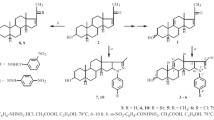
Abstract
Background
For many years, various drugs have been used for the treatment of infectious diseases but some bacterial microorganisms have induced resistance to several drugs. In a search of new antimicrobial agents, a series of new steroidal hydrazones were designed and synthesized.
Result
The structures of the compounds were established based on the spectral data. The in vitro antimicrobial activity of some newly synthesized compounds against bacteria and fungi was studied.
Conclusion
New compounds showed better or similar antimicrobial activity. Designing more efficient steroidal hydrazones from ketosteroid based on the current study may successfully lead to the development of antimicrobial agent.
Graphical abstract

Similar content being viewed by others
Background
Hydrazones are synthesized by condensation of aldehyde/ketone with hydrazine. Hydrazones are also synthesized by coupling reaction of aryl diazonium salts with active hydrogen compounds [1]. Hydrazone have gained great importance due to their diverse biological properties including antibacterial and antifungal [2], anticonvulsant [3], anti-inflammatory [4], antimalarial [5] and antituberculosis [6] activities. When they are used as intermediates, coupling products can be synthesized by using the active hydrogen component of azomethine group [7].
Searching for new molecules in the field of steroid will never end. Researchers are always been interested to do research on steroid due to its particular biological and pharmacological action. The Steroidal drugs have been widely used in traditional medicines. The versatile activity of androstene and estrane series indicates that these molecules could be a key starting material for developing a new drug. Particularly, this invention relates to therapeutically valuable steroids of androstene and estrane series having hydrazone function. Steroidal hydrazones have received extensive attention of scientists because they exhibit some biological activities such as antifungal, antibacterial, antiproliferative, antituberculosis, antiviral and anticancer [8,9,10,11,12,13,14,15,16].
Structural modification of steroids requires great synthetic effort and still a vivacious area of research. Steroidal ring modification and incorporation of heteroatom or replacing one or more carbon atoms in steroidal molecule may improves its biological activities have been researched and reported [17,18,19,20,21,22,23,24,25,26]. About preparation of steroidal derivatives, introduction of methyl group at a certain position of steroids may significantly change their bioactivities [27]. The investigation of new steroidal derivatives has been given great attention. Hydralazine plays an important role as antihypertensive drug and sold under the brand name Apresolin. Hydralazine belongs to the hydrazinophthalazine class of drugs [28]. Hydralazine derivatives have wide applications in the treatment of diseases such as tuberculosis, mental disorder [29]. Hydralazine can be used as antimicrobial, antihypertensive, antimalarial and antitumoral agents [30,31,32].
Steroidal hydrazone containing nitrogen atom has been synthesized with the aim of improving selectivity. However steroidal hydrazones with hydralazine hydrochloride were rarely reported. We decided to further explore the antimicrobial properties of steroidal hydrazone by synthesizing new analogs with suitable structural modifications (Fig. 1).
Methods
All the chemicals were used as received from commercial sources. All reaction progress were monitored by thin-layer chromatography (TLC) analysis using silica gel 60 F254 TLC plates. The melting point was determined on a Veego-matic melting point apparatus. IR spectra were recorded using potassium bromide disks on a Shimadzo IR Affinity 1S. The wave numbers are given in cm−1. 1H and C13 NMR spectra were recorded on Bruker Avance II spectrophotometer at 400 and 300 MHz and 100 and 75 MHz, respectively, with tetramethylsilane as an internal reference; the chemical shifts were measured in ppm with respect to the solvent. Mass spectra were recorded on TSQ Quantum and water make Acquity model UPLC connected with SQ detector (Single Quadra pole) software Mass Lynx (401) instrument equipped with electro spray ionization (ESI) ion source. Measurements were taken in positive (MS+) ion mode.
Experimental
General procedure for the preparation of steroidal hydrazone (3–8, 10, 11, 15, 16, 18, 20 and 22)
Ketosteroid (1, 9A, 9B, 14A, 14B, 17A, 19 and 21) (Schemes 1, 2, 4, 5, 6, 7) (2.5 mmol) and hydralazine hydrochloride (2) (2.6 mmol) with Potassium acetate (2.6 mmol) in Methanol (25 ml) was refluxed for 6 h. After the end of the reaction (monitored by TLC), the mixture was allowed to cool and added water (25 ml). On stirring, the precipitate was formed and collected by filtration. This solid was purified from Methanol (10 ml) and dried at 45–50 °C to afford the corresponding compounds as yellow solid.
Synthetic procedure of 9A (Δ6-testosterone)
To a solution of Testosterone (9) (2.0 g) in tert. butanol (20 ml) was added chloranil (1.6 g) and the reaction mixture was heated to 80 °C for 5 h. After cooling, the reaction mixture was poured into 10% Na2CO3, solution and the products were extracted with methylene dichloride. The extracts were washed with water dried over anhydrous sodium sulfate and the solvent was evaporated to afford crude crystals. Recrystallization from acetone gave (1.0 g) 9A.
