2, is a transcription factor responsible for regulating a range of cellular processes (R. Li et al. 2019). Multiple studies have discovered a decrease in the expression of Nrf-2 in PD, resulting in an elevation of oxidative stress and neuroinflammation (Ahmed et al. 2017). The demise of dopaminergic neurons is initiated by an elevation in microglial cells, which are activated by oxidative stress and serve as mediators of inflammation. This leads to the manifestation of symptoms associated with PD (Ikram et al. 2020). Caffeine can protect dopaminergic neurons by stimulating anti-oxidative signaling pathways, including Nrf2-Keap1 and peroxisome proliferator-activated receptor-gamma coactivator 1/-α (PGC1α). Caffeine has the ability to activate the Nrf2-keap1 and PGC1α signaling pathways, which in turn can drive the creation of new mitochondria, maintain a balanced redox state, and enhance cell proliferation (Schepici et al. 2020). Fig. 3.
Graphical representation of the mechanism of action of caffeine in the management of neurodegeneration in patients with PD. In patients with PD, A2AR is activated and expression of Nrf-2 is decreased. This causes increased oxidative stress, dopaminergic neurodegeneration, neuroinflammation and motor dysfunction. Caffeine blocks A2AR and it also upregulates the expression of Nrf-2. These two actions alter these consequences of PD and also alleviate other symptoms of PD
3.3 Green tea
The Camellia sinesis shrub native to Asia makes green tea, prepared by steaming and drying the leaves. It is quite a popular beverage in the UK, USA, China, Japan, and other Asian countries. Several catechins found in green tea, including Epicatechin, Epigallocatechin gallate, Epigallocatechin and Epicatechin gallate, were also discovered (Sheeja Malar et al. 2020). Among these, green tea polyphenol epigallocatechin-3-gallate (EGCG) and L-theanine have strong, multidimensional therapeutic effects against PD, primarily EGCG.
Tea drinking seems to be negatively related to the incidence of dementia and PD, according to human epidemiological data and recent animal studies. Higher intake of green tea was shown to be related to a reduced prevalence of cognitive impairment in senior Japanese participants (Dubois and Pillon 1996), while in the United States, those who drank 2 cups or more of tea per day had a lower risk of PD (Brown and Marsden 1990). A recent prospective 13-year study of over 30,000 Finnish people found that consuming three or more cups of tea per day is related to a lower risk of PD (Chaudhuri and Schapira 2009). Other studies supported this finding.
3.3.1 Mechanism of action of green tea
Dopamine reuptake and signal termination in the synaptic cleft is facilitated by DAT under normal conditions. In PD patients, EGCG also reduces dopamine synaptic reuptake by inhibiting DAT (Huot et al. 2016). Secondly, L-Dopa converts into 3-O-methyl Dopa, which decreases dopamine turnover and uptake in long-term usage for PD therapy. Catecholamine-O-methyltransferase (COMT) inhibition may limit absorption, particularly this conversion. PD sufferers may benefit from this in combination with other treatments (Kang et al. 2010). Another one is Monoamine Oxidase-B (MAO-B), which converts Dopamine to 3–4-dihydroxyphenylacetic acid and homovallinic acid through enzymatic means. EGCG prevents dopamine breakdown by blocking MAO-B, as shown in the brains of elderly animal models (Tong et al. 2017; Lin et al. 2010).
Moreover, green tea polyphenols also benefit antioxidant enzymes. Green tea uses increased antioxidant enzymes catalase and SOD and decreased protein and lipid oxidation in PD patients (D. Chen et al. 2015; Siddique et al. 2014). Protein carbonyl and 4-hydroxyl-2-nonenal (4-HNE) are oxidation by-products found in high concentrations in the postmortem brains of people with PD (Di Domenico, Tramutola, and Butterfield 2017). Lipid peroxidation promotes membrane structural degradation, apoptosis, and free reactive oxygen creates more radicals, extending the chain reaction (Liu et al. 2000). HNE may modify synuclein by creating covalent adducts that produce hazardous stable oligomers (Bae et al. 2013). Research on green tea use in Portugal found a considerable decrease in MDA and 4-HNE, lipid oxidation products (Coimbra et al. 2006). In a cell-free condition, EGCG chelates Fe3+ and inhibits fibrillation and hazardous oligomer formation (Zhao et al. 2017). EGCG also inhibits the chemical reactivity of Fe2+ in the Fenton reaction by producing Ngal–EGCG–iron complex, preventing further free radical aggravation (Bao et al. 2013). Stress-induced nitrite radicals may combine with protein tyrosine residues to generate 3-NT, which is found in various neurodegenerative disorders, including PD (Bao et al. 2013). The primary ingredient of Lewy bodies, synuclein, has been observed to be nitrated in both animal models and postmortem PD brains (Duda et al. 2000). The 3-NT alteration also inhibits synuclein binding to synthetic vesicles, slowing down breakdown and promoting fibril production (Hodara et al. 2004). Green tea polyphenols indirectly enhance the breakdown rate of synuclein, implicating a potential involvement in PD prevention.
