Abstract
Background
Acne vulgaris is a very dangerous skin disease leads to psychological disorders. Benzoyl peroxide and tretinoin in combination with topical dosage are used to improve the appearance of acne-prone skin. The study aimed to develop and validate an HPLC–PDA method for the simultaneous estimation of both drugs. Intentional modifications were implemented in the analytical method to get better optimum conditions. The final method was chosen for the reverse phase chromatographic separation by using the C18 column as a stationary phase and 0.01 M phosphate buffer adjusted pH 2.5 mixed with acetonitrile (25:75 v/v) as a mobile phase. The optimized conditions were 1.5 mL/min flow rate, 30 °C column temperature, 5 °C autosampler temperature, and 20 µL injection volume. The wavelength was chosen for detection of Benzoyl peroxide at 272 nm and Tretinoin at 353 nm by utilizing a PDA detector. All standard and sample solutions were made in methanol.
Results
The developed method exhibited peak retention times of 2.94 min for Benzoyl peroxide and 11.34 min for Tretinoin. This analytical method was proven to be robust, linear in calibration curve, accurate, precise, and specific. The forced degradation studies results showed that a high degree of specificity was obtained by separating both analytes from produced impurities completely.
Conclusions
The developed analytical method is fast, economic and stability indicating. It is useful for routine pharmaceutical analysis where the combination of benzoyl peroxide and tretinoin is formulated for their quality and safety.
Similar content being viewed by others
Background
Acne vulgaris, papules, and pustules are skin diseases with adverse effects including scarring and hyperpigmentation leading to severe psychological distress [1,2,3]. Acne can be as non-inflammatory lesions, burns, or a combination of both, and most commonly affects the face, yet additionally the back and chest [4]. Tretinoin ((2E,4E,6E,8E)-3,7-dimethyl-9-(2,6,6-trimethylcyclohexen-1-yl) nona-2,4,6,8-tetraenoic acid) a type of carboxylated retinoic acid that is an adjunct of vitamin A. In addition, it is particularly sensitive to light, intensity, and oxygen in indoor air [5, 6]. Benzoyl peroxide (benzoyl benzenecarboperoxoate) is an organic peroxide in which two benzoyl groups are bridged through a peroxide interface. It quickly decomposes into benzoic acid and hydrogen peroxide and forms free radicals that oxidize the proteins of the bacterial cell layer, exhibiting antiseptic activity [7, 8]. Both drugs in combination are used for the treatment of certain skin diseases such as acne vulgaris, psoriasis, and photoaging [4]. The chemical structure of both drugs is shown in Fig. 1.
In the United States pharmacopeia [9], the titration methods of both drugs are available while in semisolid dosage forms, HPLC methods are used for alone drug determination and no combination method is available. By the survey of the literature, a few methods of Benzoyl peroxide [10,11,12] and Tretinoin [13,14,15,16,17] were reported. One UV spectrophotometric method was reported for the simultaneous estimation of Tretinoin and Benzoyl peroxide in bulk and semisolid dosage form [18]. Although, the UV spectrophotometric method is useful for routine analysis because is fast and economic but this method is not stability indicating that the product is sensitive to temperature, and light and produces impurities. Because this method does not access the generation of impurities in the drug product. Another RP-HPLC analytical method was reported for the simultaneous determination of three components (spironolactone, benzoyl peroxide, and Tretinoin) [19]. This method also not discuss about stability indicating studies. So, according to our knowledge, there is a need for stability indicating the HPLC–PDA method for simultaneous estimation of Tretinoin and Benzoyl peroxide. Hence, our aim of the study was to develop and validate the stability indicating HPLC–PDA method for simultaneous estimation of both drugs in a topical dosage form for their quality and safety as per ICH guidelines [20].
