Abstract
Background
Neuroinflammation is a key pathological feature of a wide variety of neurological disorders, including Parkinson’s, multiple sclerosis, Alzheimer’s, and Huntington’s disease. While current treatments for these disorders are primarily symptomatic, there is a growing interest in developing new therapeutics that target the underlying neuroinflammatory processes.
Main body
Marine invertebrates, such as coral, sea urchins, starfish, sponges, and sea cucumbers, have been found to contain a wide variety of biologically active compounds that have demonstrated potential therapeutic properties. These compounds are known to target various key proteins and pathways in neuroinflammation, including 6-hydroxydopamine (OHDH), caspase-3 and caspase-9, p-Akt, p-ERK, p-P38, acetylcholinesterase (AChE), amyloid-β (Aβ), HSF-1, α-synuclein, cellular prion protein, advanced glycation end products (AGEs), paraquat (PQ), and mitochondria DJ-1.
Short conclusion
This review focuses on the current state of research on the neuroprotective effects of compounds found in marine invertebrates and the potential therapeutic implications of these findings for treating neuroinflammatory disorders. We also discussed the challenges and limitations of using marine-based compounds as therapeutics, such as sourcing and sustainability concerns, and the need for more preclinical and clinical studies to establish their efficacy and safety.
Graphical abstract

Similar content being viewed by others
1 Background
The process of neuroinflammation is intricate, encompassing the stimulation of immune cells and the discharge of inflammatory substances within the nervous system [1]. This process can destroy neurons and induce the progression of neurodegenerative diseases, such as Parkinson, multiple sclerosis, Alzheimer, and Huntington. Recently, diseases-related neuroinflammatory has become a serious problem. More than 50 million people in the world have been affected, and predicted will increase to triple in 2050 [2]. Studying neuroinflammation presents significant challenges, as it proves not only difficult to study in humans but also presents striking differences when modeled in animal systems. However, ample evidence suggests that neuroinflammation may contribute to various brain disorders, such as Alzheimer’s disease, and this area of investigation has been significantly overlooked and inadequately supported, resulting in a shortage of research in the field [3]. While current treatments for these diseases are primarily symptomatic, there is a growing interest in developing therapies that target the underlying neuroinflammatory processes.
Marine drugs have garnered attention as a promising source for developing drugs targeting neuroinflammation [4]. Scientists are exploring marine organisms as a potential source of drugs for neuroinflammatory diseases because these organisms have evolved unique defense mechanisms against pathogens and predators in their aquatic environment. As a result, they produce a wide range of bioactive compounds with potential therapeutic properties, including anti-inflammatory, antioxidant, and neuroprotective effects [5]. Certain marine invertebrate compounds have been found to target essential proteins and pathways in neuroinflammatory therapy, including prions, α-synuclein, and amyloid β, which are known to form plaques and directly activate microglia, contributing to chronic inflammation. According to reports, Lamellosterol C from Lamellodysidea cf. Chlorea exhibited a 3 times more potent anti-prion effect than Guanabenz [6]. Sycosterol A has been found to exert twice the inhibitory effect on α-synuclein compared to Epigallocatechin-3-gallate, a known potent neuroprotective agent [7, 8]. 11-Dehydrosinulariolide regulates several protective pathways by inducing DJ-1 expression and activating Akt/PI3K, Nrf2/HO-1, and p-CREB [9]. In addition to their high medicinal value, cultivating marine invertebrates has economic significance and can be utilized as a food source and daily health supplements.
Different types of compounds and marine invertebrates have distinct mechanisms of action in addressing neuroinflammation. By understanding this topic, researchers can identify the most promising combinations of compounds and marine invertebrates for potent neuroinflammatory therapy. This review focuses on compounds successfully isolated from marine invertebrates as potent anti-neuroinflammatory and anti-neurodegenerative agents. The mechanisms of action of these compounds are discussed based on the pathophysiology and key pathways of neuroinflammation, providing insight into the development of new natural-based drugs.
2 Main body
2.1 Methodology
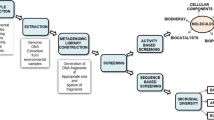
To conduct this review, Scopus, PubMed, and Google Scholar were utilized as the primary sources of literature. The search was performed using specific keywords, including ‘Marine invertebrates neuroprotection,’ ‘Sea urchins neuroprotective compounds,’ ‘Starfish anti-inflammatory properties,’ ‘Sea cucumbers neurodegenerative disorders,’ ‘Neuroinflammation marine-derived compounds,’ ‘Neuroprotection natural compounds,’ ‘Marine invertebrates Alzheimer’s disease,’ ‘Sea urchins Parkinson’s disease,’ ‘Starfish Huntington’s disease,’ ‘Sea cucumbers neuroinflammation,’ ‘Neurodegenerative disorders marine-derived compounds.’ The first search yielded 171 articles, with 109 research articles, 56 review papers, and 6 book chapters. The initial number of articles selected was only articles labeled research articles by the database (n = 109). Furthermore, a sum of 28 articles that consisted of research on the efficacy of marine invertebrate isolates as either anti-neuroinflammation or neuroprotection were included. The excluded articles were in the form of reviews, were written non-English, and solely focused on extracts or fractions activity studies. Studies that lack clear explanations and findings regarding the mechanism pathways of the utilized substance were also excluded. A flowchart of the methodology can be seen in Fig. 1.
