Abstract
Background
Medicinal plants are of great importance to researchers in the field of pharmacology as most pharmaceutical industries depend on medicinal plant for their raw materials. Hibiscus asper belongs to the family Malvaceae and is well known for its medicinal properties. The present study was carried out to evaluate the antioxidant effect and possible bioactive components present in the aqueous methanol fraction of Hibiscus asper leaves.
Results
The phytochemical of aqueous methanol fraction of Hibiscus asper leaves (AMFHAL) revealed the presence of flavonoids, tannin, phenols, saponins, alkaloids, glycosides, terpenoids, and steroids. The GC-MS analysis revealed the presence of twenty-three bioactive compounds which include 9,12,15-octadecatrien-1-ol, n-Hexadecanoic acid, octadecatrienol acid, methyl palmitate, and phytol.
Conclusion
The phytochemical and GC-MS profiling of aqueous methanol fraction of Hibiscus asper leaves revealed the presence of bioactive compounds with important medicinal properties. Hence, the presence of these phytochemicals could be responsible for the therapeutic effects of the plant.
Similar content being viewed by others
Background
Plants are used as medicines in various cultures and serve as a source of many potent drugs due to the presence of certain bioactive compounds for pharmaceutical industries [1]. Plants contain different phytochemicals, also known as secondary metabolites. Phytochemicals are useful in the treatment of certain disorders by their individual, additive, or synergic actions to improve health [2, 3]. Phytochemicals are vital in pharmaceutical industry for development of new drugs and preparation of therapeutic agents [4]. The development of new drugs starts with identification of active principles from the natural sources. The screening of plant extracts is a new approach to find therapeutically active compounds in various plant species [1, 5]. Phytochemicals such as flavonoids, tannins, saponins, alkaloids, and terpenoids have several biological properties which include antioxidant, anti-inflammatory, anti-diarrhea, anti-ulcer, and anticancer activities, among others [5].
Hibiscus asper Hook. f. (Malvaceae) is an important medicinal plant widely distributed in tropical Africa and Madagascar. The genus Hibiscus is made up of 250 species and is characterized by the presence of bioactive compounds such as phenolic acids, flavonoids, and polysaccharides [6]. This plant is mostly used in folklore medicine for treatment of depression, jaundice, inflammation, anemia, dysmenorrhea, and leucorrhoea and as poison antidote [7]. In addition, the leaves serve as potent sedative, tonic, and restorative agent. It is also used in the treatment of male infertility and skin infection and as an antioxidant [8, 9].
Gas chromatography-mass spectroscopy (GC-MS) is a combined analytical technique used to determine and identify compounds present in a plant sample [10]. GC-MS plays an essential role in the phytochemical analysis and chemotaxonomic studies of medicinal plants containing biologically active components [11].
Methods
Chemicals
All the chemicals and reagents used for the research were of analytical grade.
Plant collection and adentification
Fresh leaves of Hibiscus asper were collected from Isuofia in Aguata Local Government Area of Anambra State. The leaves were identified and authenticated by Mr. Felix Nwafor of the Pharmacognosy and Environmental Medicine Department, University of Nigeria Nsukka. The plant was deposited in the herbarium of the Department of Pharmacognosy and Environmental Medicine, University of Nigeria Nsukka, with the voucher number PCG/UNN/0350.
Preparation of plant material
Hibiscus asper leaves were air-dried at room temperature and pulverized into powder for extraction. The powder (1300 g) was macerated in 80% methanol and allowed to stand for 48 h at room temperature. The mixture was filtered with Whatman No. 1 filter paper and the filtrate was concentrated using a rotary evaporator to get a brownish black semi-solid extract.