Methods
Materials and chemicals
Benzoyl peroxide (BPO) was obtained from Cambrex Karlskoga, Sweden while Tretinoin (TRN) was obtained from Chongqing huapont shengchem pharmaceuticals, China. The Twyneo (Benzoyl peroxide 3% and Tretinoin 0.1%) cream was purchased online. All the commercial grade excipients used for the method validation study were kindly gifted by Horizon Healthcare, Pakistan. We used Honeywell-made HPLC-grade acetonitrile, methanol, and water. Potassium dihydrogen phosphate, orthophosphoric acid, and all other chemicals were used of analytical grade, manufactured by Sigma Aldrich. The mobile phase, standards, and samples were filtered through 0.22 µm polyamide membrane filters.
Chromatographic system
Shimadzu HPLC quaternary gradient LC-10AD equipped with coolant autosampler, column oven, and photodiode array multi-channel detector was used for the whole study. Chromatographic separation was accomplished by the reverse phase isocratic method using SHIMPAC® octadecyl silane C18 column, 4.6 mm internal diameter, 15 cm length, and 5 µm particle size. The final mobile phase contains a 25% volume of 0.01 M solution of potassium phosphate buffer in distilled water adjusted pH 2.5 with orthophosphoric acid and 75% volume of acetonitrile. The flow rate of the mobile phase was 1.5 mL/min, injection volume 20 µL, autosampler coolant temperature 4 °C, and the column temperature was set at 30 °C.
Preparation of standard solution
The stock standard solution of TRN drug substance was prepared by transferring 50.0 mg in a 100 mL amber-colored or low actinic volumetric flask, dissolved in methanol by using ultra sonicator, and dilute to volume. The final standard solution was prepared by transferring 30.0 mg of BPO in another 100 mL amber-colored volumetric flask, dissolved in 40 mL methanol, adding 2 mL of TRN stock solution, and mix well then diluted to volume with methanol. Hence the target concentrations were for BPO (0.3 mg/mL) and TRN (0.01 mg/mL).
Preparation of sample solution
Mix the contents of three creams, and transfer 1.0 g accurately weighed quantity of cream (nominally equivalent to 30 mg of BPO and 1 mg of TRN) into 100 mL amber-colored volumetric flask, dissolve the active ingredients by using ultra sonicator and dilute to volume. Finally, filter this solution through 0.22 µm polyamide membrane filter before injection.
The stability of the solutions was evaluated for 24 h at intervals of 0, 6, 12, and 24 h while the standard and sample solutions were both maintained in the refrigerator at 2 °C to 8 °C, protected from light.
Preparation of placebo solution
The placebo solution was prepared by transferring the excipients (anhydrous citric acid; 2.5 mg, butylated hydroxytoluene; 1 mg, carbomer homopolymer type C; 1 mg, cetrimonium chloride; 0.5 mg, cetyl alcohol; 70 mg, cyclomethicone; 10 mg, edetate disodium; 1 mg, glycerin; 20 mg, imidurea; 1 mg, (S)-lactic acid; 1 mg, macrogol stearate; 25 mg, mono and di-glycerides; 10 mg, polyquaternium-7; 10 mg, silicon dioxide; 1 mg, squalane; 20 mg, tetraethyl orthosilicate; 1.5 mg, white wax; 20 mg, and purified water; 765 mg) into 100 mL beaker. Add methanol, stir by magnetic stirrer, and heat at 50 °C for 15 min then transferred to 100 mL volumetric flask make up the volume to mark with methanol. This solution was sonicated for 20 min with intermittent shaking then filtered through 0.22 µm polyamide membrane filter before injection.
Preparation of solutions for stress studies
Stress study or Forced degradation involved the submission of samples under various environmental conditions like acid, alkali, oxidative, photolytic, and thermal with humidity to verify what are the degradation products. The control standard solution was prepared as described above. 5 mL of 1 N solution of hydrochloric acid for acidic stress, 5 mL of 1 N solution of sodium hydroxide for alkali stress, and 5 mL of 3% v/v solution of hydrogen peroxide for oxidative stress was separately added in separate samples and separate placebo solutions, then diluted with methanol to volume. All samples were kept at room temperature for 24 h. Photolytic stress was applied by keeping the sample in Laminar flow hood under UV light for 24 h. Thermal and humidity stress was applied at two different conditions for 24 h, one is at room temperature and other is 40 °C temperature with 75% relative humidity in climatic chamber.