2.2 Pathology of neuroinflammation
2.2.1 Immune cell activation
The activation of immune cells and subsequent release of inflammatory mediators in the central nervous system (CNS) is a multifaceted pathological process known as neuroinflammation [10, 11]. In this process, immune cell activation plays a pivotal role [12]. Upon exposure of the CNS to external stimuli such as injuries or infections, immune cells, such as microglia and astrocytes, get activated and secrete proinflammatory cytokines, chemokines, and reactive oxygen species (ROS) [13, 14]. Microglia are the primary immune cells in the CNS, and they become activated in response to inflammatory stimuli such as proinflammatory cytokines or damage-associated molecular patterns (DAMPs) [15, 16]. Microglia activation is characterized by morphological changes, including an increase in cell size and the development of processes [17]. Similarly, astrocytes, another type of glial cell, become activated in response to inflammatory stimuli and release cytokines, chemokines, and other inflammatory mediators, contributing to the neuroinflammatory response [18, 19].
Activated microglia and astrocytes release proinflammatory cytokines, such as interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor-α (TNF-α) [20, 21]. These cytokines can activate other immune cells in the CNS, such as monocytes and neutrophils, which can infiltrate the CNS from the bloodstream [22, 23]. These cytokines can activate neuronal receptors, such as NMDA receptors, and increase calcium influx into the neurons [24, 25]. This can lead to excitotoxicity, a process in which excessive calcium levels can cause damage and even death of neurons [26]. In addition to excitotoxicity, neuroinflammation can cause oxidative stress, leading to neuronal damage [27, 28]. Activated immune cells, particularly microglia, can generate reactive oxygen species (ROS), which contribute to the damage of neuronal and glial cells [29].
If neuroinflammation is left untreated or persists for a prolonged duration, it can cause chronic neurodegeneration, marked by progressive neuronal damage and dysfunction [30, 31]. This can pave the way for various neurodegenerative ailments like Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis [32]. Therefore, it is critical to comprehend the impact of immune cell activation in neuroinflammation pathology to develop effective therapies for these disorders [33, 34]. One of the proposed therapeutic strategies for neuroinflammatory diseases is inhibiting immune cell activation, particularly that of microglia and astrocytes. Specific inhibitors of these activation pathways [35] or nonsteroidal anti-inflammatory drugs (NSAIDs) [36] can be used to achieve this goal.
Neuroinflammation can also lead to the activation of apoptosis, a programmed cell death pathway [37]. Activated immune cells can release pro-apoptotic factors, such as caspases and Bcl-2 family proteins, which can activate apoptotic pathways in neurons [38, 39]. This can lead to the death of neurons and contribute to the progression of neurodegenerative diseases [40].
2.2.2 Blood–brain barrier dysfunction
The blood–brain barrier (BBB) is a highly selective and tightly regulated barrier that separates the central nervous system (CNS) from the peripheral circulation [41, 42]. The BBB is formed by specialized endothelial cells that line the cerebral microvasculature and pericytes, astrocytes, and extracellular matrix components [43, 44]. The BBB plays a crucial role in maintaining the homeostasis of the CNS by limiting the entry of potentially harmful substances into the brain, including immune cells [45]. The BBB can become dysfunctional and permeable in neuroinflammation, allowing immune cells and inflammatory mediators to infiltrate the CNS [46]. This can occur through several mechanisms. One mechanism is the activation of endothelial cells that form the BBB [47]. The expression of adhesion molecules by endothelial cells is a consequence of activation by inflammatory mediators, including cytokines and chemokines. Such activation enables immune cells to attach and traverse the BBB [48]. Additionally, proinflammatory cytokines can cause changes in the cytoskeleton of endothelial cells, resulting in the formation of gaps between the cells that allow molecules and cells to pass through [49, 50].
Another mechanism is the disruption of tight junctions between endothelial cells [51]. Tight junctions are specialized structures that seal the gaps between endothelial cells and prevent the diffusion of molecules and cells across the BBB [42]. In neuroinflammation, inflammatory mediators can disrupt tight junctions, leading to increased permeability of the BBB [52]. This disruption can also be caused by oxidative stress, which damages the cytoskeleton and leads to the detachment of tight junctions from the endothelial cells [53]. Finally, immune cells themselves can contribute to the disruption of the BBB [45]. When activated, immune cells, like microglia and astrocytes, secrete inflammatory mediators that can harm the endothelial cells comprising the BBB [48]. Additionally, immune cells can physically cross the BBB and infiltrate the CNS, exacerbating the neuroinflammatory response [54, 55].
The dysfunction of the BBB in neuroinflammation has important pathological consequences [47, 56]. The infiltration of immune cells and inflammatory mediators into the CNS can lead to chronic neuroinflammation and neurodegeneration, as seen in diseases such as Alzheimer’s and Parkinson’s [10, 57]. Additionally, the increased permeability of the BBB can allow the entry of pathogens into the CNS, contributing to the development of infections such as meningitis [58].
2.2.3 Neuronal damage
Another mechanism through which neuroinflammation can cause neuronal damage is through the release of glutamate [59, 60]. Glutamate is a neurotransmitter that plays a key role in excitatory signaling in the CNS. However, excessive levels of glutamate can cause excitotoxicity, leading to neuronal damage and death [60]. In neuroinflammation, immune cells can release glutamate and contribute to the excitotoxicity that damages neurons [61].
2.2.4 Neurodegeneration
Prolonged or severe neuroinflammation may bring about chronic neurodegeneration through several means. Microglia, the CNS’s resident immune cells, play a significant role in this process [10, 11]. Upon exposure to inflammatory agents, microglia become activated and release various harmful substances, including reactive oxygen species, nitric oxide, and proinflammatory cytokines, which can directly harm neurons, leading to their demise [20, 21]. Additionally, chronic neuroinflammation can promote the accumulation of misfolded proteins in neurons and glia, a characteristic feature of several neurodegenerative diseases. Misfolded proteins can trigger the innate immune system’s activation, leading to chronic inflammation and further protein misfolding and aggregation [62], generating a self-sustaining loop of neuroinflammation and neurodegeneration [63].