Synthetic procedure of 9B
5 g of Testosterone (9) in tert. butanol (50 ml) was stirred under nitrogen and charged potassium tert. butoxide (5 g) in the mixture. Stirred the reaction mass until clear solution obtained. Added drop wise solution of methyl iodide (7.0 ml) in to the reaction mass at 25–30 °C. Reaction mass allowed to stirred for 4 h (monitored by TLC) at 25–30 °C. Water (100 ml) was added, the tert-butanol removed in vacuum and cooled the suspended mass and filtered. Recrystallization from acetone to give compound (2.5 g) 9B. MS (ESI+) m/z: Calculated for C21H32O2 [M + H]+ 316.48; found, 317.31.
Method for the preparation of compound 10 and 11 according to the general procedure
See Scheme 2.
Synthetic procedure of (13)
Estrone (12) (0.75 g, 2.8 mol) and hydralazine hydrochloride (2) (0.58 g, 2.9 mol) with Potassium acetate (0.28 g, 2.8 mol) in Tetrahydrofuran (25 ml) was refluxed for 8–10 h. After the end of the reaction (monitored by TLC), Distilled off Tetrahydrofuran under reduced pressure and yellowish oily mass allowed to cool and added Methanol (15 ml). On stirring, the precipitate was formed and collected by filtration and washed with water (20 ml). This solid was purified from Methanol (10 ml) and dried at 45–50 °C to afford the corresponding compounds (13) as yellow solid (Scheme 3).
Synthetic procedure of 14A (4-methyl nandrolone) and 14B
To a solution of Nandrolone (14) in tert. butanol was added potassium tert-butoxide with stirring under inert atmosphere by nitrogen blanketing. The solution of methyl iodide (3.8 ml, 61.04 mmol) in tert-butanol (18 ml) was added drop wise over a period of 30 min. and the resulting mixture was refluxed for 30 min (monitored by TLC). After cooling, the reaction mixture was acidified with 1 M HCl and the solvent was evaporated. The crude product was extracted with Methylene dichloride, the organic layer was washed with NaHSO3 and water, dried with anhydrous sodium sulfate and distilled out solvent under vacuum to give crude product which was separated by flash chromatography over short silica column eluting with 30% ethyl acetate in n-Hexane to give white solid of 4-methyl-androst-4-en-3-one-17β-ol (14A) MS (ESI+) m/z: Calculated for C19H28O2 [M + H]+ 288.42; found, 289.16 and 4,4-dimethyl-androst-5-en-3-one-17β-ol(14B) MS (ESI+) m/z: Calculated for C20H30O2 [M + H]+ 302.45; found, 303.21.
Method for the preparation of compound 15 and 16 according to the general procedure
See Scheme 4.
Synthetic procedure of 17A (Δ6-norethisterone) according to the procedure 9A and method for the preparation of compound 18 according to the general procedure
See Scheme 5.
Method for the preparation of compound 20 according to the general procedure
See Scheme 6.
Method for the preparation of compound 22 according to the general procedure
See Scheme 7.
Results
3-(Phthalazin-1yl-hydrazono)-4-androstene-17β-ol (3)
Yellow solid, 0.63 g, Yield 67%; mp: 190 °C Dec.; IR (KBr, cm−1): 1582 (C=C), 1605(C=N), 2934 (CH), 3387(NH), 3410(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.76(s, 3H, –CH3), 1.17(s, 3H, –CH3), 3.62(s, 1H, –CH), 5.70(s, 1H, –CH) 7.51(m, 1H, Ar–H), 7.67(m, 2H, Ar–H), 7.86(s, 1H, Ar–H), 8.30 (m, 1H,Ar–H); 13C NMR (100 MHz CDCl3): 11.0, 17.4, 20.6, 23.3, 30.3, 31.5, 32.8, 33.9, 35.6, 35.7, 36.4, 42.8, 50.4, 53.9, 81.4, 123.7, 125.8, 126.9, 127.3, 128.7, 131.4, 131.7, 137.5, 146.9, 152.0, 161.4; MS (ESI+) m/z: calculated for C27H34N4O [M + H]+ 430.27; found, 431.12.
3-(Phthalazin-1yl-hydrazono)-19-nor-4-androstene-17β-ol (4)
Yellow solid, 0.70 g, Yield 62.5%; mp: > 200 °C; IR (KBr, cm−1): 1590(C=C), 1612(C=N), 2912(CH), 3352(NH), 3409 (OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.76(s, 3H, –CH3), 3.61(s, 1H, –CH), 5.78(s, 1H, –CH), 7.71(m, 1H, Ar–H), 7.81(m, 2H, Ar–H), 8.29(s, 1H, Ar–H), 8.51(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 11.1, 23.1, 26.1, 26.5, 30.3, 30.6, 35.4, 36.4, 40.4, 42.5, 43.0, 49.5, 49.7, 81.5, 122.9, 124.4, 126.2, 127.5, 128.1, 131.2, 137.6, 145.8, 166.9; MS (ESI+) m/z: Calculated for C26H32N4O [M + H]+ 416.26; found, 417.28.