Solvent partitioning of the crude methanol extract was done by using the protocol designed by Kupchan and Tsou [12] and modified version of Wagenen et al. [13]. Fractionation was carried out using n-hexane, ethylacetate, and 20% aqueous methanol (v/v). Crude extract (20 g) was weighed and dissolved in 250 ml of 20% aqueous methanol (v/v) to form a stock solution. Then, 250 ml of n hexane was added to the solution and poured into a separating funnel. The mixture was allowed to stand for 20 min for proper separation, and the upper part was collected in a beaker. The aqueous methanol part was washed repeatedly with n hexane, after which the different n hexane fractions were collected. The above procedure was repeated using ethyl acetate. At the end, ethylacetate fractions were collected and concentrated [14]. The aqueous methanol fraction was used for further studies after subjecting the different fractions to a preliminary study.
Preliminary phytochemical screening
Phytochemical profiling of crude extract and aqueous methanol fraction of Hibiscus asper leaves were carried out using the procedures as described by Harborne [15], Trease and Evans [16], Harborne [17], and Soni and Sosa [18].
Gas chromatography-mass spectrometry (GC-MS) analysis
GC-MS analysis was carried out in a combined 7890A gas chromatograph system (Agilent 19091-433HP, USA) and mass spectrophotometer, fitted with a HP-5 MS fused silica column (5% phenyl methyl siloxane 30.0 m × 250 μm, film thickness 0.25 μm), interfaced with 5675C Inert MSD with Triple-Axis detector. Helium gas was used as carrier gas and was adjusted to column velocity flow of 1.0 ml/min.
Other GC-MS conditions are ion-source temperature, 250 °C; interface temperature, 300 °C; pressure, 16.2 psi; out time, 1.8 mm; and 1 μl injector in split mode with split ratio 1:50 with injection temperature of 300 °C. The column temperature started at 36 °C for 5 min and changed to 150 V at the rate of 4 °C/min. The temperature was raised to 250 °C at the rate of 20 °C/min and held for 5 min. The total elution was 47.5 min. The relative percent amount of each component was calculated by comparing its average peak area to total areas. MS solution software provided by supplier was used to control the system and to acquire the data.
Identification of compounds
Identification of components was achieved based on their retention indices and interpretation of mass spectrum was conducted using the database of National Institute of Standards and Technology (NSIT). The database consists of more than 62,000 patterns of known compounds. The spectra of the unknown components of Hibiscus asper fraction obtained were compared with the standard mass spectra of known components stored in NIST library (NISTII).
Results
Phytochemical screening of aqueous methanol fraction of Hibiscus asper leaf revealed the presence of alkaloids, flavonoids, saponins, tannins, phenols, steroids, and terpenoids as shown in Table 1.
Gas chromatography-mass spectroscopy profiling of aqueous methanol fraction of Hibiscus asper
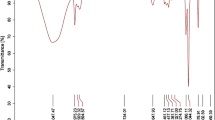
A total of 23 compounds were identified from the GC-MS analysis of methanol fraction of Hibiscus asper leaves exhibiting various phytochemical activities. The chromatogram is presented in Fig. 1, while the chemical constituents with their retention time (RT), molecular formula, molecular weight (MW), and concentration (%) in the MFHAL are presented in Table 2. The following bioactive compounds were present in the GC-MS analysis carried on methanol fraction of Hibiscus asper leaves: Benzeneacetaldehyde, Benzene, 1,2,3,5-tetramethyl-, Benzene, 1-ethyl-2,4-dimethyl-, Azulene, 1-Piperazinecarboxaldehyde, Phthalan, Benzene, 2-methoxy-1,3,4-trimethyl, Acetic acid, [bis[(trimethylsilyl)oxy]-, Pyrrolidine-5-one, 2-[3-hydroxypropyl]-, Methylester pentanoic acid, Cycloheptasiloxane, tetradecamethyl-, 3-Methyl-4-phenyl-1H-pyrrole, Hexadecamethyl cyclooctasiloxane, 5,6-Dimethoxybenzimidazole, Cyclononasiloxane, Methyl palmitate, Pentasiloxane, dodecamethyl-, 9,12-Octadecadienoic acid, methyl ester, 9,12,15-Octadecatrienoic acid, methyl ester, Phytol, 9,12,15-Octadecatrien-1-ol, (Z, Z, Z), and Amonafide.