By limiting the capacity of DATs to absorb MPP + and transfer it to presynaptic dopaminergic neurons actively, EGCG decreased neurodegeneration in CHO cells expressing DAT (Bao et al. 2013). L-theanine enhanced ERK1/2 activity in SH-SY5Y cells while lowering HO-1, caspase-3, and the production of neurotrophic factors, including BDNF and GDF (Zhao et al. 2017). In the MPTP PD model, in vivo EGCG downregulated apoptosis and decreased synuclein expression. C57BL/6 mice with increased PKC-expression and Bax expression (Hodara et al. 2004). In the Long-Evans rats' (old rats) brains, EGCG decreased MAO-B activity (Huot et al. 2016). Green tea consumption lowers oxidative stress, which shields the body against illnesses linked to oxidative stress (Di Domenico, Tramutola, and Butterfield 2017). Clinical Trials in human trials, PD patients who drank three cups of green tea daily for three months and consumed 550 g of polyphenols had a substantial increase in their antioxidant status and a reduction in oxidative damage (Kang et al. 2010) Fig. 4.
3.4 Jiawei-liujunzi tang (JLT); a Chinese herbal medicine
China is rich in natural products for the treatment of PD. Herbal products like Jiawei-Liujunzi Tang (JLT) are used for PD treatment (P. Chen et al. 2022). Some studies show that China has been using herbal medicine for 2200 years (Yu et al. 2014). The loss of dopaminergic neurons and the appearance of Lewy bodies, both of which are linked to mitochondrial dysfunction, are the fundamental pathogenic features of PD; inflammation or oxidative stress are all factors to consider (M. Li et al. 2011).
However, there are presently no medications available to prevent or slow disease exacerbation. Traditional Chinese medicine (TCM) has gained popularity due to its effectiveness. The therapy of PD has a distinct theoretical foundation and clinical results. Furthermore, numerous TCM-based treatment techniques, such as Acupuncture, Tai Chi, Chinese compound formula, and moxibustion, are examples of treatments for both prevention and treatment that have become more popular in PD (Qin et al. 2021; Jiang et al. 2018).
Jiawei-Liujunzi Tang was the subject of certain randomized controlled studies conducted in China. After 24 weeks of therapy, researchers found that JLT can relieve some non-motor complications of conventional therapy and improve the communication ability in patients with PD (M. Li et al. 2011). One of the main herbs in the JLT, Uncaria rhynchophylla (Miq.) Jacks, or "Gouteng" in Chinese, is the source of corynoxine B (Cory B), an active compound that has recently been shown to effectively promote the clearance of α-synuclein (α-syn) aggresomes in vitro and in vivo by inducing autophagy, which protects neurons in PD (Lu et al. 2012).
These studies utilized herbal granules prepared according to the formulation by Zhang Lu of the Qing Dynasty (1695), which is based on the original recipe for JLT. The herbal preparation employed in this research consisted of the following 11 herbs: Dang shen (Dried root of Codonopsis pilosula), Sheng di (Dried root tuber of Rehmannia glutinosa), Fu ling (Dried sclerotium of the fungus, Poria cocos), Gouteng (Dried hook-bearing stem branch of Uncaria rhynchophylla), Bai Zhu (Rhizome of Atractylodes macrocephala), Dang gui (Dried root of Angelica sinensis), Fa ban xia (Dried tuber of Pinelliae ternate), Chuan xiong (Dried rhizome of Ligusticum chuanxiong), Huai niu xi (Dried root of Achyranthes bidentata), Chen pi (Dried pericarp of the ripe fruit of Citrus reticulata), and Sheng gan cao (Dried root and rhizome of Glycyrrhiza uralensis) (M. Li et al. 2011; Chua et al. 2017).