17-Ethinyl-3-(phthalazin-1yl-hydrazono)-19-nor-4-androstene-17β-ol (5)
Yellow solid, 0.65 g, Yield 60%; mp: 165–167 °C; IR (KBr, cm−1): 1585(C=C), 1615(C=N), 2956(CH), 3271(≡C–H), 3331(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.97(s, 3H, –CH3), 2.86(s, 1H, –CH), 5.73(s, 1H, –CH), 7.75(m, 1H, Ar–H), 7.92(m, 2H, Ar–H), 8.30(s, 1H, Ar–H), 8.45(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 11.9, 23.1, 26.4, 26.8, 30.8, 32.6, 35.6, 36.7, 39.0, 41.2, 42.7, 47.0, 49.3, 74.3, 79.8, 87.6, 124.7, 125.9, 126.4, 127.3, 129.1, 131.9, 132.7, 135.5, 141.7, 158.5, 165.8; MS (ESI+) m/z: Calculated for C28H32N4O [M + H]+ 440.58; found, 441.38.
17-Ethinyl-3-(phthalazin-1yl-hydrazono)-7α-methyl-19-nor-4-androstene-17β-ol (6)
Yellow solid, 0.73 g, Yield 67%; mp: > 200 °C; IR (KBr, cm−1): 1580(C=C), 1608(C=N), 2934(CH), 3251 (≡C–H), 3404(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.75(d, 3H, J = 7.2 Hz, –CH3), 0.90(s, 3H, –CH3), 2.56(s, 1H, –CH), 5.82(s, 1H, –CH), 7.75(m, 1H, Ar–H), 7.92(m, 2H, Ar–H), 8.33(s, 1H, Ar–H), 8.69(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 12.6, 12.8, 22.2, 26.7, 30.6, 32.3, 36.6, 38.7, 42.0, 43.2, 43.4 45.9, 46.9, 74.1, 78.6, 87.3, 124.1, 125.2, 126.7, 127.6, 128.9, 131.5, 132.8, 135.9, 141.3, 157.9, 165.0; MS (ESI+) m/z: Calculated for C29H34N4O [M + H]+ 454.27; found, 455.19.
7α-Methyl-3-(phthalazin-1yl-hydrazono)-19-nor-4-androstene-17β-ol (7)
Yellow solid, 0.75 g, Yield 66%; mp: > 210 °C; IR (KBr, cm−1): 1587(C=C), 1610(C=N), 2927(CH), 3362(NH), 3410(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.73(d, 3H, J = 8 Hz, –CH3), 0.90(s,3H,–CH3), 3.61(s,1H,–CH), 5.72(s,1H,–CH), 7.71(m,1H,Ar–H), 7.97(m,2H,Ar–H), 8.35(s,1H,Ar–H), 8.67(m,1H,Ar–H); 13C NMR (100 MHzCDCl3): 11.8, 12.9, 22.5, 26.9, 30.4, 32.9, 36.9, 38.4, 42.1, 43.3, 43.9, 45.4, 46.3, 81.6, 123.9, 125.3, 126.5, 127.8, 128.3, 131.7, 132.6, 135.4, 141.2, 156.9, 163.7; MS (ESI+) m/z: calculated for C27H34N4O [M + H]+ 430.27; found, 431.22. Anal. Calc. C:75.31, H:7.96, N:13.01; found C:75.24, H:7.59, N:13.13.
18-Methyl-3-(phthalazin-1yl-hydrazono)-19-nor-4-androstene-17β-ol (8)
Yellow solid, 0.77 g, Yield 71.9%; mp: > 200 °C; IR (KBr, cm−1): 1575(C=C), 1609(C=N), 2923(CH), 3393(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.81(t, 3H, J = 7.1 Hz, –CH3), 1.20(m, 2H, –CH2), 3.48(s,1H,–CH), 5.73(s,1H,–CH), 7.76(m, 1H, Ar–H), 7.93(m, 2H, Ar–H), 8.33(s, 1H, Ar–H), 8.49(m, 1H, Ar–H);13C NMR (100 MHz CDCl3): 8.9, 18.6, 21.5, 26.0, 28.0, 30.4, 35.2, 35.8, 38.7, 40.5, 42.2, 47.5, 48.6, 50.2, 80.2, 123.6, 124.1, 125.9, 127.0, 128.2, 131.4, 137.3, 145.2, 168.5; MS (ESI+) m/z: Calculated for C27H34N4O [M + H]+ 430.59; found, 431.44.
3-(Phthalazin-1yl-hydrazono)-androsta-4,6-dien-17β-ol (10)
Yellow solid, 0.66 g, Yield 58.9%; mp: > 200 °C;IR (KBr, cm−1):1587(C=C), 1606(C=N), 2933(CH), 3381(NH), 3456(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.74(s, 3H, –CH3), 0.94(s, 3H, –CH3), 3.55(t, 1H, J = 16 Hz, –CH), 5.70(s, 1H, –CH), 5.98(s, 1H, –CH), 7.34–7.38(m, 1H, Ar–H), 7.49–7.53(m, 2H, Ar–H), 7.68 (s, 1H, Ar–H), 8.25–8.30(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 11.0, 16.8, 20.5, 22.3, 23.0, 30.3, 33.2, 36.1, 36.5, 37.2, 43.7, 48.7, 50.9, 81.3, 124.1, 124.2, 125.9, 127.2, 127.5, 128.6, 131.5, 131.6, 133.8, 137.8, 146.5, 1 52.0, 161.6; MS (ESI+) m/z: Calculated for C27H32N4O [M + H]+ 428.26; found, 429. 4. Anal. Calc. C:75.67, H:7.53, N:13.07; found (C:74.29, H:8.15, N:12.19).