In addition, chronic neuroinflammation can impair the brain’s ability to clear toxic substances such as amyloid-β in Alzheimer’s disease and α-synuclein in Parkinson’s disease, leading to their accumulation in the brain and further worsening neuroinflammation, neuronal dysfunction, and death [64, 65]. Moreover, chronic neuroinflammation can interfere with neurotrophic support, which is vital for neuronal survival and function. Neurotrophic factors like brain-derived neurotrophic factor (BDNF) play a crucial role in promoting neuronal growth, differentiation, and survival [66, 67]. However, chronic neuroinflammation can reduce the production and release of neurotrophic factors, leading to neuronal dysfunction and death [68].
In summary, chronic neuroinflammation can contribute to neurodegeneration by activating microglia, accumulating misfolded proteins, impairing clearance of toxic substances, and disrupting neurotrophic support. Gaining knowledge about these mechanisms can offer valuable information in devising therapeutic interventions for the management of neurodegenerative disorders.
2.3 Marine invertebrate
Marine invertebrates are diverse animals that lack a backbone and inhabit the ocean environment [69]. They include many organisms, from simple forms such as sponges, jellyfish, and sea anemones, to more complex organisms such as crustaceans, molluscs, and echinoderms. Marine invertebrates are found in all ocean habitats, from shallow coral reefs to the deep sea floor, and they play important ecological roles in marine ecosystems [70]. For example, some species of marine invertebrates, such as sea urchins and certain molluscs, are important herbivores, while others, such as crustaceans and cephalopods, are important predators [71]. Marine invertebrates have developed a variety of adaptations that allow them to survive and thrive in the ocean environment [72]. For example, some marine invertebrates have evolved unique structures, such as stinging cells or hard shells for protection, while others have developed specialized appendages for locomotion or feeding.
Cultivating and conserving marine invertebrates can be challenging, but several strategies can help promote their growth and survival [73]. Different marine invertebrates have different requirements for survival, so it is important to research the species’ specific needs [74]. Factors such as water temperature, salinity, and lighting can all affect their health and growth. To thrive, marine invertebrates need a suitable habitat that mimics their natural environment [73]. This may include a specific type of substrate, such as sand or rocks, or the presence of other organisms that they interact with in the wild. Water quality is also critical for the health of marine invertebrates [75]. Regular testing and maintenance of water parameters such as pH, temperature, and nutrient levels can help ensure a stable environment for your organisms. Many marine invertebrates require specific types of food to thrive [76]. For example, some corals require plankton or other small organisms, while certain sea urchins feed on algae. It is important to research the dietary requirements of the organisms and provide appropriate food sources.
Many marine invertebrates can be cultivated successfully, depending on the location and environmental conditions [77]. Coral reefs are found in many tropical and subtropical regions worldwide, and many different species of coral can be successfully cultivated in aquariums or the wild [78]. Some popular species for cultivation include brain coral, mushroom coral, and stony coral. Oysters are grown commercially in many coastal regions and are an important food source [79, 80]. They are typically grown in mesh bags or cages suspended in the water and are harvested when they reach maturity [81]. Sea urchins are commonly cultivated in aquaculture systems in many regions, particularly in Japan, where they are an important food source [82]. They require specific environmental conditions, including cool water temperatures and high-quality seawater. Clams are another important food source that can be cultivated in many coastal regions [83,84,85]. They require specific conditions for growth, including a sandy or muddy substrate and high-quality seawater. Lobsters are commercially harvested in many regions, particularly North America and Europe [86, 87]. They require specific environmental conditions, including cool water temperatures and rocky substrate for shelter [88]. Sea cucumbers are cultivated commercially in many regions, particularly in Asia, where they are an important food source [89, 90]. They require specific environmental conditions, including a sandy substrate and high-quality seawater. The specific types of marine invertebrates that can be cultivated successfully depend on the environmental conditions and available resources in a particular region. With proper research and management practices, many different types of marine invertebrates can be grown successfully and sustainably.
Marine invertebrates are important sources of food, medicine, and other resources for humans. For example, many molluscs and crustaceans are commercially harvested for food, while some marine invertebrates, such as sponges and corals, contain compounds with potential medicinal properties. Overall, marine invertebrates are a fascinating and important group of organisms that contribute to the diversity and functioning of marine ecosystems and have significant value to human societies.
2.4 Therapeutic targets of marine invertebrate bioactive compounds
Marine invertebrates have become a fascinating source for discovering bioactive compounds with therapeutic potential. These organisms have evolved a wide range of mechanisms to protect themselves against predators and to interact with their environment. Scientists have discovered that some of these compounds can target specific molecular pathways involved in different diseases, including neuroinflammation and neurodegenerative disorders. Table 1 provides an overview of the therapeutic activities of marine invertebrate bioactive compounds, highlighting their potential as a source of new treatments.
Marine invertebrate bioactive compounds have shown potential therapeutic activities in treating neurological disorders. Table 1 summarizes the therapeutic activities of these compounds, which target essential proteins and pathways involved in neuroinflammatory and neurodegenerative processes. However, a better understanding of the underlying mechanisms is needed to harness their potential as natural drug candidates fully. Figure 2 provides an overview of the anti-neuroinflammatory and anti-neurodegenerative mechanisms of these marine invertebrate compounds, highlighting their potential as a source for developing new drugs to combat neurological disorders.
2.4.1 Oxidative stress
Oxidative stress is a condition that arises when the production of reactive oxygen species (ROS) exceeds the cell’s capacity to detoxify them [115]. This can harm various parts of cells, such as proteins, lipids, and DNA, causing cells to malfunction or die [116, 117]. Oxidative stress can be triggered by hydrogen peroxide (H2O2) and tert-butyl hydroperoxide (t-BHP), both types of ROS that damage mitochondria [118]. The damage to mitochondria can lead to the release of lactate dehydrogenase (LDH) and a decrease in the overall antioxidant capacity (T-AOC) and activity of superoxide dismutase (SOD) [119].
