Abstract
Background
Monkeypox virus (MPV), an endemic pathogen in Africa, shares clinical similarities with smallpox. Recent reports indicate a concerning increase in the number of MPV cases detected outside its endemic region, highlighting the emergence of a multi-country outbreak. Given the importance of the cell surface-binding protein E8L in facilitating viral attachment to host cells, this study aimed to identify potential small interfering RNAs (siRNAs) capable of silencing E8L and thereby serving as a basis for therapeutic development.
Results
siRNAs have emerged as promising candidates for genetic therapies and antiviral and antibacterial treatments. In this investigation, we employed computational assays, including GC content analysis, binding free energy assessment, folding properties evaluation, melting temperature determination, and siRNA efficacy prediction. Our comprehensive analysis identified five siRNAs with high potential for effectively silencing the cell surface-binding protein of the monkeypox virus. Among these siRNAs, molecular docking revealed that “S8” (Guide-UUAUGGAUCCAAUCACUUGAU, Passenger-CAAGUGAUUGGAUCCAUAAUC) demonstrated the strongest affinity with the human argonaute-2 protein.
Conclusions
The siRNA “S8” represents a promising therapeutic target for developing treatments against monkeypox virus infection by specifically silencing the cell surface-binding protein E8L. Our research lays the foundation for future endeavors in genome-level therapies. It can potentially create chemically produced RNA molecules as effective antiviral drugs targeting Monkeypox virus infection. These findings contribute to advancing therapeutic strategies and offer new avenues for combating the spread of MPV, particularly in regions affected by the multi-country outbreak.
Similar content being viewed by others
1 Background
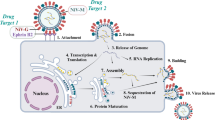
The Orthopoxvirus genus within the Poxviridae family consists of several human pathogens, including cowpox (CPXV), Vaccinia (VACV), monkeypox (MPV), and Variola (VARV) viruses. Among these, the scientific community is particularly concerned about the recent global outbreak of monkeypox viruses. Monkeypox is a viral illness that closely resembles smallpox, causing symptoms such as fever, muscle aches, and blistering [1]. In May 2022, the Monkeypox virus was identified as the primary cause of a widespread epidemic spanning multiple countries, with approximately one hundred distinct cases reported outside of Africa's endemic regions [1]. Although research is still in its preliminary stages, there are indications that the monkeypox virus (MPV) could pose a significant public health threat in the USA and worldwide, particularly in the aftermath of the COVID-19 pandemic.
The Monkeypox and Variola viruses are members of the Orthopoxvirus family, which are double-stranded DNA viruses. Monkeypox is responsible for the disease of the same name, while smallpox is caused by the Variola virus. Remarkably, smallpox was successfully eradicated worldwide in 1980, but monkeypox continues to be prevalent in Sub-Saharan Africa [2]. Beyond Africa, monkeypox has been reported in nearly a hundred cases across 12 countries, including Australia, Belgium, Canada, France, Germany, Italy, the Netherlands, Portugal, Spain, Sweden, the United Kingdom, and the USA [3, 4]. It is worth noting that occurrences of monkeypox outside of Africa have been documented since 2003. However, the current outbreak stands out as unprecedented due to the substantial number of affected individuals [5,6,7].
There are two distinct genetic clades of the Monkeypox virus, known as the West African clade and the Central African (or Congolese) clade. Both clades demonstrate similar clinical and pathological characteristics, as documented in references [8, 9]. The World Health Organization has reported on a concerning situation, titled "Multi-country monkeypox epidemic in non-endemic nations" [10]. According to this article, as of May 21, there have been a total of 92 laboratory confirmed cases of Monkeypox virus in twelve countries where it is not commonly found. Notably, the cases confirmed through PCR testing belong to the MPV lineage observed in West Africa.
The orthopoxviridae have various distinguishing characteristics. For example, they have the biggest genome (186 kilobase pairs for VARV) and the most genes (200 open reading frames) [11]. In terms of etiology and genetic measures, the Monkeypox virus is thought to be closely linked to the Variola and Cowpox viruses [12,13,14]. The MPXV genome is made up of 197 kb of linear double-stranded DNA that is covalently connected at its terminals by palindromic hairpins, and the inverted terminal repeats (ITRs) are made up of hairpin loops, tandem repeats, and some open reading frames (ORFs) that include 191 non-overlapping genes [15].
RNA interference (RNAi) is a natural biological process that regulates gene expression by suppressing mRNA through post-transcriptional gene silencing. This mechanism shows great potential in combating human viral infections [16, 17]. Within the RNAi pathway, small interfering RNAs (siRNAs) play a crucial role. They bind to complementary mRNA molecules and effectively neutralize them, leading to the suppression of gene expression [18]. Typically, siRNAs consist of a 19–25 base pair long RNA duplex with two nucleotide overhangs at the 3' end. Through their attachment to target mRNA, siRNAs initiate post-translational gene silencing (PTGS), resulting in enzymatic degradation of the mRNA molecule [19].