3-(Phthalazin-1yl-hydrazono)-4,4′-dimethyl-5-androstene-17β-ol (11)
Yellow solid, 0.6 g, Yield 55.0%; mp: 208–210 °C; IR (KBr, cm−1): 1584(C=C), 1607(C=N), 2931(CH), 3367(NH), 3426 (OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.72(s,3H,–CH3), 0.96(s, 3H, –CH3), 1.22(s, 6H, –CH3), 3.67(d, 1H, J = 8.03 Hz, –CH), 5.63(m,1H,–CH), 7.65(m, 1H, Ar–H), 7.87(m, 2H, Ar–H), 8.11(s, 1H, Ar–H), 8.39(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 12.0, 18.7, 20.8, 23.1, 25.4, 28.4, 30.1, 31.3, 39.2, 42.4, 48.7, 50.2, 81.5, 124.2, 126.1, 126.5, 127.3, 128.1, 144.6, 149.5, 163.2; MS (ESI +) m/z: calculated for C29H38N4O [M + H]+ 458.64; found, 459.39.
3-Hydroxy-1,3,5(10)-estratrien-17-(phthalazine-1yl-hydrazono) (13)
Yellow solid, 0.75 g, Yield 66%; mp: 167–169 °C; IR (KBr, cm−1): 1585(C=C), 1602, 1649(C=N), 2935(CH), 3055(aromatic CH), 3383(NH); 1H NMR (400 MHz DMSO) δ, ppm: 0.98(s, 3H, –CH3), 6.51(d, 1H, J = 4 Hz, Ar–H, estrone), 6.57(d, 1H, J = 4 Hz, estrone), 7.14(d, 1H, J = 8.48 Hz, Ar–H, estrone), 7.70 (m, 3H, Ar–H, hydralazine), 8.00(s, 1H, Ar–H, hydralazine), 8.23(s, 1H, Ar–H, hydralazine), 9.08(s, 1H, –OH), 11.21(s, 1H, –NH); 13C NMR (100 MHz DMSO): 16.9, 25.9, 27.2, 29.1, 30.7, 35.1, 38.1,43.8, 44.3, 52.1, 112.86, 114.9, 115.9, 123.3, 126.2, 126.8, 131.4, 136.5, 137.1, 145.7, 168.0, 176.6; MS (ESI+) m/z: Calculated for C26H28N4O [M + H]+ 412.53; found, 413.4. Anal. Calc. C:75.70, H:6.84, N:13.58; found (C:74.35, H:6.72, N:13.59).
3-(Phthalazin-1yl-hydrazono)-4-methyl-19-nor-4-androstene-17β-ol (15)
Yellow solid, 0.4 g, Yield 36%; mp: > 200 °C; IR (KBr, cm−1): 1579(C=C), 1607(C=N), 2932(CH), 3366(NH), 3415(OH); 1HNMR (400 MHz CDCl3) δ, ppm: 0.93(s, 3H, –CH3), 1.22(s, 3H, –CH3), 3.53(s, 1H, –CH), 7.71(m, 1H, Ar–H), 7.87(m, 2H, Ar–H), 8.18(s, 1H, Ar–H), 8.51(m, 5H, Ar–H); 13C NMR (100 MHz CDCl3):11.3, 18.0, 23.3, 24.7, 26.1, 26.5, 29.7, 30.1, 30.6, 35.4, 36.4, 40.4, 42.5, 43.0, 49.5, 49.7, 81.5, 124.4, 129.2, 138.4, 166.9; MS (ESI+) m/z: Calculated for C27H34N4O [M + H]+ 430.27; found, 431.18.
3-(Phthalazin-1yl-hydrazono)-4,4′-dimethyl-19-nor-5-androstene-17β-ol (16)
Yellow solid, 0.6 g, Yield 66.6%; mp: 109–108 °C; IR (KBr, cm−1): 1588(C=C), 1606(C=N), 2954(CH), 3371(NH), 3456(OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.97(s, 3H, –CH3), 1.21 (s, 6H, –CH3), 3.57(s, 1H, –CH), 5.56 (d, 1H, J = 7.1 Hz, –CH), 7.51(m, 1H, Ar–H), 7.71(m, 2H, Ar–H), 8.21(s,1H,Ar–H), 8.41(m, 1H, Ar–H); 13C NMR (100 MHz CDCl3): 13.6, 21.4, 23.9, 24.7, 27.4, 30.7, 31.1, 31.8, 32.1, 33.8, 37.1, 38.4, 44.2, 48.9, 80.8, 119.9, 124.3, 124.6, 125.5, 127.0, 127.4, 128.3, 131.2, 131.8, 133.4, 137.9, 146.6, 152.3, 162.3; MS (ESI +) m/z: calculated for C28H38N4O [M + H]+ 446.63; found, 447.47.