The cell has two key antioxidant defense systems, namely total antioxidant capacity (T-AOC) and superoxide dismutase (SOD), that help to safeguard it from oxidative harm [120]. The regulation of these antioxidant enzymes is determined by various factors, among which Nrf2 plays a crucial role [121]. Nrf2 binds to the promoter region of the SOD gene, leading to an upsurge in its expression and subsequently, SOD activity [122]. Moreover, Nrf2 also regulates the expression of other genes that participate in the oxidative stress response, such as catalase and glutathione peroxidase [123].
When there is oxidative stress, the mRNA level of Bcl-2 may decrease, which tips the balance in favor of pro-apoptotic proteins like Bax [124]. Bax can cause the release of cytochrome c from the mitochondria, kickstarting the caspase cascade [125]. In the intrinsic pathway of apoptosis, caspase-9 plays the role of the initiator caspase, and its activation leads to the activation of other effector caspases, including caspase-3. The end result of this pathway is cell death [126].
Compound 13 shows potential as a neuroprotective agent by blocking the mitochondrial dysfunction induced by H2O2 or t-BHP, limiting the release of lactate dehydrogenase (LDH) caused by H2O2 or t-BHP, and increasing intracellular total antioxidant capacity (T-AOC) and superoxide dismutase (SOD) activity compared to the H2O2 or t-BHP group [97]. Together with 13, Sea Cucumber cerebrosides (14) and Star Fish Cerebrosides (20) have been found to increase the activity of SOD and reduce the content of NO, NOS, 8-OHdG, 8-oxo-G, and MDA. They can also increase the survival rate of PC12 cells, recover cellular morphology, and regulate the expression of caspase-9, cleaved caspase-3, total caspase-3, Bax, and Bcl-2, indicating their potential as neuroprotective agents [98,99,100].
Compounds 15, 16, 39, and 40 were shown to reduce 6-hydroxydopamine (6-OHDA). 6-OHDA is a neurotoxin commonly used to destroy dopaminergic neurons in the brain selectively. 6-OHDA is often used in animal models of Parkinson’s disease to simulate the degeneration of these neurons in human disease [101, 108, 109]. Compound 40 has been discovered to have the ability to reverse the downregulation of the PI3K/Akt signaling pathway induced by 6-OHDA and boost the translocation of Nrf2 to aid downstream protein translation of HO-1 and SOD-1. Moreover, it was observed to impede the cleavage of caspase-3 protein by increasing the levels of p-Akt and p-ERK and reducing the levels of p-P38. These findings suggest that compound 40 might be a promising therapeutic agent for treating neurodegenerative diseases [109]. Marine invertebrate compounds regulating ROS can be seen in Fig. 3.
Marine invertebrate compounds regulating reactive oxygen species. Pseudopterosin A (1); Sarboettgerin E (9); Eicosapentaenoic acid (13); Frondoside A (15); Ginsenoside Rg3 (16); Gracilin A (22); Gracilin H (23); Gracilin J (24); Gracilin K (25); Tetrahydroaplysulphurin-1 (27); (22E)-24-nor-cholesta-5,22-diene-3β,7β-diol (39); Stellettin B (40); Narrabeenamine B (41); 5,6-Dibromo-7-methoxykynuramine (42); 7-bromoquinolin-4(1H)-one (43); 5,6-dibromo-N,N-dimethyltryptamine (44); 5,6-dibromotryptamine (45); 6-bromo-N-methyltryptamine (46); 3-bromo-4-methoxytyramine (47); 5,6-dibromo-N-methyltryptamine (48); and 6-bromotryptamine (49)
2.4.2 Acetylcholinesterase
Acetylcholinesterase (AChE) is an enzyme that plays a critical role in regulating cholinergic neurotransmission [127]. This substance is mainly located within the synaptic clefts of cholinergic neurons, and its primary function is to quickly break down the neurotransmitter acetylcholine (ACh) into choline and acetate [128]. In Alzheimer’s disease, the activity of AChE is often increased, leading to ACh’s breakdown and the depletion of cholinergic neurotransmission [129]. This depletion of cholinergic neurotransmission is thought to contribute to the cognitive deficits seen in Alzheimer’s disease. Therefore, AChE is an important enzyme that regulates the activity of the cholinergic system and plays a critical role in normal nervous system function [130]. It is also involved in the pathogenesis of certain neurological disorders, making it an important target for therapeutic interventions.
Previous studies investigated anti-AChE from active compounds of sea urchins, Scaphechinus mirabilis (10), and sponge, Xestospongia testudinaria (28) (see Fig. 4) [95, 106]. Compound 28 calculated significant IC50 of AChE inhibition (0.64 μM) [106]. Furthermore, compound 10 is a strong acetylcholinesterase (AChE) inhibitor, and its mode of inhibition is both uncompetitive and irreversible [94].
2.4.3 Amyloid-β accumulation
Amyloid beta (Aβ) is a protein that accumulates in the brains of patients with Alzheimer’s disease (AD) [131]. Aβ is produced by the cleavage of a larger protein called amyloid precursor protein (APP) by β-secretase 1 (BACE1) and gamma-secretase [132]. The accumulation of Aβ in the brain is thought to play a central role in the pathogenesis of AD. BACE1 is an enzyme that cleaves APP to generate Aβ, and its activity is essential for producing Aβ. Inhibiting BACE1 activity has been proposed as a therapeutic strategy for AD [133]. Neuroprotective effects against Aβ1–42-induced synaptic dysfunction are related to preventing the loss of synaptic function and neuronal damage that occurs in AD [134]. Several studies have demonstrated that certain compounds and interventions can protect against Aβ1–42-induced synaptic dysfunction [99, 135]. For example, resveratrol, a natural compound found in grapes, has been shown to protect against Aβ1–42-induced synaptic dysfunction by reducing oxidative stress and inflammation [136].