The process of gene expression suppression through siRNA is a complex phenomenon. Once the siRNA duplex enters the cell, it undergoes a series of intricate steps. To begin with, a dicer enzyme, resembling RNase III, initiates the breakdown of the siRNA duplex. Subsequently, the resulting fragments integrate into the RNA-induced silencing complex (RISC), a protein complex [20, 21]. Within RISC, the RNA helicase domain, which relies on ATP, carries out the task of separating the RNA strands. The sense strand undergoes degradation within RISC, while the antisense single-stranded RNA facilitates RISC's alignment with the target mRNA. This alignment triggers the catalytic activity of RISC, particularly through an argonaut protein, leading to the cleavage of the target mRNA strands [22]. In summary, the suppression of gene expression by siRNA involves a complex series of events, starting from siRNA entry into the cell, dicer-mediated breakdown, integration into RISC, strand separation, and ultimately, the slicing of the target mRNA by the catalytic RISC protein.
In our research, we have discovered siRNAs that target the Monkeypox virus, suggesting their potential to effectively silence genes of related viruses in living organisms. Currently, there are no officially approved vaccines or drugs available for treating the Monkeypox virus. However, dedicated scientists are actively working toward developing a targeted treatment for this virus. For our study, we focused on a specific protein found on the surface of Monkeypox virus cells, known as the chondroitin sulfate (CS) binding protein. This protein is well-characterized, located on the viral membrane, and plays a vital role in viral infection [23].
Furthermore, binding to cell-surface carbohydrates is required for poxvirus cell infection. In addition, blocking the CS binding protein (CSBP) found on Vaccinia intracellular mature virus reduces infectivity [24]. Thus, the characteristics that make chondroitin sulfate binding proteins (CSBPs) excellent candidates for the creation of affinity probes also make them viable targets for treatments development. Many prior research employed similar antiviral techniques against other orthopoxviruses, such as inhibiting viral interaction to cell receptors and HIV [25,26,27]. We hypothesized this study will aid in the development of a similar therapeutic technique for this viruses in vivo.
2 Methods
The complete methodology for predicting the potential siRNA molecules against Monkeypox virus is shown in Fig. 1.
2.1 Sequence retrieval of CDS of cell surface binding protein
The genomic sequence of cell surface binding protein was obtained from the NCBI virus database under the accession number NC_063383, which was reported by Mauldin,M.R., et al. This is the refseq of monekypox virus whose Geo Location is Nigeria: Rivers State. Then, we identified the CDS of E8L or cell surface chondroitin sulfate gene from the whole mRNA sequence. This is the cell surface binding protein gene of Monkeypox virus. We then selected and saved the CDS of this gene for further siRNA prediction analysis.
2.2 Designing of siRNA from the CDS of cell surface binding protein
To identify potential siRNA molecules derived from the coding sequences (CDS) of the cell surface binding protein, we utilized the siDirect version 2.0 webserver [28]. The process involved inputting the fasta sequence of the cell surface binding protein CDS into the siDirect webserver, which employed three distinct rules for siRNA prediction: Ui-Tei, Amarzguioui, and Reynolds [29,30,31]. By default, the webserver ensured that the melting temperature (Tm) of the seed duplex was below 21 °C, optimizing siRNA efficacy and minimizing off-target effects [28]. The Ui-Tei algorithm applies specific rules to identify potential siRNAs: (1) the 5′ terminus of the antisense/guide strand should contain an A/U nucleotide, (2) the 5′ end of the sense/passenger strand must have a G/C nucleotide, (3) the 5′ terminal 7 base pairs of the sense/passenger strand should include at least 4 A/U nucleotides, and (4) the GC stretch should not exceed 9 nucleotides [29]. On the other hand, the Amarzguioui rules encompass the following parameters: (1) strong binding of the 5′ sense/passenger strand, (2) a positive A/U differential at the duplex end, (3) an A at position 6, (4) any base except U at position 1, (5) weak binding of the 3′ sense/passenger strand, and (6) any base except G at position 19 [30]. The Reynolds algorithm follows several criteria: (1) the sense/passenger strand must maintain ≥ 3 base pairs between positions 15 and 19, (2) the designed siRNA should have a GC content between 30 and 52%, (3) positions 19 and 3 of the sense/passenger strand must contain an A, (4) low internal stability at the target site, (5) a U at position 10 of the sense/passenger strand, and (6) any base except G at position 13 of the sense/passenger strand [31]. By utilizing these three algorithms, we aimed to design effective siRNAs for targeting the cell surface binding protein CDS. Investigation of parameters for siRNA refinement.
To identify the most effective siRNAs from a large pool initially obtained through the siDirect webserver, we applied various refinement techniques to select highly potent siRNAs. Initially, we assessed the GC content of the siRNA molecules using the OligoCalc webserver [32]. Any siRNAs with a GC content below 30% were excluded from the study. Next, we used the RNA structure website [33] to predict the secondary structure and free energy of folding of the siRNAs. SiRNAs with a negative free energy of folding, as predicted by the webserver, were removed from further analysis. To evaluate the RNA-RNA interaction between the target and guide strands of the siRNAs, we employed the Bifold tool from the RNA structure webserver [33]. A stronger interaction between the target and guide strands indicated higher siRNA efficacy. Additionally, we generated heat capacity and concentration plots using the DINA Melt webserver [34]. The detailed heat capacity figure displayed the ensemble heat capacity (Cp) as a function of temperature, with the melting temperature Tm (Cp) indicated. The concentration plot helped us determine the melting temperature Tm (Conc), which corresponds to the point where the concentration of double-stranded molecules is half of its maximum value. Lastly, we utilized the SMEpred webserver [35] to further analyze the selected siRNAs and refine our pool of candidates.