17-Ethinyl-3-(phthalazin-1yl-hydrazono)-estr-4,6-dien-17β-ol (18)
Yellow solid, 0.55 g, Yield 74%; mp: 167–169 °C; IR (KBr, cm−1):1582(C=C), 1619(C=N), 2977(CH), 3322(NH), 3401 (OH); 1H NMR (400 MHz CDCl3) δ, ppm: 0.95(s,3H,–CH3), 2.86(s, 1H, CH), 5.80(d, 1H, J = 10.7 Hz, CH), 5.92(s, 1H, –CH), 6.23(d, 1H, J = 10.7 Hz, –CH), 7.60(m,1H,Ar–H), 7.79(m,2H,Ar–H), 8.28(s,1H,Ar–H), 8.43(m,1H,Ar–H); 13C NMR (100 MHz CDCl3): 12.0, 22.5, 25.1, 26.9, 32.3, 37.8, 38.7, 40.8, 41.9, 45.7, 47.3, 48.7, 74.3, 80.4, 87.0, 124.4, 125.6, 126.9, 127.5, 128.8, 131.6, 132.1, 136.5, 141.5, 158.9, 161.8; MS (ESI +) m/z: Calculated for C28H30N4O [M + H]+; 438.56; found, 439.49.
3-(Phthalazin-1yl-hydrazono)-1α-methyl-5α-androstan-17β-ol (20)
Yellow solid, 0.67 g, Yield 61%; mp: > 200 °C; IR (KBr, cm−1):1586(C=C), 1629(C=N), 2930(CH), 3392(OH); 1H NMR (300 MHz CDCl3) δ, ppm: 0.74–1.0(m,14H,Mesterolone), 1.0–1.79(m,11H,Mesterolone), 3.58 (t, 1H, J = 9.0 Hz, –CH), 7.40–7.45(m, 1H, Ar–H), 7.56–7.59(m, 2H, Ar–H), 7.72(d, 1H, J = 6 Hz, Ar–H), 8.30–8.35 (m, 1H, Ar–H);13C NMR (75 MHz CDCl3): 11.3, 14.0, 14.8, 20.0, 23.5, 28.7, 30.5, 30.9, 31.3, 35.6, 36.7, 38.6, 38.9, 39.2, 39.6, 40.6, 43.1, 48.7, 48.9, 51.0, 81.8, 124.1, 126.0, 127.2, 131.4, 131.6, 137.4, 146.4, 166.8; MS (ESI+) m/z: Calculated for C28H38N4O [M + H]+ 446.63; found, 447.92.
4′-[17-(Phthalazin-1-yl-hydrazone)-1,3,5(10)-estratrien-3-yloxymethyl]-biphenyl-2-carbonitrile (22)
Yellow solid, 0.50 g, Yield 76.9%; mp: > 200 °C; IR (KBr, cm−1): 1586(C=C), 1602(C=N), 1648, 2223(C≡N), 2937(CH), 3064(CH aromatic), 3398(NH); 1H NMR (400 MHz CDCl3) δ, ppm: 1.1(s, 3H, –CH3), 5.16(s, 2H, –OCH2–), 6.80–8.85(m, 20H, Ar–H);13C NMR (100 MHz CDCl3):17.0, 25.1, 27.3, 29.1, 30.7, 35.1, 38.1, 43.8, 44.3, 52.1, 112.86, 114.9, 115.9, 123.3, 126.2, 126.8, 131.4, 136.5, 137.1, 137.8, 141.4, 145.7, 149.7, 154.0, 167.1, 183.5; MS (ESI+) m/z: calculated for C40H37N5O [M + H]+ 603.75; found, 604.86.
Discussion
Chemistry
The literature survey was done by focusing on steroidal hydrazone where ketosteroid used as a starting material. Allah HMF synthesized phthalazinohydrazone of 17α-methyltestosterone [33]. Rasras et al. [34] conveyed the efficient procedure for the synthesis of novel hydrazide–hydrazone of cholic acid and tested them for antibacterial activity. Mohareb et al. reported synthesis of hydrazide‑hydrazone, pyrazole, pyridine, thiazole, thiophene derivatives and their cytotoxicity evaluations [35]. A method conveyed by Nadaria et al. [36, 37] for the synthesis and biological activity of hydrazone of 5α-steroids and synthesis and cytotoxicity of epiandrosterone hydrazones. Jaben et al. [38] specified the synthesis of anticancer agents of progesterone and testosterone. Zickovic et al. [39] synthesized steroidal thiosemicarbazones and evaluated their cytotoxic activity.
The structural chemistry of these steroidal hydrazones involves the condensation of hydralazine hydrochloride at C3 and C17 of ketosteroid. The reaction is catalyzed by potassium acetate in methanol as solvent. Androgen and estrogen scaffold used as ketosteroid for the synthesis of title compounds. The synthesis of steroidal hydrazones was developed without chromatographic purification (column/flash chromatography).