The inhibition of Aβ toxicity by preventing its aggregation through an autophagic pathway regulated by HSF-1 involves the activation of heat shock factor 1 (HSF-1). HSF-1 is a transcription factor that controls the expression of genes involved in the cellular stress response [103]. Studies have demonstrated that activating HSF-1 can enhance the clearance of Aβ aggregates through autophagy, leading to a decrease in Aβ toxicity. Some active compounds from marine invertebrates were investigated for inhibitors of Aβ, including 11, 12, 14, 19, 54, and 55 [96, 98,99,100, 103, 112, 113]. Compound 14 has been found to have neuroprotective effects against Aβ1–42-induced synaptic dysfunction in the rat hippocampus, possibly by promoting synaptic function and protecting against neuronal damage through the upregulation of proteins involved in synaptic plasticity and neuronal survival [98,99,100]. Compound 54 inhibits amyloid β-42 production induced by aftin-5 [112]. Compound 19, conversely, demonstrated that can safeguard C. elegans from the harmful effects of Aβ by inhibiting its aggregation through an autophagic pathway regulated by HSF-1. As a result, this compound could be a potentially valuable therapeutic option for Alzheimer’s disease [103].
Finally, compounds 11 and 12 from Urechis unicinctus were studied for anti-BECE-1 in vitro [96]. Compound 55 has been found to directly reduce Aβ oligomer formation and produce less neuronal toxicity in SH-SY5Y cells, indicating its potential as a therapeutic agent for AD [113]. Despite different mechanisms of action, all compounds have demonstrated potential as treatments for Alzheimer’s (Fig. 5).
2.4.4 α-synuclein aggregations
The involvement of α-synuclein in the degeneration of dopaminergic neurons within the substantia nigra is believed to contribute to the motor-related symptoms seen in Parkinson’s disease [137]. α-synuclein aggregation is believed to cause neuronal dysfunction and death by disrupting normal cellular processes, including mitochondrial function, vesicle trafficking, and protein degradation [138]. On the other hand, reducing α-synuclein levels has been shown to improve dopamine-dependent behavioral functions. Dopamine is a neurotransmitter critical in regulating movement and reward, and its depletion is a hallmark of PD [139]. Studies have suggested that reducing α-synuclein levels may increase dopamine release and improve dopaminergic neuron function, thereby improving movement and other dopamine-dependent behaviors [140, 141].
Reduction of α-synuclein levels can be achieved through various mechanisms, including the activation of autophagy [142]. Autophagy is critical in maintaining cellular homeostasis and is essential for neuronal survival. Autophagic signaling mediated through lgg-1 and atg-7 activity has been shown to reduce α-synuclein levels and protect dopaminergic neurons in animal models of PD [143]. The activation of autophagy promotes the clearance of misfolded or aggregated proteins, including α-synuclein, thereby reducing their toxicity.
Compounds from Holothuria scabra (17 and 18) may have therapeutic potential through their effects on autophagic signaling mediated through lgg-1 and atg-7 activity [102], while compounds like 21, 56, and 65 have been investigated for the treatment and monitoring of α-synuclein-mediated neurodegeneration and suggested potential strategies.
These compounds have been tested for α-synuclein aggregation inhibitory activity using the Thioflavin T (ThT) dye assay method and demonstrated significant α-synuclein aggregation inhibitory activity [6, 8, 104]. Compound 15 was able to decrease the aggregation of α-synuclein, increase the lifespan in NL5901, and promote the upregulation of regulators of protein degradation, such as ubh-4, hsp-16.2, hsp-16.1, and hsf-1 [101]. Structure of these compounds can be seen in Fig. 6.
2.4.5 Prion aggregations
The activity of prions in the brain can lead to the formation of protein aggregates, which are toxic to neurons and can cause neurodegeneration [144, 145]. These protein aggregates can disrupt the brain’s normal function and lead to symptoms such as cognitive impairment, motor dysfunction, and behavioral changes [146]. This enzyme is usually linked to the transformation of regular cellular prion protein (PrPc) into the abnormal and infectious form (PrPSc) [147]. PrPSc is resistant to degradation by cellular machinery and can accumulate in the brain over time [148]. The aggregation of PrPSc results in the formation of plaques and fibrils, which are believed to be responsible for the damage to neurons and subsequent neurodegeneration [149].
Prion diseases can be transmitted through ingestion, contact with infected tissue or blood, or genetic inheritance [150]. Prion diseases have no cure, and treatment options are limited [151]. Therapeutic approaches focus on reducing prion activity, such as inhibiting the conversion of PrPc to PrPSc or promoting the clearance of PrPSc from the brain [152].
Lamellosterols A-C (56–58) were examined for their ability to inhibit prion activity and were found to be more effective than the known anti-prion compound guanabenz. The EC50 values for these compounds as anti-prion agents against the [PSI+] yeast prion were 12.7, 13.8, and 9.8 μM, respectively [6]. Similarly, procerolide A (59) and procerone A (63), isolated from the Ascidian Polycarpa procera, showed potential anti-prion activity against the [PSI+] yeast prion with EC50 values of 23 and 29 μM, respectively [114]. PrPSc can form protein aggregates in the yeast S. cerevisiae, including Sup35, related to the [PSI+] phenotype. Several studies have also shown that PrPSc can interact with Sup35 in the yeast S. cerevisiae and can influence the formation of protein aggregates caused by Sup35 [153]. However, the exact connection between PrPSc and [PSI+] remains unclear, and additional investigation is necessary. Structure of these compounds can be seen in Fig. 7.