SMEpred is a website specifically designed for predicting the efficiency of chemically modified siRNAs and has been validated using different datasets, including a normal siRNA dataset (2182) and a cm-siRNA dataset (3031 cm-siRNAs), both of which have been experimentally validated. In addition, we used the SVM algorithm and performed a tenfold cross-validation using SMEpred. Overall, these refinement procedures enabled us to identify the most effective siRNAs from the initial pool, incorporating considerations such as GC content, secondary structure, thermodynamic interaction, and chemically modified siRNA prediction.
2.3 Conservancy checking against other strains and human genomic transcript
In the final step of siRNA prediction, a conservancy checking was performed against the 83 strains of 2022 monkeypox outbreak through NCBI Blastn search and multiple sequence alignment through CLC Drug Discovery Workbench 3.0 software [36, 37]. In the NCBI Blastn database, we manually uploaded the CDS of cell surface binding protein of all strains and all other parameters were selected as default for Blast search. For phylogenetic tree construction, we employed neighbor joining phylogenetic tree with a bootstrap value of 500. Jukes-Cantor nucleotide distance measurement was selected while constructing the phylogenetic tree [38]. CLC Drug Discovery Workbench 3.0 software was used for alignment and phylogenetic tree construction [39]. Finally, we did a single blast analysis in NCBI to compare the resulting siRNAs to human genomic transcripts. The e-value was adjusted to 1e−10 in order to lessen the stringency of the search criterion and hence increase the likelihood of arbitrary matches.
2.4 Molecular docking of guide siRNA and argonaute-2 protein
The right interaction between siRNA duplex (primarily guide strand) and RISC complex protein (mostly human argonaute protein) is required to initiate an adequate antiviral response via RNAi-mediated viral gene silencing [40]. Molecular docking of the siRNA guide strand with argonaute-2 protein was conducted with HDOCK webserver [41]. Before molecular docking, we predicted the 3D model of the siRNA’s and argonaute-2 protein. For identifying the 3D structure of human argonaute-2 Robetta webserver was used [42]. This is a homology modeling webserver that employs deep learning algorithms, RoseTTAFold and TrRosetta, as well as an integrated reporting facility for specific sequence alignments for homology modeling. For predicting the 3D structure of siRNA guide strand, we used Mfold and RNA Composer webserver [43]. The mfold web server, which is used to calculate the folding pattern of DNA/RNA at 37 °C, is one of the oldest known online servers in computational molecular biology. The RNAComposer system, on the other hand, provides a new user-friendly technique to fully autonomous modeling of huge RNA 3D structures. The method relies on the automatic translation concept and uses the RNA FRABASE database as a lexicon to connect RNA secondary and tertiary design components. Finally, after modeling of the guide siRNA and human argonaute-2 protein, we docked the siRNA with RISC complex (argonaute-2) through molecular docking. After docking we visualize the interaction pattern through PDBsum webserver and discovery studio visualizer [44]. PDBsum is a web server that provides structural information on Protein Data Bank entries (PDB). Protein secondary structure, protein ligand and proteinDNA interactions, PROCHECK structural quality evaluations, and other image-based analysis are all included in the PDBsum analysis.
3 Results
3.1 Sequence retrieval and 35 siRNA prediction through siDirect
The complete CDS (coding sequence) of monkeypox cell surface binding protein was retrieved from the NCBI virus webserver (refseq ID: NC_063383). Here, we selected the refseq strain of the virus (Geo Location: Nigeria: Rivers State) for siRNA design. After that siDirect webserver was used to identify the potential siRNA’s from CDS of cell surface binding protein. siDirect used several parameters including Ui-Tei, Renold and Amarguioui rules to identify potential siRNA’s with melting temperature below 21.5 °C to reduce the seed dependent off-target binding. Initially, siDirect webserver predicted 35 potential siRNA’s from CDS of omicron spike protein. We, then, filtered this 35 siRNA’s to 10 siRNa’s by combining the three parameters (Ui-Tei, Renold and Amarguioui rules) and by also selected those siRNA’s whose melting temperature is below 21.5 °C. So, this 10 siRNA’s are highly off target reduced siRNA’s (Table 1).
3.2 GC content calculation of the predicted 10 siRNA’s
The exact amount of GC content in the predicted 10 siRNA molecules were identified though GC-content calculator. It is generally recommended to pick siRNA sequences with low GC content (between 30 and 52%) [45]. This is because smaller GC content may limit the efficacy of target mRNA identification and hybridization. On the contrary, higher GC content may cause to take longer time to unwind the siRNA duplex [30]. GC content of all our ten siRNA’s predicted in the range of 26.1–39.1%. We then filtered the rest of the siRNA’s whose GC content is under 30% from this study. This subsequently reduces our siRNA’s from 10 to 8 (Table 2).
3.3 Secondary structure prediction of the 8 siRNA’s
The calculated free energy of folding as well as the secondary structure of the 8 siRNA’s was predicted through RNA Structure webserver. According to previous research, an RNA molecule should have the highest free energy of folding [46]. Study found that formation of secondary structure in siRNA molecules owing to lower folding free energy may prevent target cleavage by RISC complex [46]. That’s why free energy of folding with associate secondary structure prediction is crucial for functional siRNA selection. In our study, the calculated free energy of folding of the 8 siRNA’s ranged from − 0.1 to 1.8 (Table 3). Among them two siRNA’s molecule (S4 and S10) has no pair calculated by the webserver. We then filtered those non-pair and negative free energy of folding molecules from this study. This ultimately resulted in 5 siRNA molecule after analysis (Fig. 2).