Results
Development of analytical method and system suitability
Various deliberate changes were done to get a better-optimized condition of the chromatographic system. Firstly, we used methanol as an organic modifier but the asymmetry and theoretical plates of the BPO peak were obtained at 1.43 and 1790 which was poor, also the retention time of the TRN peak was obtained at 23.5 min, which was far. Then we consider the acetonitrile with a ratio of 50% which provided the retention time of TRN 23.69 min and BPO 6.91 min with less theoretical plates value, finally, we selected 75%. As per above said chromatographic system parameters, we injected the six replicates of the standard solution and got the system suitability as shown in Table 1.
The detection wavelength was chosen for BPO 272 nm and TRN 353 nm by scanning from 200 to 500 nm as shown in Fig. 2.
Stability of solutions
Solutions stability studies were assessed by keeping the standard and sample solution at 2 – 8 °C for 24 h and analyzed by comparing it with a freshly prepared standard solution at time intervals of 0, 6, 12, and 24 h. The results are provided in Table 2.
Method validation
Specificity
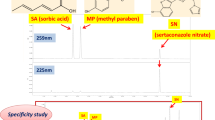
The specificity was done by injecting standard, sample, blank, and placebo solutions. The average peak purity index for the TRN sample was observed at 1.0000 and for BPO 0.99994 indicated no interference of any impurity. Also, there was no interference of diluent and placebo was observed in the retention time of both drugs. The chromatograms of specificity are shown in Fig. 3. The forced degradation studies revealed no interference of generated impurities in the Rt of main analytes peaks as shown in Fig. 3.
Linearity with limit of quantification and detection
The Linearity of analytical method was analyzed by preparing standard solutions in the range of 70–130% for both drugs. The concentration range for BPO (0.21 – 0.39 mg/mL) and for TRN (0.007–0.013 mg/mL) was produced. The quantification and detection limits were calculated by using the standard deviation of intercept and slope of calibration curve from regression data. The calibration curves for both drugs are shown in Fig. 4. The regression data are provided in Table 3.
Accuracy
Accuracy was assessed by spiking both drug substances in placebo solution approximately at 80%, 100%, and 120% spiked levels. Three replicates of each solution were injected as per above said method and percent recovery with percent relative standard deviation between the spiked levels is calculated as shown in Table 4.
Precision
The system precision was performed by injecting six replicates of standard solution as described in the system suitability results. In method precision, repeatability was analyzed by preparing six sample solutions on the same day while the reproducibility was assessed by preparing six sample solutions by two analysts on three consecutive days and three replicates of each preparation were injected. The average percent recovery and relative standard deviation is calculated as provided in Table 5.
Robustness/rigidness
Robustness was performed by deliberating the few variations in instrumental and method parameters such as variation in column, wavelength, column temperature, auto sampler temperature and organic modifier in mobile phase. The robustness results are provided in Table 6.
Forced degradation studies
Forced degradation studies were employed by applying various environmental conditions like acid, alkali, oxidative, photolytic, and thermal with humidity on laboratory-prepared samples as well as commercial samples. The chromatograms of stress studies are provided in Fig. 3 while the results are shown in Table 7.