2.4.6 Advanced glycation end products (AGEs) formation
Advanced glycation end products (AGEs) refer to a cluster of molecule created by the reaction of amino groups of proteins, lipids, and nucleic acids with reducing sugars, independent of enzymatic activity [154, 155]. These molecules can accumulate in tissues over time and are believed to play a role in the development of chronic illnesses, including neurodegenerative conditions such as Alzheimer’s disease and Parkinson’s disease (PD) [156]. AGEs can have several deleterious effects on the brain, including increased oxidative stress, inflammation, and disruption of normal cellular function [157]. In neurodegenerative disorders, AGEs can contribute to the aggregation of misfolded proteins, such as amyloid β in AD and α-synuclein in PD [158]. AGEs can also impair the clearance of these proteins, leading to their accumulation and subsequent neurodegeneration [159].
Furthermore, AGEs have the ability to activate a variety of signaling pathways, including RAGE and NF-kB, which are implicated in inflammation and cell death processes [160]. The activation of these pathways can result in the discharge of harmful substances such as proinflammatory cytokines and reactive oxygen species, intensifying the progression of neurodegenerative disorders [161]. Compounds 31–38 are mirabamides isolated from Siliquariaspongia mirabilis and Stelletta clavosa. These compounds have shown calculations via Conceptual Density Functional Theory (DFT) to generate information about the reactive nature of these compounds and the active points for electrophilic, nucleophilic, and radical attacks and the Solvation Model based on the Density (SMD) for the molecular and structural properties of compounds [107]. The computational results accurately predict the compounds’ ability to inhibit the formation of AGEs, which could be valuable in developing drugs for combating diseases like Parkinson’s and Alzheimer’s [162, 163]. Structure of these compounds can be seen in Fig. 8.
2.4.7 Paraquat activity
Paraquat is an herbicide commonly used that has been linked to the occurrence of Parkinson’s disease and other neurodegenerative diseases [164]. The compound is extremely harmful and induces oxidative stress by generating reactive oxygen species (ROS), resulting in neuronal damage and ultimately leading to neurodegeneration [165]. The mechanisms by which PQ induces neurodegeneration are complex and multifaceted. PQ can accumulate in the brain and cause damage to dopaminergic neurons, which are particularly vulnerable to oxidative stress [166]. PQ can also induce the formation of protein aggregates, such as α-synuclein, a hallmark of PD pathology [167]. In addition to its direct effects on neurons, PQ can induce inflammation and activate microglia, immune cells in the brain [168]. Microglia that have been activated can secrete cytokines that promote inflammation, as well as molecules that are neurotoxic and reactive oxygen species, all of which can worsen neurodegeneration. Compound 53 increased the survival of Neuro-2a cells when exposed to PQ and decreased the number of harmful ROS within these cells. Additionally, acetylpenasterol induced the expression of Hsp70, a protein that helps protect cells from stress, in PQ-treated cells. Furthermore, it was observed that acetylpenasterol prevented the loss of neurites and increased the number of cells with neurites in PQ-exposed cells [111] (Fig. 9).
2.4.8 Mitochondria DJ-1 expression
The protein DJ-1 is present in both the cytoplasm and mitochondria of cells [169] and plays a crucial role in maintaining mitochondrial function and safeguarding cells against oxidative stress and mitochondrial dysfunction [170]. Its dysfunction or loss may cause neurodegenerative diseases like PD by compromising mitochondrial function and elevating oxidative stress. In addition to its role in maintaining mitochondrial function, DJ-1 is also involved in the Akt signaling pathway, which is essential for cell survival and growth [171]. Research has shown that DJ-1 is capable of activating the PI3K/Akt pathway, which promotes cell survival and reduces cell death [172]. Conversely, the loss or dysfunction of DJ-1 can lead to a decrease in Akt activity and an increase in susceptibility to oxidative stress and apoptosis [173]. These findings suggest that the interaction between DJ-1 and the Akt pathway is crucial for the neuroprotective effects of DJ-1. Further exploration of this relationship could potentially uncover new targets for the treatment of neurodegenerative diseases.
11-Dehydrosinulariolide (compound 2, see Fig. 10) is a natural compound that has been extracted from the soft coral Sinularia leptoclados and has been found to have multiple biological effects. One of its mechanisms for providing neuroprotection is its ability to increase DJ-1 expression. According to one research, the treatment of SH-SY5Y human neuroblastoma cells with 2 increased the expression of DJ-1 in a dose-dependent manner and also stimulated mitochondrial complex I activity [92]. Another study reported that 2 could activate p-CREB and the downstream of Akt/PI3K and promote Nrf2/HO-1 translocation in SH-SY5Y cells [9].
2.5 Author perspective
From our perspective, this review article on using marine invertebrate compounds for anti-neuroinflammation and anti-neurodegenerative purposes provides an opportunity to shed light on an emerging area of drug discovery research. The potential benefits of marine invertebrates as a source of novel therapeutic agents for neurodegenerative diseases are increasingly recognized by researchers [174]. However, several challenges must be addressed to harness the potential of marine invertebrate compounds fully. One of the main challenges is optimizing the bioavailability and activity of these compounds. Many marine invertebrate compounds have poor solubility and bioavailability, which can limit their effectiveness as therapeutic agents [175]. Targeted delivery systems that can improve these compounds’ bioavailability and therapeutic efficacy could solve this problem.