3.4 Computation of RNA-RNA binding, heat capacity, concentration plot, and validation
The RNA structure webserver was utilized to compute the free energy of hybridization between the guide and target strand of the last five siRNAs. The efficacy of RNA interference (RNAi) is closely associated with the binding energies of siRNAs to their corresponding target mRNAs. Therefore, the free energy of binding with the target (referred to as computational RNA-RNA interaction) serves as a significant metric [47]. A lower binding energy indicates stronger interactions, thereby increasing the likelihood of inhibiting the target. In our study, all five siRNAs from the previous analysis exhibited improved hybridization efficacy, with binding energies ranging between − 29.5 and − 34.5 (as depicted in Table 4 and Fig. 3). This further confirms that the guide strand of these five siRNAs could effectively hybridize with the target strand in the mRNA.
Subsequently, following the binding energy calculation, we determined the heat capacity (TmCp) and duplex concentration (TmConc). The higher values of Tm (Cp) and Tm (Conc) also indicate the enhanced effectiveness of the siRNAs. The heat capacity plot represents Cp as a function of temperature, while TmCp is denoted when Cp is a function of Tm. Similarly, the concentration plot illustrates mole fractions as a function of temperature, with Tm (Conc) representing the point where the concentration of the double-stranded molecule is half of its maximum value [34]. The DINAMelt web server was employed to calculate the complete equilibrium melting profiles across a range of temperatures. In our case, the higher the TmCp and Tm (Conc) values, the more efficient the RNAi molecules were, as evidenced by the significant melting profiles listed in Table 4. Finally, we validated the efficacy of both siRNA molecules by assessing their effectiveness using the SMEpred webserver. All five siRNAs demonstrated the highest inhibitory efficacy as calculated by the SMEpred webserver, as presented in Table 4.
3.5 Calculation of off-target effect and conservancy search against other strains
Despite the fact that our siRNAS has reduced off-target binding (as the seed-duplex Tm of all of these siRNA’s is under 21.5 °C), we BLASTn the final two siRNAs against the human genomic transcript to find out possible homology. This finding revealed that our projected siRNAs are unique and have no link to any human genomic target.
After, that, we employed conservancy analysis of the 5 siRNA molecules target against 83 strains of monkeypox virus from recent outbreak of 2022 through multiple sequence alignment and NCBI Blast search. All of the siRNA molecule’s target except S2 and S8 molecules showed 100% conservancy after conservancy analysis (Table 4 and Fig. 4). S2 and S8 molecules showed 98.80% conservancy (out of 83 sequences, 82 sequences matched with target). We also build a phylogenetic tree of the 83 strains for cell surface binding protein coding sequences. No strains showed any significant divergence after tree analysis (bootstrap value ≥ 100%) (Fig. 5). These results stated that our predicted siRNA’s are mostly conserved among the other strains of recent monkeypox outbreak.
3.6 Molecular modeling and docking analysis of final siRNA’s and Ago2
Molecular modeling of the final siRNA molecules was conducted by Mfold and RNA Composer webserver. First of all we imputed the five guide siRNA sequences in the Mfold webserver to form the RNAdraw format. Then we used this RNAdraw format in the RNA Composer webserver to compose the final 3D structure of the final siRNA molecules. After designing the 3D models of siRNA’s, we modeled the 3D structure of the human Ago2 (argonaute 2) protein through Robetta homology modeling webserver. We used the refseq sequence of the human Ago2 e.g., UniprotKB: Q9UKV8 to model the Ago2 protein. The template for the homology modeling selected was 4Z4D crystal structure (Human argonaute protein bound to t1-G target RNA) as this protein showed maximum sequence similarity with our Ago2 sequence. The modeled protein was then refined with the GalaxyRefine webserver. The quality of the model was checked using Ramachandran plot analysis of ZLab webserver [48]. Ramachandran analysis of Ago2 3D structure revealed good plot with 99.062% residues laid in the highly preferred observation. Only 0.938% residues laid in the preferred region and no residues found lied in the questionable region (Fig. 6).