The structures of the synthesized compounds (3–8, 10, 11, 15, 16, 18, 20 and 22) were established using 1H, 13C-NMR and mass spectral data. In 1H NMR spectrum of steroidal hydrazones (3–8, 10, 11, 15, 16, 18, 20 and 22) singlet signals of 4-CH3, 4,4′-CH3, 18-CH3 and 19-CH3 groups were present at δ 1.22 ppm, 0.76–0.96 ppm and 0.81 ppm and doublet signal of 7-CH3 group was present at δ 0.73–0.75 ppm. Aromatic protons of hydralazine were noted in the interval at δ7.5–8.5 ppm. In the 13C NMR spectra of steroidal hydrazones (5, 6 and 18) peaks of ≡CH carbon existence at around δ 79.0 ppm and –C≡ at around 87 ppm. Signals of C=N bond at 161.6 ppm and 168.0 ppm. The C17 peaks of steroidal hydrazones (3–8, 10, 11, 15, 16, 18, 20 and 22) were observed at around δ 81 ppm. In 1H NMR spectrum of steroidal hydrazones (3–8, 11, 15, 16 and 18) and singlet signal of –CH were present at around δ 2.48–3.61 ppm. Where as in compound 10 and 20, –CH gave triplet at δ 3.55 and 3.58 ppm. In the mass spectral analysis [M + H]+ of 9A, 14A, 14B, 10, 13 and 20 matched with theoretical values.
In the 1H NMR spectra (in DMSO) of steroidal hydrazone (13) singlet signals of angular 18-CH3 group was present at δ 0.98 ppm. The signals of aromatic protons were present in the range of δ 6.57–7.14 ppm. The singles of aromatic protons of hydralazine were present in the range of δ7.70–8.23 ppm. Singlet signal of the proton of –OH group at δ 9.08 ppm. The protons of the –NH at δ 11.21 ppm. The IR spectrum of the steroidal hydrazone (13) contained absorption bands the NH– group at 3358 cm−1, C=N bond at 1649 cm−1, bands at 1602, 1585 cm−1for –C=C–. The infrared spectra of steroidal hydrazones (3–8, 10, 11, 15, 16, 18, 20 and 22) showed the NH-band in the range of 3381–3209 cm−1.
Biological activity
In vitro antimicrobial activity
We have selected compounds 3 (testosterone), 6 (iso tibolone), 7 (7-methyl nandrolone), 10 (δ6-testosterone), 11 (4,4-dimethyl steroid) and 18 (δ6-norethisterone) for the antimicrobial screening based on the steroidal skeleton. The difference in the structure of above compounds is the presence of -CH3, Unsaturation and ethinyl group. The position of groups are encourages us that what will be the activity of the selected compounds among the all.
The in vitro antimicrobial activity of some of the synthesized compounds was accomplished by broth microdilution method [40]. It is one of the non-automated in vitro bacterial susceptibility tests. This classic method yields a quantitative result for the amount of antimicrobial agents that is needed to inhibit growth of specific microorganisms. It is carried out in tubes. Mueller–Hinton broth was used as nutrient medium to grow and dilute the compound suspension for the test bacteria and Sabouraud Dextrose broth used for fungal nutrition.
Each synthesized drug was diluted obtaining 2000 µg /ml concentration, as a stock solution.
Primary screen
In primary screening 1000 micro/ml, 500 micro/ml and 250 micro/ml concentrations of the synthesized drugs were taken. The active synthesized drugs found in this primary screening were further tested in a second set of dilution against all microorganisms.
Secondary screen
The drugs found active in primary screening were similarly diluted to obtain 200 micro/ml, 100 micro/ml, 50 micro/ml, 25 micro/ml, 12.5 micro/ml, 6.250 micro/ml and concentrations.
Reading result
The highest dilution showing at least 99% inhibition zone is taken as MIC. The result of this is much affected by the size of the inoculum. The test mixture should contain 108 organism/ml. Inoculum size for test strain was adjusted to 108 CFU [Colony Forming Unit] per milliliter by comparing the turbidity. The strains employed for the activity were procured from [MTCC—Micro Type Culture Collection] Institute of Microbial Technology, Chandigarh.
The compounds 3, 6, 7, 10, 11 and 18 were screened for their antibacterial activity against Escherichia coli (E. coli), Pseudomonas aeruginosa (P. aeruginosa), Staphylococcus aureus (S.aureus), Streptococcus pyogenes (S. pyogenes) and antifungal activity against Candida albicans (C. albicans), Aspergillus niger (A. Niger) and Aspergillus clavatus (A. Clavatus). DMSO was used as media to get desired concentration of compounds to test upon microbial strains. The lowest concentration, which showed no visible growth after spot subculture was considered as MIC for each compound. The standard antibiotics used for comparison in the present study were gentamycin, ampicillin, chloramphenicol, ciprofloxacin and norfloxacin for evaluating antibacterial activity while nystatin and griseofulvin for antifungal activity. The results are summarized in Tables 1 and 2.
From the antimicrobial data, steroidal hydrazones 3, 7, 10 and 18 showed better activity (MIC 116–289 µM) against gram-positive bacteria Staphylococcus aureus (S. aureus) as compare to ampicillin (MIC 715 µM). Compounds 7 and 10 showed excellent activity (MIC 145 and 116 µM) against gram-negative bacteria Escherichia coli (E. coli) as compared to ampicillin (MIC 286 µM), compound 6 (MIC 55 µM) is found active against Streptococcus pyogenes (S. pyogenes) as compare to chloramphenicol (MIC 154 µM) and ciprofloxacin (MIC 150 µM). Compound 6 (MIC 110 µM) and 11 (MIC 142 µM) exhibited powerful activity against Pseudomonas aeruginosa (P. aeruginosa) as compare to ampicillin (MIC 286 µM). Compound 6 showed equivalent potency against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) as compared to ampicillin.