Discussion
Phytochemical screening of aqueous methanol fraction of Hibsicus asper revealed the presence of phyto-compounds that have been documented to have antioxidant and other activities. Flavonoids have been shown to be highly effective scavengers of most oxidizing molecules, including singlet oxygen, and various free radicals [19] implicated in several diseases. Flavonoids have anti-oxidative and mucosal protective effect [20, 21]. Flavonoid-rich vegetables are widely used functional foods since they can be used to treat cardiovascular diseases [22]. They are characterized by their good bioavailability and, hence, constant dietary consumption of flavonoids has been reported to give pharmacologically relevant plasma concentrations in humans [23]. In addition, several studies have reported the possible cardioprotective effects of flavonoids against ischemia reperfusion [24, 25]. Saponins may activate mucous membrane protective factors, while tannins reduce the permeability of mucosa to chemical irritation. Consequently, they reduce inflammation, exert astringent and protective action on the stomach mucosa, and curb excess acidity. In addition, terpenoids and alkaloid compounds have also been reported to have potent activity against gastric ulcers [26, 27]. Terpenoids have been reported to relax cardiovascular smooth muscle by inhibition of Ca2+ influx in vascular smooth muscle or via quenching of reactive oxygen species (ROS) and stimulation of nitric oxide (NO) synthesis [28]. The presence of these phytochemicals in methanol fraction of H. asper leaves possibly indicates its numerous medicinal properties such as anti-inflammatory, anti-ulcer, and anti-oxidative properties, among others.
Among the identified bioactive components, 9, 12, 15-Octadecatrien-1-ol (Z, Z, Z) has highest percent peak area. This compound has antioxidant and antibacterial properties [1]. n-Hexadecanoic acid has antioxidant, 5- alpha-reductase inhibitor, anti-fibrinolytic, hemolytic, antimicrobial activity, hypocholesterolemic nematicide, pesticide, antiandrogenic flavor, and hemolytic properties [5]. 9, 12, 15-Octadecatrienoic acid, methyl ester (Z, Z, Z) has anti-inflammatory, cancer preventive, hepatoprotective, antioxidant, and hypocholesterolemic properties [5]. Phenolic compounds, esters, alkanes, aldehydes, alkenes, and ketones are the other major volatile compounds present which have antiulcer, anti-inflammatory, anti-arthritic, antidiabetic, hypolipidemic, and cytotoxic activities [29]. Phytol was reported with antioxidant and neuroprotective, antimicrobial, anticancer, anti-inflammatory, and anti-diuretic activities [29, 30]. 9,12- Octadecadienoic acid, methyl ester has anti-inflammatory, anti-arthritic, hepatoprotective, antiandrogenic, hypocholesterolemic, nematicide, 5-alpha-reductase inhibitor, antihistaminic, anticoronary, insectifuge, antieczemic, and antiacne properties [31]. Methyl palmitate reported as antioxidant, hypocholesterolemic, nematicide, flavoring agents, hemolytic, and 5-alpha-reductase inhibitor [32]. Cycloheptasiloxane, tetradecamethyl- has antimicrobial, antiseptic, hair-conditioning agent, and skin-conditioning agent-emollient properties [33].
Conclusion
In the present study, Hibiscus asper leaves have shown to have various secondary metabolites which possess many pharmacological properties of which antioxidant activity is one. The GC-MS analysis showed the presence of 23 phytochemical constituents which contribute the activities like antimicrobial, antioxidant, anticancer, hypercholesterolemic, anti-inflammatory, and other activities. Hence, the presence of phytochemicals is responsible for their therapeutic effects. Further investigation is required for possible development of novel drugs using some of the bioactive compounds found in H. asper.
Availability of data and materials
All data and material are available upon request.