According to scientific evidence, most PD patients may benefit from JLT if treatment is feasible. Another double-blind, randomized, placebo-controlled clinical experiment examined the same JLT efficacy and safety in treating nonmotor symptoms in PD patients. 111 idiopathic PD patients were randomly assigned to JLT or placebo for 32 weeks. The Nonmotor Symptoms Assessment Scale total score, mood/cognition, and hallucinations improved (p = 0.019, 0.005, and 0.024). After post hoc analysis, constipation decreased significantly. Overall findings showed that, long-term JLT is well tolerated and improves NMS mood, cognition, and constipation (Chua et al. 2017).
4 Future perspectives
Natural products and plants with medicinal properties have been used in the management of various kinds of diseases for a long time. Many individuals nowadays still use herbal nutraceuticals as their primary form of medicine. More than half of the medications now being used in clinical trials are made from natural products. Many researchers have looked into the use of numerous herbs and natural substances in the treatment of PD in recent years (J. Li et al. 2019). Countless in vivo and in vitro research have shown how different natural substances or synthetic compounds can protect against neurodegenerative disorders. In the future, natural products may be a very promising option for treating PD. Some plants have been shown to be more trustworthy and effective than typical synthetic medications. One of the most significant sources of currently accessible medications to treat neurological problems is natural products (Tathagata Sengupta et al. 2016a, b). In modern times, more than 50% of medications related to PD treatment are extracted from Natural Products (Leonoudakis et al. 2017). Natural products have been used for long as a reliable source for the treatment of different neurological diseases (Corona 2018). This century may see lots of new techniques, technology, and models for using natural products in medical science.
Additionally, many of these natural compounds have anti-PD capabilities due to their well-known anti-oxidative and anti-inflammatory properties, as well as their inhibitory functions in protein misfiling and the regulatory impacts of PD-related pathways (Grkovic et al. 2014). The future will rely heavily on new drugs obtained from natural products not only for PD treatment but also for all neurodegenerative disorders. However, more sophisticated studies are required to determine their safety, optimal dosage, and actual benefits in treating various types of neurological diseases. Additionally, exploring and identifying better natural products for potential therapeutic use is a valid and important endeavor. To do this, researchers can consider several approaches, such as bioprospecting, ethnobotanical and ethnopharmacological studies, biodiversity conservation, advanced analytical techniques, computer-aided drug discovery (CADD), and open data sharing. Besides, purification/enrichment or chemical synthesis of certain components from natural products can have several potential benefits, which may enhance their effects in various applications.
5 Concluding remarks
People with neurological diseases are frequently sensitive to some severe cases in the body and sometimes cause death. This moribund condition demands an alternative therapy that has a minimum side effect and encourages researchers to develop new drugs from natural products. This review suggests natural products are alternative options for relieving and managing PD. Drug design and development authorities should consider the suggested natural products of this review article to develop new drugs.
Availability of data and materials
Not applicable.
Abbreviations
- PD:
-
Parkinson's disease
- ADHD:
-
Attention deficit hyperactivity disorder
- MP:
-
Mucuna pruriens
- NF-kB:
-
Nuclear transcription factor-Kb
- 6-OHDA:
-
6-Hydroxydopamine
- JLT:
-
Jiawei-Liujunzi Tang
- TCM:
-
Traditional Chinese medicine
- A2AR:
-
Adenosine A2A receptors
References
Adepoju GKA, Odubena OO. Effect of Mucuna Pruriens on Some Haematological and Biochemical Parameters. J Med Plants Res. 2009;3(2):73–6.
Ahmed SMU, Luo L, Namani A, Wang XJ. Nrf2 Signaling Pathway: Pivotal Roles in Inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–97.
Altman RD, Lang AE, Postuma RB. Movement disorders, and undefined 2011. Caffeine in Parkinson’s Disease: A Pilot Open-label, Dose-escalation Study. Wiley Online Library. 2011;26(13):2427–31. https://doi.org/10.1002/mds.23873.
Ascherio A, Zhang SM, Hernán MA, et al. Prospective Study of Caffeine Consumption and Risk of Parkinson’s Disease in Men and Women. Ann Neurol. 2001;50(1):56–63. https://doi.org/10.1002/ana.1052.