Moreover, combining several compounds with different action mechanisms may result in a more potent, efficient, and low side-effect therapy. Implementing a multicompartment delivery system would be beneficial to regulate the release and maintain the stability of the biomolecular mixture during storage. For instance, liposomes, novasomes, and polymers could also be an option for multi-delivery synergistic drug biomolecules [176,177,178,179,180,181,182]. As derived from marine sources, invertebrate extracts or isolates typically have a less appealing aroma and taste. Instant granule technology and practical dosage forms must also be implemented to mask the unpleasant aroma and taste [115, 183].
Another challenge is identifying more effective and selective compounds that target specific neuroinflammatory and neurodegenerative pathways. While several promising compounds have been identified from marine invertebrates, further research is needed to understand their mechanisms of action and potential clinical applications fully. There is also a need for more extensive in vitro and in vivo studies to evaluate the safety, efficacy, and pharmacokinetic properties of marine invertebrate compounds. This could involve investigating the effects of these compounds on animal models of neurodegenerative diseases and conducting clinical trials to evaluate their potential as therapeutic agents.
3 Conclusion
Based on the reviewed literature, it is evident that compounds isolated from marine invertebrates have shown potential therapeutic mechanisms in inhibiting neuroinflammation caused by amyloid β, paraquat, α-synuclein, AGEs, prion activity, and oxidative stress. These bioactive compounds have demonstrated promising anti-neuroinflammatory and anti-neurodegenerative properties through various pathways and proteins such as DJ-1, Akt/PI3K, p-CREB, Nrf2/HO-1, Nrf-1, and HSF-1. Therefore, marine invertebrates can serve as a valuable source for developing natural compounds that could be utilized to treat neuroinflammatory disorders. Further research and investigation are needed to explore these bioactive compounds’ full potential and underlying mechanisms for developing novel therapeutic drugs.
Availability of data and materials
Not applicable.
Abbreviations
- CNS:
-
Central nervous system
- AZ:
-
Alzheimer’s disease
- ROS:
-
Reactive oxygen species
- DAMPs:
-
Damage-associated molecular patterns
- IL:
-
Interleukin
- TNF-α:
-
Tumor necrosis factor-α
- PD:
-
Parkinson’s disease
- MS:
-
Multiple sclerosis
- NSAIDs:
-
Nonsteroidal anti-inflammatory drugs
- BBB:
-
Blood–brain barrier
- BDNF:
-
Like brain-derived neurotrophic factor
- NO:
-
Nitric oxide
- BACE1:
-
Beta-site amyloid cleaving enzyme
- PQ:
-
Paraquat
- AChE:
-
Acetylcholinesterase
- t-BHP:
-
Tert-butyl hydroperoxide
- LDH:
-
Lactate dehydrogenase
- SOD:
-
Superoxide dismutase
- T-AOC:
-
Total antioxidant capacity
- ThT:
-
Thioflavin T
- PrP:
-
Prion protein
- AGEs:
-
Advanced glycation end products
- DFT:
-
Conceptual Density Functional Theory
- SMD:
-
Solvation Model based on the Density
References
Vezzani A, Balosso S, Ravizza T (2019) Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat Rev Neurol 15(8):459–472
Li T, Lu L, Pember E, Li X, Zhang B, Zhu Z (2022) New Insights into neuroinflammation involved in pathogenic mechanism of Alzheimer’s disease and its potential for therapeutic intervention. Cells 11(12):1925
Gallin EK, Franko M, Bond E. Philanthropy’s role in advancing biomedical research. In: Principles and practice of clinical research. Elsevier; 2018. p. 611–30.
Karthikeyan A, Joseph A, Nair BG (2022) Promising bioactive compounds from the marine environment and their potential effects on various diseases. J Genet Eng Biotechnol 20(1):14
Pohnert G. Chemical defense strategies of marine organisms. 2004. p. 179–219.
Jennings LK, Prebble DW, Xu M, Ekins MG, Munn AL, Mellick GD et al (2020) Anti-prion and α-synuclein aggregation inhibitory sterols from the sponge Lamellodysidea cf. chlorea. J Nat Prod. 83(12):3751–7
Youn K, Ho C-T, Jun M (2022) Multifaceted neuroprotective effects of (-)-epigallocatechin-3-gallate (EGCG) in Alzheimer’s disease: an overview of pre-clinical studies focused on β-amyloid peptide. Food Sci Hum Wellness 11(3):483–493
Prebble DW, Xu M, Mellick GD, Carroll AR (2021) Sycosterol A, an α-Synuclein Inhibitory Sterol from the Australian Ascidian Sycozoa cerebriformis. J Nat Prod 84(12):3039–3043
Feng C-W, Hung H-C, Huang S-Y, Chen C-H, Chen Y-R, Chen C-Y et al (2016) Neuroprotective effect of the marine-derived compound 11-dehydrosinulariolide through DJ-1-related pathway in in vitro and in vivo models of Parkinson’s disease. Mar Drugs 14(10):187
DiSabato DJ, Quan N, Godbout JP (2016) Neuroinflammation: the devil is in the details. J Neurochem 139:136–153
Kölliker-Frers R, Udovin L, Otero-Losada M, Kobiec T, Herrera MI, Palacios J et al (2021) Neuroinflammation: an integrating overview of reactive-neuroimmune cell interactions in health and disease. Mediators Inflamm 31(2021):1–20
Tohidpour A, Morgun AV, Boitsova EB, Malinovskaya NA, Martynova GP, Khilazheva ED et al (2017) Neuroinflammation and infection: molecular mechanisms associated with dysfunction of neurovascular unit. Front Cell Infect Microbiol 20:7