Entire steroidal hydrazones (MIC 580–2319 µM) showed inferior activity against all gram-positive and gram-negative bacteria as compare to gentamycin (MIC 0.10–2 µM) and norfloxacin (MIC 31 µM). Compound 7 (MIC 580 µM) is found active against C. albicans as compare to griseofulvin (MIC 1417 µM) and rest of the steroidal hydrazones exhibited less potency than standard fungicidal nystatin and griseofulvin against Candida albicans, Aspergillus niger and Aspergillus clavatus.
Conclusions
The antimicrobial activities of steroidal hydrazone were studied by the broth microdilution method. Compound 6, 7 and 10 displayed excellent antibacterial activity among the tested compounds due to bearing an ethinyl at C-17 of compound 6, methyl at C-7 of compound 7. Compound 10 showed excellent antibacterial activity due to the compounds bearing an additional –C=C– in the structure. Hence, these substituted steroidal skeleton considered for the development of the new antimicrobial agent.
Availability of data and materials
Data and materials are available upon request.
References
Rollas S, Kucukguzel SG (2007) Biological activities of hydrazone derivatives. Molecules 12:1910–1939
Subhashini NJP, Janaki P, Bhadraiah B (2017) Synthesis of hydrazone derivatives of benzofuran and their antibacterial and antifungal activity. Russ J Gen Chem 87:2021–2026
Sridhar KS, Pandeya SN, Stables JP, Atmakuru R (2002) Anticonvulsant activity of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Pharm Sci 16:129–132
Debnatha U, Mukherjee S, Joardar N, Sinha BS, JanaMisra KAK (2019) Aryl quinolinyl hydrazone derivatives as anti-inflammatory agents that inhibit TLR4 activation in the macrophages. Eur J Pharm Sci 134:102–115
Kumar P, Kadyan K, Duhan M, Sindhu J, Singh V, Singh B, Kumar S (2017) Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem Cent J 11:115
Nogueira TM, Cruz LS, Lourenço M, Nora de Souza MV (2019) Design, synthesis and anti-tuberculosis activity of hydrazones and N-acylhydrazones containing vitamin b6 and different heteroaromatic nucleus. Lett Drug Des Discov 16:7
Singh V, Srivastava VK, Palit G, Shanker K (1992) Coumarin congeners as antidepressants. Arzneim-Forsch Drug Res 42(8):993–996
Gan C, Liu L, Cui J, Liu Z, Shi H, Lin Q, Sheng H, Yang C, Huang Y (2017) Synthesis of some steroidal derivatives with side chain of 20- and 22-hydrazone aromatic hetero cycles and their antiproliferative activity. Med Chem 13(4):375–383
Loncle C, Brunel JM, Vidal N, Dherbomez M, Letourneux Y (2004) Synthesis and antifungal activity of cholesterol-hydrazone derivatives. Eur J Med Chem 39:1067–1071
Visbal G, San-Blas G, Maldonado A, Alvarez-Aular A, Capparelli MV, Murgich J (2011) Synthesis, in vitro antifungal activity and mechanism of action of four sterol hydrazone analogues against the dimorphic fungus Paracoccidioides brasiliensis. Steroids 76:1069–1081
Khan SA, Kumar P, Joshi R, Iqbal PF, Saleem K (2008) Synthesis and in vitro antibacterial activity of new steroidal thiosemicarbazone derivatives. Eur J Med Chem 43(9):2029–2034
Gan C, Cui J, Su S, Lin Q, Jia L, Fan L, Huang Y (2014) Synthesis and antiproliferative activity of some steroidal thiosemicarbazones, semicarbazones and hydrozones. Steroids 87:99–107
Merlani MI, Kemertelidze EP, Papadopoulos K, Men’shova NI (2004) Some derivatives of 5α ketosteroid hydrazones: synthesis from tigogenin and antituberculosis activity. Russ J Bioorgan Chem 30(5):497–501
Nadaraia NS, Onashvili EO, Kakhabrishvili ML, Barbakadze NN, Sylla B, Pichette A (2016) Synthesis and antiviral activity of several N-containing 5α-steroids. Chem Nat Compd 52(5):853–855
Nadaraia NS, Barbakadze NN, Kakhabrishvili ML, Sylla B, Pichette A, Makhmudov US (2018) Synthesis and biological activity of several modified 5α-androstanolone derivatives. Chem Nat Compd 54(2):310–314
Wang HJ, Bu M, Wang J, Liu L, Zhang S (2019) Synthesis and biological evaluation of novel steroidal 5α, 8α-endoperoxide steroidal derivatives with aromatic hydrazone side chain as potential anticancer agents. Russ J Bioorg Chem 45:585–590
Dhingra N, Bhardwaj TR, Mehta N, Mukhopadhyay T, Kumar A, Kumar M (2010) Synthesis, antiproliferative, acute toxicity and assessment of antiandrogenic activities of some newly synthesized steroidal lactams. Eur J Med Chem 45:2229–2236