Atanasov AG, Zotchev SB, Dirsch VM, et al. Natural Products in Drug Discovery: Advances and Opportunities. Nat Rev Drug Discovery. 2021;20(3):200–16.
Bae EJ, Ho DH, Park E, et al. Lipid Peroxidation Product 4-Hydroxy-2-Nonenal Promotes Seeding-Capable Oligomer Formation and Cell-to-Cell Transfer of α-Synuclein. Antioxid Redox Signal. 2013;18(7):770–83. https://doi.org/10.1089/ARS.2011.4429.
Bao GH, Jie Xu, Feng Lin Hu, et al. EGCG Inhibit Chemical Reactivity of Iron through Forming an Ngal-EGCG-Iron Complex. Biometals. 2013;26(6):1041–50. https://doi.org/10.1007/S10534-013-9681-8.
Benedetti MD, Bower JH, Maraganore DM, et al. Smoking, Alcohol, and Coffee Consumption Preceding Parkinson’s Disease: A Case-Control Study. Neurology. 2000;55(9):1350–8. https://doi.org/10.1212/WNL.55.9.1350.
Biswas P, Dey D, Biswas PK, et al. A Comprehensive Analysis and Anti-Cancer Activities of Quercetin in ROS-Mediated Cancer and Cancer Stem Cells. Int J Mol Sci. 2022;23(19):11746.
Brown RG, Marsden CD. Cognitive Function in Parkinson’s Disease: From Description to Theory. Trends Neurosci. 1990. https://doi.org/10.1016/0166-2236(90)90058-I.
Butler MS. Natural Products to Drugs: Natural Product-Derived Compounds in Clinical Trials. Nat Prod Rep. 2008;25(3):475–516. https://doi.org/10.1039/b514294f.
Cano-Marquina A, Tarín JJ, Cano A. The Impact of Coffee on Health. Maturitas. 2013;75(1):7–21. https://doi.org/10.1016/j.maturitas.2013.02.002.
Chaudhuri KR, Schapira AHV. Non-Motor Symptoms of Parkinson’s Disease: Dopaminergic Pathophysiology and Treatment. The Lancet Neurology. 2009. https://doi.org/10.1016/S1474-4422(09)70068-7.
Chawla J, Suleman A. Neurologic effects of caffeine. Medscape Reference. Retrieved on June 2018. http://emedicine.medscape.com/article/1182710-overview.
Chen D, Zhou Y, Lyons KE, et al. Green Tea Consumption Reduces Oxidative Stress in Parkinson’s Disease Patients. J Behav Brain Sci. 2015;05(06):194–202. https://doi.org/10.4236/JBBS.2015.56020.
Chen JF, Xu K, Petzer JP, Staal R, Xu YH, Beilstein M, Sonsalla PK, Castagnoli K, Castagnoli N Jr, Schwarzschild MA. Neuroprotection by caffeine and A(2A) adenosine receptor inactivation in a model of Parkinson's disease. J Neurosci. 2001;21(10):RC143. https://doi.org/10.1523/JNEUROSCI.21-10-j0001.2001.
Chen P, Zhang J, Wang C, et al. The Pathogenesis and Treatment Mechanism of Parkinson’s Disease from the Perspective of Traditional Chinese Medicine. Phytomedicine. 2022;100:154044.
Chua KK, Wong A, et al. A Randomized Controlled Trial of Chinese Medicine on Nonmotor Symptoms in Parkinson’s Disease. Parkinson’s Disease. 2017. https://doi.org/10.1155/2017/1902708.
Coimbra S, Castro E, Rocha-Pereira P, et al. The Effect of Green Tea in Oxidative Stress. Clin Nutr. 2006;25(5):790–6. https://doi.org/10.1016/J.CLNU.2006.01.022.
Corona JC. Natural Compounds for the Management of Parkinson’s Disease and Attention-Deficit/Hyperactivity Disorder. Biomed Res Int. 2018. https://doi.org/10.1155/2018/4067597.
DeMaagd G, Philip A. Parkinson’s Disease and Its Management: Part 1: Disease Entity, Risk Factors, Pathophysiology, Clinical Presentation, and Diagnosis. P T. 2015;40(8):504–32.
Dexter DT, Jenner P. Parkinson Disease: From Pathology to Molecular Disease Mechanisms. Free Radic Biol Med. 62:132–44. https://doi.org/10.1016/j.freeradbiomed.2013.01.018.