Abbreviations
- H. asper :
-
Hibiscus asper
- GC-MS:
-
Gas chromatography-mass spectroscopy
- AMFHAL:
-
Aqueous methanol fraction of Hibiscus asper leaves
- Ca2+ :
-
Calcium ion
- RT:
-
Retention time
References
Gopalakrishnan K, Udayakumar R (2014) GC-MS analysis of phytocompounds of leaf and stem of marsilea quadrifolia (L). Int J Biochem Res Rev 4(6):517–526
Patel DK (2015) Plant as a source of medicine. Med Aromat Plants S 3:1
Mahomoodally MF (2013) Traditional medicines in Africa: an appraisal of ten potent African medicinal plants. Evid Based Complementary Altern Med 1:1–14
Nisha K, Darshana M, Madhu G, Bhupendra MK (2011) GC-MS analysis and anti- microbial activity of Psidium guajava (leaves) grown in Malva region of India. Int J Drug Dev Res 3(4):237–245
Starlin T, Prabha PS, Thayakumar BKA, Gopalakrishnan VK (2019) Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. Biomed Inform 15(6):425–429
Vasudeva N, Sharma SK (2008) Biologically active compounds from the genus Hibiscus. Pharm Biol 46:145–153
Schippers RR, Bosch CH (2004) Hibiscus asper hook F. In: Grubben GJH, Denton OA (eds) PROTA (plant resources of tropical Africa/Ressources végétales de l’Afrique tropicale), vol 2004, Wageningen, pp 314–315
Foyet HS, Abdou BA, Ponka R, Asongalem AE, Kamtchouing P, Nastasa V (2011) Effects of Hibiscus asper leaves extracts on carrageenan induced oedema and complete Freund’s adjuvant-induced arthritis in rats. J Cell Anim Biol 5(5):69–75
Lucian H, Veronica B, Harquin SF, Alin C, Ionela LS, Daniel T, Emil A (2014) Antioxidative effects of the methanolic extract of Hibiscus asper leaves in mice. Rom Biotechnol Lett 19(3):9376–9383
Uma G, Balasubramaniam V (2012) GC-MS analysis of Nothapodytes nimmoniana, Mabberly leaves. J Chem Pharm 4(9):4417–4419
Héthelyi E, Tétényi P, Dabi E, Dános B (1987) The role of mass spectrometry in medicinal plant research. Biomed Environ Mass Spectrom 14(11):627–632
Kupchan SM, Tsou G (1973) Tumor inhibitors: a new antileukemic simaroubolid from Brucea antidysenterica. J Organomet Chem 38:178–179
Wagenen BCV, Larsen R, Cardellina JH, Ran-Dazzo D, Lidert ZC, Swithenbank C (1993) Ulosantoin, a potent insecticide from the sponge Ulosa ruetzleri. J Organomet Chem 58:335–337
Suganya R, Thangaraj M (2014) Isolation and characterization of leaf extract of Derris trifoliate. Int J ChemTech Res 6(9):4115–4122
Harborne JB (1973) Phytochemical methods: a guide to modern techniques of plant analysis, 1st edn. Chapman and Hall, London, p 279
Trease GE, Evans WC (2002) Pharmacognosy, 15th edn. Saunders, London, pp 42–393
Harborne JB (1998) Phytochemical methods: a guide to modern technique of plant analysis, vol 3. Champman and Hall, London, pp 60–66
Soni A, Sosa S (2013) Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. J Pharmacogn Phytochem 2(4):22–24
Saeed N, Khan MR, Shabbir M (2012) Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC complement Altern. Med 12(1):221–232
Sharath SS, Preethy J, Kumar GS (2015) Screening for anti-ulcer activity of Convolvulus pluricaulis using pyloric ligation method in Wistar rats. Int J Pharm Sci 6(1):89–99
Abebaw M, Mishra B, Gelayee DA (2017) Evaluation of anti-ulcer activity of the leaf extract of Osyris quadripartita Decne (Santalaceae) in rats. J Exp Pharmacol 9:1–11
Stoclet JC, Schini-Kerth V (2011) Dietary flavonoids and human health. Ann Pharmacother 69:78–90