Finally, molecular docking of the final five siRNA molecule and human Ago2 was conducted by HDOCK webserver (Fig. 7). Human Ago2 is selected for docking as targeting the coding sequence (CDS) with siRNA is suggested for modulating transcript levels via Argonaute 2 (Ago2) mediated transcript cleavage. And complementary siRNA targeting the 3′untranslated region (UTR) of mRNA causes translational repression, which is mediated by Ago1, Ago3, and Ago4 [45]. As we have targeted the CDS of Monkeypox virus, that’s why we docked our siRNA’s with human Ago2 protein [49, 50]. In addition, we also docked a 20nt length RNA (UUCACAUUGCCCAAGUCUUU) with our Ago2 receptor as a control. The RNA that was used as a control is the ligand of human argonaute-2 protein of PDB ID: 4Z4D. We docked this 20nt RNA with our modeled Ago2 protein to find out if the docking is successful or not. Our docking analysis through HDOCK docking server revealed control RNA binds in the same pocket (Energy: − 1081.52, ligand RMSD: 0.21) as resembled to 4Z4D Human Argonaute2 with t1-G Target RNA (Table 5). After docking of the control RNA we docked the five siRNA molecules with our modeled human Ago2 protein. Among the five docked complex, siRNA complex S8-Ago2 showed lowest docking energy (− 408.24) with lowest ligand RMSD (27.29). The others complex also showed docking energy lower than − 300 except complex no S6 (Table 5). We then selected this best docked complex (S8) for interaction analysis through Discovery studio and PDBsum webserver. Interaction analysis with receptor protein showed that our predicted siRNA molecule S8 binds in the same cavity as like as the control and spanning mostly between the PAZ and PIWI domain of Ago2 (Fig. 8 and Table 6). The interacting residues are also found similar to the control RNA bound with Ago2 e.g., LYS65, CYS66, PRO67, LYS124, ASP125, ARG277, LYS278, TYR279, PHE294, TYR311, ARG315, GLN332, LYS335, HIS336, THR337, ARG351, ILE365, THR368, LYS525, LYS550, VAL598, THR599, LYS709, ARG710, HIS753, GLN757, GLY758, THR759, SER760, ARG761, TYR790, ARG792, CYS793, ARG795, VAL797, SER798, TYR804, PHE811, TYR815. Some residues of the docked complex are also found similar with previously reported studies which are ARG277, ARG315, ARG351, ILE353, ILE365, LYS709, ARG710, GLN757, THR759, ARG761, ARG792, SER798, TYR804 [51,52,53]. So, it can be stated that these residues are conserved for binding of the siRNA’s with human Ago2 protein. While identifying promising siRNA candidates in silico represents a significant step toward developing therapeutic interventions against the Monkeypox virus, several challenges remain to be addressed for successful translation into clinical applications. These include siRNA instability within biological fluids, limited cellular absorption by target cells, and the need for reliable and targeted delivery methods. To overcome these hurdles, future research will focus on investigating the efficacy of various siRNA modifications, such as 2'-O-methyl or LNA, to enhance their stability and in vivo persistence. Additionally, the application of diverse delivery systems, including liposomes, nanoparticles, and cell-penetrating peptides, will be explored to facilitate efficient cellular uptake of the siRNAs. Furthermore, strategies such as ligand conjugation and microfluidic devices will be investigated for targeted delivery to specific cell types infected by the Monkeypox virus. Through a multifaceted approach combining in vitro and in vivo studies, we aim to identify the most promising siRNA modifications and delivery methods, paving the way for the development of effective siRNA-based therapies against the Monkeypox virus.
4 Discussion
The Poxviridae family's Orthopoxvirus genus contains multiple human diseases, including monkeypox (MPV), Vaccinia (VACV), cowpox (CPXV), and Variola (VARV) viruses [54]. Vaccination is now the only way to protect against exposure with these viruses, and no authorized antiviral medication therapy is accessible [54]. Monkeypox virus (MPV) was first isolated in cynomolgus monkeys bred in laboratories [55]. The virus is thought to be propagating throughout central and western Africa for a long period in a variety of animal hosts, including squirrels. Early human infestations with MPV, which were discovered in Zaire and later in Liberia and Sierra Leone, were caused by exposure to infected animals [56]. However, individual transmission has lately been reported [57]. Monkeypox illness symptoms are comparable to smallpox, but with fewer mortality and a more concentrated pustular rash distribution [58]. Because of the ceasing of smallpox vaccination in the early 1980s, contemporary public immunity to poxviruses is termed non-protective, and younger generations are called immunological naive [59]. There are currently no licensed medications to treat poxvirus infections, and the use of antiviral Cidofovir and ST-246 could slowly decline as resistant viral strains evolve or therapeutic adverse effects are detected [60,61,62,63]. As a result, there is an urgent need for more effective medications and unique therapy procedures that can survive field application conditions. Furthermore, recently the outbreak of Monkeypox virus The World Health Organization just published a paper entitled "Multi-country monkeypox epidemic in non-endemic countries" that describes the current scenario [10]. According to the post, as of May 21, 13:00, "92 laboratory confirmed cases of MPV also have been submitted to WHO" from twelve countries where the virus is not prevalent. So far, the majority of these cases actually have been reported in Western Europe and North America. According to the WHO article, the cases "confirmed by PCR" are from the MPV lineage seen in West African clade. So, an updated and new protecting method is necessary against the virus and which is our concern.