Di Domenico F, Tramutola A, Allan D, Butterfield. Role of 4-Hydroxy-2-Nonenal (HNE) in the Pathogenesis of Alzheimer Disease and Other Selected Age-Related Neurodegenerative Disorders. Free Radical Biol Med. 2017;111(October):253–61. https://doi.org/10.1016/j.freeradbiomed.2016.10.490.
Dias V, Junn E, MaralMouradian. M. The Role of Oxidative Stress in Parkinson’s Disease. J Parkinson’s Dis. 2013;3(4):461–91. https://doi.org/10.3233/JPD-130230.
Dorsey ER, Elbaz A, Nichols E, et al. Global, Regional, and National Burden of Parkinson’s Disease, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–53.
Dubois B, Pillon B. Cognitive Deficits in Parkinson’s Disease. J Neurol. 1996;244(1):2–8. https://doi.org/10.1007/PL00007725.
Duda JE, Giasson BI, Chen Q, et al. Widespread Nitration of Pathological Inclusions in Neurodegenerative Synucleinopathies. Am J Pathol. 2000;157(5):1439–45. https://doi.org/10.1016/S0002-9440(10)64781-5.
Duke JA. Handbook of Medicinal Herbs. CRC Press; 2002.
Fredholm BB, Bättig K, Holmén J, et al. Actions of Caffeine in the Brain with Special Reference to Factors That Contribute to Its Widespread Use. Pharmacol Rev. 1999;51(1):83–133.
Gelb DJ, Oliver E, Gilman S. Diagnostic Criteria for Parkinson Disease. Arch Neurol. 1999;56(1):33–9. https://doi.org/10.1001/archneur.56.1.33.
Gonzalez, de Mejia E, and Marco V Ramirez-Mares. Impact of Caffeine and Coffee on Our Health. Trends Endocrinol Metab. 2014;25(10):89–492.
Grkovic T, Pouwer RH, Vial M-L, et al. NMR Fingerprints of the Drug-like Natural-Product Space Identify Iotrochotazine A: A Chemical Probe to Study Parkinson’s Disease. Wiley Online Library. 2014;53(24):24. https://doi.org/10.1002/anie.201402239.
Guerreiro S, Toulorge D, Hirsch E, et al. Paraxanthine, the Primary Metabolite of Caffeine, Provides Protection against Dopaminergic Cell Death via Stimulation of Ryanodine Receptor Channels. Mol Pharmacol. 2008;74(4):980–9. https://doi.org/10.1124/mol.108.048207.
Gyawali R, Ibrahim SA. Natural Products as Antimicrobial Agents. Food Control. 2014. https://doi.org/10.1016/j.foodcont.2014.05.047.
Hald A, Lotharius J. Oxidative Stress and Inflammation in Parkinson’s Disease: Is There a Causal Link? Exp Neurol. 2005;193(2):279–90.
Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s Disease: A Target for Neuroprotection? Lancet Neurol. 2009;8(4):382–97.
Hodara R, Norris EH, Giasson BI, et al. Functional Consequences of α-Synuclein Tyrosine Nitration: Diminished Binding to Lipid Vesicles and Increased Fibril Formation. J Biol Chem. 2004;279(46):47746–53. https://doi.org/10.1074/JBC.M408906200.
Huot P, Fox SH, Brotchie JM. Dopamine Reuptake Inhibitors in Parkinson’s Disease: A Review of Nonhuman Primate Studies and Clinical Trials. J Pharmacol Exp Ther. 2016. https://doi.org/10.1124/jpet.116.232371.
Iarkov A, Barreto GE, Alex Grizzell J, et al. Strategies for the Treatment of Parkinson’s Disease: Beyond Dopamine. Front Aging Neurosci. 2020. https://doi.org/10.3389/fnagi.2020.00004.
Ikram M, Park TJ, et al. Antioxidant and Neuroprotective Effects of Caffeine against Alzheimer’s and Parkinson’s Disease: Insight into the Role of Nrf-2 and A2AR Signaling. Mdpi.Com. 2020:902. https://doi.org/10.3390/antiox9090902.
Jami MABS, Biswas K. A Cross-Sectional Study Regarding the Knowledge, Attitude and Awareness about Self-Medication among Bangladeshi People. Health Policy and Technology. 2023. https://doi.org/10.1016/j.hlpt.2022.100715.