Discussion
The review of the literature indicated that there was no stability-indicating liquid chromatographic method available in a combination of benzoyl peroxide and tretinoin [10,11,12,13,14,15,16,17,18,19]. So, according to our aim, we developed and validated the stability-indicating liquid chromatographic-PDA method for simultaneous determination of both drugs. The summary of results indicated that the method was developed by optimizing the various suitability parameters and finally selected parameters provided the method and system suitability results as shown in Table 1. The solution stability results are provided in Table 2 showed the solutions were stable at 2–8 °C for 24 h. The method validation data indicated the specificity results in Fig. 3 provided, that there was no interference of excipients, diluent and impurities observed at the retention time of both analytes. Regression data in Table 3 provided the linearity of the method having a correlation coefficient for both analytes 0.9997, measurable quantification limits, and detection limits. Accuracy results in Table 4 revealed the obtained recovery reflects the spiked level and average recovery BPO 101.52%, TRN 99.88%. The precision data in Table 5 showed the percent relative standard deviation for repeatability of BPO 0.70% and TRN 0.94% while for reproducibility of BPO 0.52% and TRN 0.76%, indicating the method was repeatable and reproducible. Various deliberate changes were done in the robustness study, and the provided results in Table 6 indicated the using of octylsilyl-silane C8 column cause increasing the retention time of BPO while decreasing the TRN. It also given the less theoretical plates value for both analytes and peak purity index for BPO. The variation in wavelength, column temperature having no significant effect except peak areas. Variation in auto sampler temperature revealed the decreasing of recovery. The change in organic modifier cause increasing the tailing factor and decreasing the theoretical plates value for both analytes. The stress studies showed that BPO was observed unstable against acidic environment and produce impurity at Rt 1.815 min as shown in Fig. 3C while TRN also influenced and generate the impurities as shown in Fig. 3C. In alkali environment the BPO also generate the impurity at Rt 1.821 min but as compared to acidic environment it was less while TRN generate three impurities at Rt 4.74, 5.23 and 10.01 min indicated it is sensitive to alkali conditions as shown in Fig. 3D. In oxidative environment, BPO slightly degraded while TRN produce impurities at Rt 4.78, 5.21 and 10.04 as shown in Fig. 3E. In photolytic conditions TRN reduced and shows impurities as shown in Fig. 3F. In thermal and humidity environment there were little bit degradation of both analytes observed. The summery of degradation studies shows that BPO is unstable against acidic while TRN is unstable against alkali and oxidative conditions. The BPO is stable at photolytic while TRN is stable in an acidic medium, but both are stable in a mild thermal humidity medium. But the all generated impurities were separated and shows no interaction in the Rt of main analytes provided the selectivity and stability—indicating activity of method.
Conclusion
The developed and validated analytical stability-indicating liquid chromatographic method for simultaneous determination of benzoyl peroxide and tretinoin is specific, accurate, precise, linear, and robust observed. It is the simple and fast isocratic method that can be used in routine pharmaceutical analysis where the combination of benzoyl peroxide and tretinoin formulated.
Availability of data and materials
All data and materials are available upon request.
Abbreviations
- HPLC:
-
High performance liquid chromatography
- PDA:
-
Photodiode array
- RP:
-
Reverse phase
- UV:
-
Ultraviolet
- ICH:
-
International conference on harmonization
- BPO:
-
Benzoyl peroxide
- TRN:
-
Tretinoin
- R t :
-
Retention time
- T f :
-
Tailing factor
- N:
-
Number of theoretical plates
- R:
-
Resolution
- RSD:
-
Relative standard deviation
- R 2 :
-
Correlation coefficient
- m:
-
Slope
- C:
-
Intercept
- SDI:
-
Standard deviation of intercept
- RH:
-
Relative humidity
- RT:
-
Room temperature
References
Ghodsi SZ, Orawa H, Zouboulis CC (2009) Prevalence, severity, and severity risk factors of acne in high school pupils: a community-based study. J Invest Derm 129:2136–2141. https://doi.org/10.1038/jid.2009.47
Leyden JJ (1995) New understandings of the pathogenesis of acne. J Am Acad Derm 32:S15-25. https://doi.org/10.1016/0190-9622(95)90416-6
Magin P, Adams J, Heading G, Pond D, Smith W (2006) Psychological sequelae of acne vulgaris: results of a qualitative study. Can Fam Phy 52:978–979