In this study, we have used the RNAi interference technology to identify the possible siRNA’s against the Monkeypox virus E8L or cell surface binding protein gene. Because viruses have relatively tiny genomes with a limited number of targetable genes, the utilization of the RNAi pathway as a new method in antiviral drug discovery is especially promising. Furthermore, the genetic difference among mammalian and viral genomes offers a benefits in terms of decreasing off-target hits and adverse effects [64]. RNAi studies have being very useful in understanding gene functions in a wide range of prokaryotic and eukaryotic cells [65]. Recent developments in siRNA delivery technologies have also emphasized the RNAi pathway's enormous value as a therapeutic option for infectious, neurological, cancer, and genetic disorders [66]. Thus, in this study we thus identified the possible siRNA’s against the Monkeypox virus by analyzing through different webservers and molecular docking. We also did a literature review of the previous work of siRNA prediction against other orthopox viruses. In a 2009 study, Abdulnaser Alkhalil and his colleagues reported the efficiency of RNA interference in inhibiting Monkeypox viral reproduction [54]. They discovered two siRNA pools with significant antiviral characteristics that reduced virus replication to less than 10% of its proliferation in control untreated cells. They targeted the E8L and A6R gene of Monkeypox virus and finally identified 4 siRNA’s for each of the gene. Among them two siRNA’s of E8L gene e.g., siE8L-a (CGACAATAGTGTTCTGAAT) and siE8L-d (GAATAGCGGTGAGTATAAA) is also found in our study (S2 and S4). They showed that siE8L-d severly disrupted MPV replication in their study. Furthermore, another study against Vaccinia virus double-stranded RNA binding protein [E3L] displays significant antiviral activities which was published in 2006 [67]. Rajnish S. Dave et al. targeted the E3L gene of vaccinia virus for siRNA prediction in the study, and their data show that E3L-C siRNAs are capable of suppressing Vaccinia viral replication by 97% and 98%, respectively, when compared with the control infection in two human cell types, HeLa and 293T cells [67]. In another study, Solenne vignee demonstrated the selective suppression of orthopoxvirus replication by a short interfering RNA targeting the D5R gene [68]. They discovered that whether applied prophylactically or therapeutically, a 100 nM siRNA (siD5R-2) targeting the D5 protein reduced vaccinia virus strain western reserve (VACVWR) multiplication by up to 90% in human lung cancer A549 cells. At a dose of 100 Nm, this siRNA inhibited VACVWR replication in a concentration-dependent manner and had a 72-h preventive antiviral effect. They also confirmed siD5R-2's antiviral effectiveness against additional harmful orthopoxviruses including cowpox and monkeypox, which were suppressed up to 70% at the lowest dose (1 nM). In another work, Solenne Vigne et al. [69] employed RNA interference, by itself or in conjunction with cidofovir, to limit orthopoxvirus replication. They employed plaque reduction and virus yield tests to test the antiviral efficacy of two chosen small interfering RNAs (siRNAs) designated siB1R-2 and siG7L-1, as well as a previously published siRNA, siD5R-2, against vaccinia virus (VACV). They discovered that siB1R-2 and siG7L-1, when given before to or after viral infection, inhibited VACV multiplication by more than 90%. Furthermore, at a dose of 1 nM, these two siRNAs reduced monkeypox viral multiplication by 95%. Also when siB1R-2, siG7L-1, or siD5R-2 was coupled with cidofovir, strong synergistic effects were seen. This finding shown that siRNAs are effective in vitro inhibitors of not only wild-type VACV but also numerous cidofovir-resistant VACV.
The COVID-19 pandemic stressed the significance of developing effective vaccinations quickly. While research is still in its early stages, the monkeypox virus (MPV) has the potential to become a serious public health hazard in the USA and throughout the world. Today, there are various vaccinations available now that give some protection against monkeypox as well as smallpox. However, a newer smallpox vaccine (MVA-BN, also known as Imvamune, Imvanex, or Jynneos) was authorized in 2019 for use in treating monkeypox but is not yet readily accessible [70]. Vaccines for smallpox and JYNNEOS provide protection against MPV, although vaccine development should continue because to vaccination escape. Furthermore, because smallpox immunization was discontinued in 1980, many younger individuals were never even immunized and so lack immunity [70]. That is why a newer protection method is necessary against the Monkeypox virus. Although fewer studies is now begin to conduct against the monkepox virus, (For example, Andrew Gao et al identified non-cross reactive epitopes for 2022 outbreak of Monkeypox cell surface binding protein) much more study is needed to fully protected against this virus [23]. Thus, our study could be a promising outcome in this sector.
However, the potential of siRNAs for targeted gene silencing in therapeutic applications may face various challenges, including siRNA instability, limited cellular absorption, and the absence of a reliable delivery method [71]. To enable effective gene therapy, a promoter-controlled vector can facilitate the transport of therapeutic genes to the intended cells [72]. In order to evaluate the effectiveness of newly generated siRNA, a vector-based siRNA in plasmid form can be employed to specifically target genes within a cell line [73]. In our research, we have identified potential siRNA molecules for RNAi activity against the cell surface binding protein of Monkeypox virus. Further in vitro investigations using vector-based approaches are required to assess the efficacy of our proposed siRNAs. We anticipate that our research will make a valuable contribution to this field. Ultimately, the discovery of this siRNA therapeutic approach could offer a promising alternative to traditional vaccine design in mitigating the spread of Monkeypox.
5 Conclusions
While the current study has identified potential siRNA candidates against the Monkeypox virus through in silico analysis, it is important to acknowledge the limitations inherent to such computational approaches. The identified siRNAs require further validation and testing in experimental settings, including cell culture and animal models, to confirm their efficacy and address concerns related to potential off-target effects, siRNA instability, and delivery efficiency. Despite these limitations, this research highlights the potential of siRNA technology as a promising avenue for developing novel therapeutic strategies against the Monkeypox virus. By targeting the E8L gene or the cell surface-binding protein gene, siRNAs offer a targeted approach with potentially reduced off-target effects compared to conventional antiviral drugs. This study builds upon prior research demonstrating the effectiveness of RNAi against poxviruses, adding to the growing body of evidence supporting its potential as a valuable tool in the fight against emerging viral threats. In light of the urgent need for effective interventions against the Monkeypox virus, further research is crucial to translate the findings of this study into practical applications. This includes in vitro validation using vector-based siRNA delivery systems, addressing challenges related to siRNA stability and delivery efficiency, and demonstrating efficacy in animal models. By overcoming these hurdles, siRNA-based therapeutics have the potential to offer a viable alternative to existing preventive measures, such as vaccination, and contribute significantly to the management of the Monkeypox pandemic.