Jankovic J, Tan EK. Parkinson’s Disease: Etiopathogenesis and Treatment. J Neurol Neurosurg Psychiatry. 2020;91(8):795–808. https://doi.org/10.1136/jnnp-2019-322338.
Jiang X, Zhou J, Lin Q, et al. Anti-Angiogenic and Anticancer Effects of Baicalein Derivatives Based on Transgenic Zebrafish Model. Bioorg Med Chem. 2018;26(15):4481–92.
Jo MG, Ikram M, Jo MH, et al. Gintonin Mitigates MPTP-Induced Loss of Nigrostriatal Dopaminergic Neurons and Accumulation of $α$-Synuclein via the Nrf2/HO-1 Pathway. Mol Neurobiol. 2019;56(1):39–55. https://doi.org/10.1007/s12035-018-1020-1.
Joghataie MT, Roghani M, Negahdar F, Hashemi L. Protective effect of caffeine against neurodegeneration in a model of Parkinson's disease in rat: behavioral and histochemical evidence. Parkinsonism Relat Disord. 2004;10(8):465–8. https://doi.org/10.1016/j.parkreldis.2004.06.004.
Joglar B, Rodriguez-Pallares J, Rodriguez-Perez AI, et al. The Inflammatory Response in the MPTP Model of Parkinson’s Disease Is Mediated by Brain Angiotensin: Relevance to Progression of the Disease. J Neurochem. 2009;109(2):656–69.
Kalda A, Yu L, Oztas E, Chen JF. Novel neuroprotection by caffeine and adenosine A(2A) receptor antagonists in animal models of Parkinson's disease. J Neurol Sci. 2006b;248(1-2):9–15. https://doi.org/10.1016/j.jns.2006.05.003.
Kalda, A, L Yu, E Oztas, JF Chen - Journal of the neurological sciences, and undefined 2006. “Novel Neuroprotection by Caffeine and Adenosine A2A Receptor Antagonists in Animal Models of Parkinson’s Disease.” Elsevier.
Kang KS, Wen Y, Yamabe N, et al. Dual Beneficial Effects of (-)-Epigallocatechin-3-Gallate on Levodopa Methylation and Hippocampal Neurodegeneration. In Vitro and in Vivo Studies. PLoS ONE. 2010;5(8):e11951.https://doi.org/10.1371/journal.pone.0011951.
Khan MM, Kempuraj D, Thangavel R, et al. Protection of MPTP-Induced Neuroinflammation and Neurodegeneration by Pycnogenol. Neurochem Int. 2013;62(4):379–88.
Leonoudakis D, Rane A, et al. Anti-Inflammatory and Neuroprotective Role of Natural Product Securinine in Activated Glial Cells: Implications for Parkinson’s Disease. Mediators Inflamm. 2017. https://doi.org/10.1155/2017/8302636.
Li J, Long X, Hu J, et al. Multiple Pathways for Natural Product Treatment of Parkinson’s Disease: A Mini Review. Phytomedicine. 2019;60:152954. https://doi.org/10.1016/J.PHYMED.2019.152954.
Li M, Kum WF, et al. Treatment of Idiopathic Parkinson’s Disease with Traditional Chinese Herbal Medicine: A Randomized Placebo-Controlled Pilot Clinical Study. Evidence-Based Complementary and Alternative Medicine. 2011. https://doi.org/10.1093/ecam/nep116.
Li R, Jia Z, Zhu H. Regulation of Nrf2 Signaling. React Oxyg Species (Apex). 2019;8(24):312–22. PMID: 31692987.
Lin SM, Wang SW, Ho SC, et al. Protective Effect of Green Tea (-)-Epigallocatechin-3-Gallate against the Monoamine Oxidase B Enzyme Activity Increase in Adult Rat Brains. Nutrition. 2010;26(11–12):1195–200. https://doi.org/10.1016/j.nut.2009.11.022.
Lindqvist D, Kaufman E, Brundin L, Hall S, Surova Y, Hansson O. Non-motor symptoms in patients with Parkinson's disease - correlations with inflammatory cytokines in serum. PLoS One. 2012;7(10):e47387. https://doi.org/10.1371/journal.pone.0047387.
Liu, W, M Kato, AA Akhand, … A Hayakawa - Journal of cell, and undefined 2000. 2000. “4-Hydroxynonenal Induces a Cellular Redox Status-Related Activation of the Caspase Cascade for Apoptotic Cell Death.” Journals.Biologists.Com. https://doi.org/10.1242/jcs.113.4.635.