Leyden JJ (2003) A review of the use of combination therapies for the treatment of acne vulgaris. J Am Acad Dermatol 49:S200-210. https://doi.org/10.1067/s0190-9622(03)01154-x
Rosso JD, Harper J, Pillai R, Moore R (2013) Tretinoin photostability: comparison of micronized tretinoin gel 0.05% and tretinoin gel 0.025% following exposure to fluorescent and solar light. J Clin Aesthet Dermatol 6:25–28
National Center for Biotechnology Information. "PubChem Compound Summary for CID 444795, Tretinoin" PubChem
Swauger JE, Dolan PM, Zweier JL, Kuppusamy P, Kensler TW (1991) Role of the benzoyloxyl radical in DNA damage mediated by benzoyl peroxide. Chem Res Toxicol 4:223–228. https://doi.org/10.1021/tx00020a016
Matin T, Goodman MB (2021) Benzoyl Peroxide. In: StatPearls. Treasure Island (FL): StatPearls Publishing. http://www.ncbi.nlm.nih.gov/books/nbk537220/
United States Pharmacopeial Convention (2007) The United States Pharmacopeia 30; The National Formulary 25, 30th edn. United States Pharmacopeial Convention, Rockville
Roy C, Panigrahi L, Chakrabarty J (2015) Validated stability-indicating RP-HPLC method for the estimation of degradation behaviour of organic peroxide and third-generation synthetic retinoids in topical pharmaceutical dosage formulation. Sci Pharm 83:321–338. https://doi.org/10.3797/scipharm.1412-10
Wankhade R, Bhalerao S, Panchory HI, Pundir AR, Pradhan R (2012) Analysis of erythromycin and benzoyl peroxide in combined dosage form by UV-visible spectrophotometry. Int J Pharm Sci 4:527–531
RB D, Patel BH (2012) Development and validation of HPLC method for simultaneous estimation of clindamycin phosphate, clotrimazole and tinidazole in pharmaceutical dosage form. Inventi Rapid: Pharm Analysis & Quality Assurance.
Ye YR, Bektic E, Buchta R, Houlden R, Hunt B (2004) Simultaneous determination of tretinoin and clindamycin phosphate and their degradation products in topical formulations by reverse phase HPLC. J Sep Sci 27:71–77. https://doi.org/10.1002/jssc.200301652
Mahmoudi A, Boukhechem MS (2020) Simplified HPLC method for simultaneous determination of erythromycin and tretinoin in topical gel form. Sep Sci Plus 3:86–93. https://doi.org/10.1002/sscp.201900093
Sarkis N, Sawan A (2022) Development and validation of derivative UV spectroscopic method for simultaneous estimation of nicotinamide and tretinoin in their binary mixtures and pharmaceutical preparations. BMC chem 16:1–1. https://doi.org/10.1186/s13065-022-00809-x
Barazandeh Tehrani M, Namadchian M, Fadaye Vatan S, Souri E (2013) Derivative spectrophotometric method for simultaneous determination of clindamycin phosphate and tretinoin in pharmaceutical dosage forms. Daru 21:29. https://doi.org/10.1186/2008-2231-21-29
Sheliya K, Shah K, Kapupara P (2014) Development and validation of analytical method for simultaneous estimation of mometasone furoate, hydroquinone and tretinoin in topical formulation by RP-HPLC. J Chem Pharm Res 6:934–940
Gupta A, Gulati M, Pandey NK (2009) A validated UV spectrophotometric method for simultaneous estimation of tretinoin and benzoyl peroxide in bulk and semisolid dosage form. Rasayan J Chem 2:649–654
Wu T, Wang C, Li N (2006) Simultaneous determination of inhibitive components in anti-acne cosmetics by reversed-phase high performance liquid chromatography. Chin J Chrom 24:589–591
ICH Harmonised Tripartite Guidline. (2005) Validation of analytical procedures: text and methodology Q2(R1), Geneva. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
Acknowledgements
The authors are thankful to the Horizon Healthcare (Pvt) Limited, Lahore, for providing the materials and necessary equipment.
Funding
The present research project was not funded by any external grant.
Author information
Authors and Affiliations
Contributions
MU conceptualized and collected the necessary data from literature. MU designed the methodology and TA performed the whole experiment; both are responsible for the data curation. MU and TA collaboratively wrote the manuscript draft. MU proof read the manuscript. The authors declare that they have read and agreed to the published version of the manuscript. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The authors declare no conflict of interest.
Competing interests
The authors declare no conflict of interest regarding this publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usman, M., Abbas, T. Development and validation of stability indicating HPLC–PDA method for the simultaneous determination of benzoyl peroxide and tretinoin in topical dosage form. Futur J Pharm Sci 9, 26 (2023). https://doi.org/10.1186/s43094-023-00473-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-023-00473-5