Availability of data and materials
All data supporting the findings of this study are available within the article and its supplementary materials.
References
Giorgi FM et al (2022) Genomic characterization of the recent monkeypox outbreak
Parrino J, Graham BS (2006) Smallpox vaccines: past, present, and future. J Allergy Clin Immunol 118(6):1320–1326
Mahase E (2022) Seven monkeypox cases are confirmed in England. British Medical Journal Publishing Group
Quarleri J, Delpino M, Galvan VJG (2022) Monkeypox: considerations for the understanding and containment of the current outbreak in non-endemic countries. Geroscience 44:1–9
Bunge EM et al (2022) The changing epidemiology of human monkeypox—a potential threat? A systematic review. PLoS Negl Trop Dis 16(2):e0010141
Mauldin MR et al (2022) Exportation of monkeypox virus from the African continent. J Infect Dis 225(8):1367–1376
Velavan TP, Meyer CG (2022) Monkeypox 2022 outbreak: an update
Karumathil S et al (2018) Evolution of synonymous codon usage bias in west African and central African strains of monkeypox virus. Evol Bioinform 14:1176934318761368
Chen N et al (2005) Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 340(1):46–63
Multi-country monkeypox outbreak in non-endemic countries: Update. World Health Organization [cited September 5, 2022]. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON388.
Esposito JJ et al (2006) Genome sequence diversity and clues to the evolution of variola (smallpox) virus. Science 313(5788):807–812
Douglass N, Dumbell K (1992) Independent evolution of monkeypox and variola viruses. J Virol 66(12):7565–7567
Shchelkunov SN et al (1998) The genomic sequence analysis of the left and right species-specific terminal region of a cowpox virus strain reveals unique sequences and a cluster of intact ORFs for immunomodulatory and host range proteins. Virology 243(2):432–460
Shchelkunov SN et al (2001) Human monkeypox and smallpox viruses: genomic comparison. FEBS Lett 509(1):66–70
Kugelman JR et al (2014) Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis 20(2):232
Levanova A, Poranen MM (2018) RNA interference as a prospective tool for the control of human viral infections. Front Microbiol 9:2151
Sharif Shohan MU, Paul A, Hossain M (2018) Computational design of potential siRNA molecules for silencing nucleoprotein gene of rabies virus. Future Virol 13(3):159–170
Hamilton AJ, Baulcombe DCJS (1999) A species of small antisense RNA in posttranscriptional gene silencing in plants. Science 286(5441):950–952
Elbashir SM et al (2001) RNA interference is mediated by 21-and 22-nucleotide RNAs. Genes Dev 15(2):188–200
Bernstein E et al (2001) Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 409(6818):363–366
Hammond SM et al (2000) An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells. Nature 404(6775):293–296
Dana H et al (2017) Molecular mechanisms and biological functions of siRNA. Int J Biomed Sci IJBS 13(2):48
Gao A, Gao S (2022) In silico identification of non-cross-reactive epitopes for Monkeypox cell surface-binding protein
Schoeniger JS et al (2008) Structural basis of poxvirus interaction with cell-surface receptors and synthetic ligands. Sandia National Lab.(SNL-CA), Livermore, CA (United States)
De Clercq E (2001) Vaccinia virus inhibitors as a paradigm for the chemotherapy of poxvirus infections. Clin Microbiol Rev 14(2):382–397
De Clercq E et al (1994) Highly potent and selective inhibition of human immunodeficiency virus by the bicyclam derivative JM3100. Antimicrob Agents Chemother 38(4):668–674
Grande F, Garofalo A, Neamati N (2008) Small molecules anti-HIV therapeutics targeting CXCR4. Current Pharm Des 14(4):385–404
Naito Y et al (2009) siDirect 2.0: updated software for designing functional siRNA with reduced seed-dependent off-target effect. BMC Bioinform 10(1):1–8
Ui-Tei K et al (2004) Guidelines for the selection of highly effective siRNA sequences for mammalian and chick RNA interference. Nucleic Acids Res 32(3):936–948
Amarzguioui M, Prydz H (2004) An algorithm for selection of functional siRNA sequences. Biochem Biophys Res Commun 316(4):1050–1058
Reynolds A et al (2004) Rational siRNA design for RNA interference. Nat Biotechnol 22(3):326–330
Kibbe WA (2007) OligoCalc: an online oligonucleotide properties calculator. Nucleic Acids Res 35(suppl_1):W43–W46
Bellaousov S et al (2013) RNAstructure: web servers for RNA secondary structure prediction and analysis. Nucleic Acids Res 41(W1):W471–W474
Markham NR, Zuker M (2005) DINAMelt web server for nucleic acid melting prediction. Nucleic Acids Res 33(suppl_2):W577–W581
Dar SA et al (2016) SMEpred workbench: a web server for predicting efficacy of chemicallymodified siRNAs. RNA Biol 13(11):1144–1151
Camacho C et al (2009) BLAST+: architecture and applications. BMC Bioinform 10(1):1–9
Home - QIAGEN digital insights. [cited September 5, 2022]. https://digitalinsights.qiagen.com/.