Logroscino G. The Role of Early Life Environmental Risk Factors in Parkinson Disease: What Is the Evidence? Environ Health Perspect. 2005;113(9):1234–8. https://doi.org/10.1289/ehp.7573.
Lu J-H, Tan J-Q, Durairajan SSK, et al. Isorhynchophylline, a Natural Alkaloid, Promotes the Degradation of Alpha-Synuclein in Neuronal Cells via Inducing Autophagy. Autophagy. 2012;8(1):98–108. https://doi.org/10.4161/auto.8.1.18313.
Malar DS, Prasanth MI, Brimson JM, Sharika R, Sivamaruthi BS, Chaiyasut C, Tencomnao T. “Neuroprotective Properties of Green Tea (Camellia Sinensis) in Parkinson’s Disease: A Review.” Molecules. 2020;25(17):3926. https://doi.org/10.3390/molecules25173926. Mdpi.Com.
Mitra S, Dash R, Sohel M, et al. Targeting Estrogen Signaling in the Radiation-induced Neurodegeneration: A Possible Role of Phytoestrogens. Curr Neuropharmacol. 2023;21(2):353–79. https://doi.org/10.2174/1570159X20666220310115004.
Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. https://doi.org/10.1371/journal.pmed.1000097.
Nawrot P, Jordan S, Eastwood J, et al. Effects of Caffeine on Human Health. Food Addit Contam. 2003;20(1):1–30. https://doi.org/10.1080/0265203021000007840.
O’Keefe JH, Bhatti SK, Patil HR, et al. Effects of Habitual Coffee Consumption on Cardiometabolic Disease, Cardiovascular Health, and All-Cause Mortality. J Am Coll Cardiol. 2013;62(12):1043–51. https://doi.org/10.1016/j.jacc.2013.06.035.
Paul P, Biswas P, Dey D, et al. Exhaustive Plant Profile of ‘Dimocarpus Longan Lour’ with Significant Phytomedicinal Properties: A Literature Based-Review. Processes. 2021. https://doi.org/10.3390/pr9101803.
Postuma RB, Lang AE, Munhoz RP, Charland K, Pelletier A, Moscovich M, Filla L, Zanatta D, Rios Romenets S, Altman R, Chuang R, Shah B. Caffeine for treatment of Parkinson disease: a randomized controlled trial. Neurology. 2012;79(7):651–8. https://doi.org/10.1212/WNL.0b013e318263570d.
Qin N, Lu X, Liu Y, et al. Recent Research Progress of Uncaria Spp. Based on Alkaloids: Phytochemistry, Pharmacology and Structural Chemistry. Eur J Med Chem. 2021;210:112960.
Rabiei Z, Solati K, Amini-Khoei H. Phytotherapy in Treatment of Parkinson’s Disease: A Review. Pharm Biol. 2019;57(1):355–62.
Rai SN, Birla H, Zahra W, et al. Immunomodulation of Parkinson’s Disease Using Mucuna Pruriens (Mp). J Chem Neuroanat. 2017;85:27–35.
Rai SN, Chaturvedi VK, Singh P, et al. Mucuna Pruriens in Parkinson’s and in Some Other Diseases: Recent Advancement and Future Prospective. 3 Biotech. 2020;10(12):1–11. https://doi.org/10.1007/s13205-020-02532-7.
Ren, Xiangpeng, and Jiang Fan Chen. 2020. “Caffeine and Parkinson’s Disease: Multiple Benefits and Emerging Mechanisms.” Frontiers in Neuroscience 14 (December). https://doi.org/10.3389/FNINS.2020.602697/FULL.
de Rijk MC, Breteler MMB, den Breeijen JH. Dietary Antioxidants and Parkinson Disease: The Rotterdam Study. Arch Neurol. 1997;54(6):762–5. Jamanetwork.Com.
Ritchie K, Carrière I, De Mendonca A, et al. The Neuroprotective Effects of Caffeine: A Prospective Population Study (the Three City Study). Neurology. 2007;69(6):536–45.
Roni, MAH, Jami MABS, et al. Traditional Herbal Interventions for Premenstrual Syndrome Management: A Comprehensive Literature Review. Int J Chem Biol Sci (IJCBS). 25(18):120–40. https://www.iscientific.org/wp-content/uploads/2024/03/10-IJBCS-24-25-18-010.pdf.