Cao J, Zhang Y, Chen W, Zhao X (2010) The relationship between fasting plasma concentrations of selected flavonoids and their ordinary dietary intake. Br J Nutr 103(2):249–255
Njoku UO, Nwodo OFC, Ogugofor MO (2017) Cardioprotective potential of methanol extract of Costus afer leaf on carbon tetrachloride-induced cardiotoxicity in albino rats. Asian J Pharm Res Health Care 9(2):51–58
Lecour S, Lamont KT (2011) Natural polyphenols and cardioprotection. Mini-Rev Med Chem 11(14):1191–1199
Abdelhak R, Soraya B (2018) Phytochemical characterization, anti-inflammatory and anti-ulcer activity of a spontaneous succulent Delosperma reseii. Univers J Agr Res 6(3):113–117
Sreeja PS, Arunachalam K, Saikumar S, Kasipandi M, Dhivya S, Murugan R, Parimelazhagan T (2018) Gastroprotective effect and mode of action of methanol extract of Sphenodesme involucrata var. paniculata (C.B. Clarke) Munir (Lamiaceae) leaves on experimental gastric ulcer models. Biomed Pharmacother 97:1109–1118
Alves-Silva JM, Zuzarte M, Marques C, Ligia S, Girao H (2016) Protective effects of terpenes on the cardiovascular system: current advances and future perspectives. Curr Med Chem 23(40):1–42
Kumar PP, Kumaravel P, Lalitha C (2010) Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr J Biomed Res 4(7):191–195
Banjare J, Salunke M, Indapurkar K, Ghate U, Bhalerao S (2017) Estimation of serum malondialdehyde as a marker of lipid peroxidation in medical students undergoing examination-induced psychological stress. J Sci Soc 44:137–139
Nishanthini A, Mohan VR, Jeeva S (2014) Phytochemical, FT-IR, and GC-MS analysis of stem and leaf of Tiliacora acuminata (lan.) hook f and Thomas (menispermaceae). Int J Pharm Sci Res 5(9):3977–3986
Rajeswari G, Murugan M, Mohan VR (2013) GC-MS analysis of bioactive components of Hugonia mystax L. bark (Linaceae). J Pharm Biomed Sci 29:818–824
Mary APF, Giri RS (2018) GC-MS analysis of bioactive compounds of Achyranthes aspera. World J Pharm Res 7(1):1045–1056
Acknowledgements
The authors wish to acknowledge Mr. Felix Nwafor who authenticated the plant material, and Mr. Mbaoji of Department of Pure and Industrial Chemistry, University of Nigeria Nsukka, for their assistance.
Plant authentication
The leaves were identified and authenticated by Mr. Felix Nwafor of Pharmacognosy and Environmental Medicine Department, University of Nigeria Nsukka. The plant was deposited in the herbarium of Department of Pharmacognosy and Environmental Medicine, University of Nigeria Nsukka, with the voucher number PCG/UNN/0350.
Funding
No funding received
Author information
Authors and Affiliations
Contributions
All the authors contributed in the design of the study. NUO and OMO sourced the plant materials, while UCG dried and extracted the plant material. All the authors contributed in the fractionation of the plant extract. All the authors contributed in the phytochemical profiling of the plant fraction. NUO and OMO contributed in the GC-MS evaluation of the plant sample, and in the interpretation of the results. All the authors contributed in preparing the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing interest.
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Olivia, N.U., Goodness, U.C. & Obinna, O.M. Phytochemical profiling and GC-MS analysis of aqueous methanol fraction of Hibiscus asper leaves. Futur J Pharm Sci 7, 59 (2021). https://doi.org/10.1186/s43094-021-00208-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-021-00208-4