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10(3):512–526
Tamura K et al (2021) MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38(7):3022–3027
Jana S, Chakraborty C, Nandi S (2004) Mechanisms and roles of the RNA-based gene silencing. Electron J Biotechnol 7(3):15–16
Yan Y et al (2020) The HDOCK server for integrated protein–protein docking. Nat Protoc 15(5):1829–1852
Kim DE, Chivian D, Baker D (2004) Protein structure prediction and analysis using the Robetta server. Nucleic Acids Res 32(2):W526–W531
Zuker M (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res 31(13):3406–3415
Laskowski RA et al (2018) PDBsum: structural summaries of PDB entries. Protein Sci 27(1):129–134
Safari F, Barouji SR, Tamaddon AM (2017) Strategies for improving siRNA-induced gene silencing efficiency. Adv Pharm Bull 7(4):603
Vickers TA, Wyatt JR, Freier SM (2000) Effects of RNA secondary structure on cellular antisense activity. Nucleic Acids Res 28(6):1340–1347
Schubert S et al (2005) Local RNA target structure influences siRNA efficacy: systematic analysis of intentionally designed binding regions. J Mol Biol 348(4):883–893
Anderson RJ et al (2005) Main-chain conformational tendencies of amino acids. Proteins Struct Funct Bioinform 60(4):679–689
Rivas FV et al (2005) Purified Argonaute2 and an siRNA form recombinant human RISC. Nat Struct Mol Biol 12(4):340–349
Su H et al (2009) Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev 23(3):304–317
Elkayam E et al (2012) The structure of human argonaute-2 in complex with miR-20a. Cell 150(1):100–110
Shawan MMAK et al (2021) Designing an effective therapeutic siRNA to silence RdRp gene of SARS-CoV-2. Infect Genet Evol 93:104951
Chowdhury UF et al (2021) A computational approach to design potential siRNA molecules as a prospective tool for silencing nucleocapsid phosphoprotein and surface glycoprotein gene of SARS-CoV-2. Genomics 113(1):331–343
Alkhalil A et al (2009) Inhibition of Monkeypox virus replication by RNA interference. Virol J 6(1):1–10
Magnus P et al (1959) A pox‐like disease in cynomolgus monkeys 46(2):156–176
Marennikova S et al (1972) Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull World Health Organ 46(5):599
Learned LA et al (2005) Extended interhuman transmission of monkeypox in a hospital community in the Republic of the Congo, 2003. Am J Trop Med Hyg 73(2):428–434
Breman JG et al (1980) Human monkeypox, 1970–79. Bull World Health Organ 58(2):165
Breman JG et al (1980) The confirmation and maintenance of smallpox eradication. World Health Organization
De Clercq E (2007) Acyclic nucleoside phosphonates: past, present and future: bridging chemistry to HIV, HBV, HCV, HPV, adeno-, herpes-, and poxvirus infections: the phosphonate bridge. Biochem Pharmacol 73(7):911–922
Becker MN et al (2008) Isolation and characterization of cidofovir resistant vaccinia viruses. Virol J 5(1):1–8
Yang G et al (2005) An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol 79(20):13139–13149
Broekema FI, Dikkers FG (2008) Side-effects of cidofovir in the treatment of recurrent respiratory papillomatosis. Eur Arch Oto-Rhino-Laryngol 265(8):871–879
Qiu S, Adema CM, Lane T (2005) A computational study of off-target effects of RNA interference. Nucleic Acids Res 33(6):1834–1847
Wang Q-C, Nie Q-H, Feng Z-H (2003) RNA interference: antiviral weapon and beyond. World J Gastroenterol WJG 9(8):1657
Nguyen T et al (2008) RNAi therapeutics: an update on delivery. Curr Opin Mol Ther 10(2):158–167
Dave RS et al (2006) siRNA targeting vaccinia virus double-stranded RNA binding protein [E3L] exerts potent antiviral effects. Virology 348(2):489–497
Vigne S et al (2008) Specific inhibition of orthopoxvirus replication by a small interfering RNA targeting the D5R gene. Antiviral Ther 13(3):357–368
Vigne S et al (2009) Inhibition of vaccinia virus replication by two small interfering RNAs targeting B1R and G7L genes and their synergistic combination with cidofovir. Antimicrob Agents Chemother 53(6):2579–2588
Monkeypox. World Health Organization [cited September 10, 2022]. https://www.who.int/news-room/questions-and-answers/item/monkeypox?gclid=CjwKCAjwqauVBhBGEiwAXOepkbjHW6NaRPj4BU4MCdQZmJQAY91cdy9ZSFYOXiVZ0l61AEhKJ4GyzBoCvFcQAvD_BwE.
Tanaka K et al (2010) Disulfide crosslinked stearoyl carrier peptides containing arginine and histidine enhance siRNA uptake and gene silencing. Int J Pharm 398(1–2):219–224
Glorioso J, DeLuca N, Fink DJ (1995) Development and application of herpes simplex virus vectors for human gene therapy. Annu Rev Microbiol 49:675–711
ElHefnawi M et al (2016) In silico design and experimental validation of siRNAs targeting conserved regions of multiple hepatitis C virus genotypes. PLoS ONE 11(7):e0159211
Acknowledgements
Not applicable.
Funding
No specific grant was received for this study.
Author information
Authors and Affiliations
Contributions
RI designed this study, AS and RI conducted this study and wrote the manuscript, MRU and NF revised the manuscript and helped with writing and editing.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Islam, R., Shahriar, A., Uddin, M.R. et al. Immunoinformatic and molecular docking approaches: siRNA prediction to silence cell surface binding protein of monkeypox virus. Beni-Suef Univ J Basic Appl Sci 13, 17 (2024). https://doi.org/10.1186/s43088-024-00472-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-024-00472-2