Ross GW, Abbott RD, Petrovitch H, et al. Association of Coffee and Caffeine Intake with the Risk of Parkinson Disease. J Am Med Assoc. 2000;283(20):2674–9. https://doi.org/10.1001/jama.283.20.2674.
Schepici G, Silvestro S, Bramanti P. Caffeine: An Overview of Its Beneficial Effects in Experimental Models and Clinical Trials of Parkinson’s Disease. Int J Mol Sci. 2020;21(13):4766. https://doi.org/10.3390/ijms21134766. Mdpi.Com.
Schwarzschild MA, Chen JF, Ascherio A. Caffeinated Clues and the Promise of Adenosine A2A Antagonists in PD. Neurology. 2002;58(8):1154–60.
Sengupta T, Vinayagam J, Singh R, et al. Plant-Derived Natural Products for Parkinson’s Disease Therapy. Springer. 2016b;12:415–96. https://doi.org/10.1007/978-3-319-28383-8_23.
Sengupta T, Vinayagam J, Singh R, et al. Plant-Derived Natural Products for Parkinson’s Disease Therapy. Adv Neurobiol. 2016;12:415–96.
Siddique YH, Jyoti S, Naz F. Effect of Epicatechin Gallate Dietary Supplementation on Transgenic Drosophila Model of Parkinson’s Disease. J Diet Suppl. 2014;11(2):121–30. https://doi.org/10.3109/19390211.2013.859207.
Sohel Md, Partha Biswas Md, Al Amin Md, et al. Genistein, a Potential Phytochemical against Breast Cancer Treatment-Insight into the Molecular Mechanisms. Processes. 2022a. https://doi.org/10.3390/pr10020415.
Sohel Md, Islam MN, Hossain MA, et al. Pharmacological Properties to Pharmacological Insight of Sesamin in Breast Cancer Treatment: A Literature-Based Review Study. Int J Breast Cancer. 2022b. https://doi.org/10.1155/2022/2599689.
Sohel Md, Sultana Habiba, Tayeba Sultana Md, et al. Chemotherapeutic Potential of Hesperetin for Cancer Treatment, withMechanistic Insights: A Comprehensive Review. Heliyon. 2022;8(1):e08815.
Tong J, Rathitharan G, Meyer JH, et al. Brain Monoamine Oxidase B and A in Human Parkinsonian Dopamine Deficiency Disorders. Brain. 2017;140(9):2460–74. https://doi.org/10.1093/BRAIN/AWX172.
Voon V, Mehta AR, Hallett M. Impulse Control Disorders in Parkinson’s Disease: Recent Advances. Curr Opin Neurol. 2011;24(4):324.
Waltenberger B, Mocan A, Šmejkal K, et al. Natural Products to Counteract the Epidemic of Cardiovascular and Metabolic Disorders. Molecules. 2016;21(6):807. https://doi.org/10.3390/molecules21060807.
Yu C-H, Liu P-H, Van Y-H, et al. Traditional Chinese Medicine for Idiopathic Precocious Puberty: A Hospital-Based Retrospective Observational Study. Complement Ther Med. 2014;22(2):258–65.
Zhao J, Xu L, Liang Q, et al. Metal Chelator EGCG Attenuates Fe (III)-induced Conformational Transition of Α-synuclein and Protects AS-PC 12 Cells against Fe (III)-induced Death. J Neurochem. 2017;143(1):136–46. https://doi.org/10.1111/jnc.14142.
Zhou C, Huang Y, Przedborski S. Oxidative Stress in Parkinson’s Disease: A Mechanism of Pathogenic and Therapeutic Significance. Ann N Y Acad Sci. 2008;1147(December):93–104. https://doi.org/10.1196/annals.1427.023.
Acknowledgements
Not applicable.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MAHR, MABSJ, SH, MAH, MS and FA equally participated in writing and draft preparation; MABSJ, MS, MA, KHB, R and FJ equally participated in searching relevant articles or databases; MSH, NH, MSM and MYBMY contributed to figure generation; MNA was in conceptualization, study design and final editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Roni, M.A.H., Jami, M.A.B.S., Hoque, S. et al. Clinically proven natural products in aid of treating Parkinson's disease: a comprehensive review. Curr Med 3, 6 (2024). https://doi.org/10.1007/s44194-024-00033-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s44194-024-00033-w