Huang Y, Cui J, Zheng Q, Zeng C, Chen Q, Zhou A (2012) 6-Hydroximino-4-aza-Ahomo-cholest-3-one and related analogue as a potent introducer of apoptosis in cancer cells. Steroids 77:829–834
Duha CY, Loa IW, Wang SK, Dai CF (2007) New cytotoxic steroids from the soft coral Clavulariaviridis. Steroids 72:573–579
Malika IO, Maurice S (2006) Recent advances in thiasteroids chemistry. Steroids 71:1025–1044
Hanson JR (2006) Steroids: partial synthesis in medicinal chemistry. Nat Prod Rep 23:100–107
Chen SJ, Cui JG, Li Y, Fan LH (2011) Recent advance of steroidal hydrazone with biological activities. Chin J Org Chem 31(2):187–192
Cui J, Liu L, Zhao D, Gan C, Huang X, Xiao Q, Qi B, Yang L, Huang Y (2015) Synthesis, characterization and antitumor activities of some steroidal derivatives with side chain of 17-hydrazone aromatic heterocycle. Steroids 95:32–38
Cui JG, Liu L, Gan CF, Xiao Q (2014) Synthesis and biological activity of steroids bearing aromatic rings and heterocycles. Prog Chem 26(2/3):320–333
Xu H, Su X, Liu XQ, Zhang KP, Hou Z, Guo C (2019) Design, synthesis and biological evaluation of novel semicarbazone-selenochroman-4-ones hybrids as potent antifungal agents. Bioorg Med Chem Lett 29:126726
Stulov SV, Misharin AYu (2013) Synthesis of steroids with nitrogen-containing substituent’s in ring D. Chem Heterocycl Compd 48(10):1431–1472
Li C, Qiu W, Yang Z, Luo J, Yang F, Liu M, Xie J, Tang J (2010) Stereoselective synthesis of some methyl-substituted steroid hormones and their in vitro cytotoxic activity against human gastric cancer cell line MGC-803. Steroids 75:859–869
Schroder NA (1952) The effect of 1-hydrasinophthalasine in hypertension. Circulation 5(1):28–37
Sousa C, Freire C, De Castro B (2003) Synthesis and characterization of benzo-15-crown-5 ethers with appended N2O Schiff bases. Molecules 8:894–900
Kajal A, Bala S, Kamboj S, Saini V (2014) Synthesis, characterization, and computational studies on phthalic anhydride-based benzylidene-hydrazide derivatives as novel, potential anti-inflammatory agents. Med Chem Res 23:2676–2689
Cikla P, Tatar E, Kucukguzel I, Sahin F, Yurdakul D, Basu A, Krishnan R, KNicholsKaushik-BasuKucukguzel DBNSG (2013) Synthesis and characterization of flurbiprofen hydrazide derivatives as potential anti-HCV, anticancer and antimicrobial agents. Med Chem Res 22:5685–5699
Bedia KK, Oruc-Emre EE, Unsalan S, Rollas S (2009) Synthesis and anticancer activity of new hydrazide-hydrazones and their Pd(II) complexes. Med Chem Res 18:277–286
Allah HMF, Soliman R (1987) Synthesis and spectra of some triazolo and triazin phthalazines of possible hypotensive activity. J Hetero Cycl Chem 24:667–671
Rasras AJM, Al-Tel TH, Al-Aboudi AF, Al-Qawasmeh RA (2010) Synthesis and antimicrobial activity of cholic acid hydrazone analogues. Eur J Med Chem 45:2307–2313
Mohareb RM, Al-Omran F (2012) Novel synthesis of hydrazide-hydrazone, pyrazole, pyridine, thiazole, thiophene derivatives and their cytotoxicity evaluations. Steroids 77:1551–1559
Nadaraia NS, Barbakadze NN, Kakhabrishvili ML, Mshvildadze VD (2019) Synthesis and biological activity of hydrazones of 5α-steroids. Res J Pharm Biol Chem Sci 10(1):238–242
Nadaraia NS, Barbakadze NN, Mshvildadze VD, Sylla B, Legault J, Pichette A (2020) Synthesis and cytotoxicity of epiandrosterone hydrazones. Chem Nat Compd 56(2):274–277
Muafia J, Muhammad IC, Ghulam AM, Khondaker MR, Umer R, Khan HU, Arshiab F, Abdul S (2018) Synthesis, pharmacological evaluation and docking studies of progesterone and testosterone derivatives as anticancer agents. Steroids 136:22–31
Zivkovic MB, Matic IZ, Rodic MV, Novakovic IT, Sladic DM, Krstic NM (2016) Synthesis, characterization and in vitro cytotoxic activities of new steroidal thiosemicarbazones and thiadiazolines. RSC Adv 6:34312–34333
Wiegand I, Hilpert K, Hancock REW (2008) Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 3(2):165
Acknowledgements
We are thankful to D. K. Pharma, Powoi, Mumbai for generous gift of hydralazine hydrochloride.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
SM contributed to synthesis, characterization and activity. AKS contributed to analytical work. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mistry, S., Singh, A.K. Synthesis and in vitro antimicrobial activity of new steroidal hydrazone derivatives. Futur J Pharm Sci 8, 7 (2022). https://doi.org/10.1186/s43094-021-00391-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00391-4