Matejuk A, Vandenbark AA, Offner H (2021) Cross-talk of the CNS with immune cells and functions in health and disease. Front Neurol 31:12
Wang W-Y, Tan M-S, Yu J-T, Tan L (2015) Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann Transl Med. 3(10):136–51
Serna-Rodríguez MF, Bernal-Vega S, de la Barquera JAO-S, Camacho-Morales A, Pérez-Maya AA (2022) The role of damage associated molecular pattern molecules (DAMPs) and permeability of the blood-brain barrier in depression and neuroinflammation. J Neuroimmunol. 371:577951
Zhu X, Huang H, Zhao L (2022) PAMPs and DAMPs as the bridge between periodontitis and atherosclerosis: the potential therapeutic targets. Front Cell Dev Biol 25:10
Woodburn SC, Bollinger JL, Wohleb ES (2021) The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J Neuroinflamm 18(1):258
Cordiglieri C, Farina C (2010) Astrocytes exert and control immune responses in the brain. Curr Immunol Rev 6(3):150–159
Reid JK, Kuipers HF (2021) She doesn’t even go here: the role of inflammatory astrocytes in CNS disorders. Front Cell Neurosci 3:15
Barany Z, Toth I, Jocsak G, Frenyo LV, Bartha T, Sterczer A et al (2021) Differential production of interleukin-6 and tumor necrosis factor-α in primary rat astrocyte cultures using two distinct methods of microglia elimination. Clin Exp Neuroimmunol 12(3):192–201
Ishijima T, Nakajima K (2021) Inflammatory cytokines TNFα, IL-1β, and IL-6 are induced in endotoxin- stimulated microglia through different signaling cascades. Sci Prog 104(4):003685042110549
Arima Y, Kamimura D, Sabharwal L, Yamada M, Bando H, Ogura H et al (2013) Regulation of immune cell infiltration into the CNS by regional neural inputs explained by the gate theory. Mediators Inflamm 2013:1–8
Huang X, Hussain B, Chang J (2021) Peripheral inflammation and blood–brain barrier disruption: effects and mechanisms. CNS Neurosci Ther 27(1):36–47
Orellana DI, Quintanilla RA, Gonzalez-Billault C, Maccioni RB (2005) Role of the JAKs/STATs pathway in the intracellular calcium changes induced by interleukin-6 in hippocampal neurons. Neurotox Res 8(3–4):295–304
Turovskaya MV, Turovsky EA, Zinchenko VP, Levin SG, Godukhin OV (2012) Interleukin-10 modulates [Ca2+]i response induced by repeated NMDA receptor activation with brief hypoxia through inhibition of InsP3-sensitive internal stores in hippocampal neurons. Neurosci Lett 516(1):151–155
Mattson MP. Excitotoxicity. In: Stress: physiology, biochemistry, and pathology. Elsevier; 2019. p. 125–34.
Crews FT, Vetreno RP. Neuroimmune basis of alcoholic brain damage. 2014. p. 315–57.
Liao R, Wood TR, Nance E (2020) Nanotherapeutic modulation of excitotoxicity and oxidative stress in acute brain injury. Nanobiomedicine 1(7):184954352097081
Simpson DSA, Oliver PL (2020) ROS generation in microglia: understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 9(8):743
Agrawal M. Molecular basis of chronic neurodegeneration. In: Clinical molecular medicine. Elsevier; 2020. p. 447–60.
Gorman AM (2008) Neuronal cell death in neurodegenerative diseases: recurring themes around protein handling. J Cell Mol Med 12(6a):2263–2280
Diack A, Alibhai J, Barron R, Bradford B, Piccardo P, Manson J (2016) Insights into mechanisms of chronic neurodegeneration. Int J Mol Sci 17(1):82
Lenz KM, Nelson LH (2018) Microglia and beyond: innate immune cells as regulators of brain development and behavioral function. Front Immunol 13:9
Singh D (2022) Astrocytic and microglial cells as the modulators of neuroinflammation in Alzheimer’s disease. J Neuroinflammation 19(1):206
Liu C-Y, Wang X, Liu C, Zhang H-L (2019) Pharmacological targeting of microglial activation: new therapeutic approach. Front Cell Neurosci 19:13
Mathew E, Kim E, Zempsky W (2016) Pharmacologic treatment of pain. Semin Pediatr Neurol 23(3):209–219
Rajesh Y, Kanneganti T-D (2022) Innate immune cell death in neuroinflammation and Alzheimer’s disease. Cells 11(12):1885
Hussar P (2022) Apoptosis regulators Bcl-2 and Caspase-3. Encyclopedia 2(4):1624–1636
Ola MS, Nawaz M, Ahsan H (2011) Role of Bcl-2 family proteins and caspases in the regulation of apoptosis. Mol Cell Biochem 351(1–2):41–58
Pemberton JM, Pogmore JP, Andrews DW (2021) Neuronal cell life, death, and axonal degeneration as regulated by the BCL-2 family proteins. Cell Death Differ 28(1):108–122
Hubbard JA, Binder DK. Blood–brain barrier disruption. In: Astrocytes and epilepsy. Elsevier; 2016. p. 291–311.
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17(1):69
Baeten KM, Akassoglou K (2011) Extracellular matrix and matrix receptors in blood-brain barrier formation and stroke. Dev Neurobiol 71(11):1018–1039
Gastfriend BD, Palecek SP, Shusta EV (2018) Modeling the blood–brain barrier: Beyond the endothelial cells. Curr Opin Biomed Eng 5:6–12
Patabendige A, Janigro D (2023) The role of the blood–brain barrier during neurological disease and infection. Biochem Soc Trans. 51:613–626
Salimi H, Klein RS. Disruption of the blood–brain barrier during neuroinflammatory and neuroinfectious diseases. 2019. p. 195–234.
Takata F, Nakagawa S, Matsumoto J, Dohgu S (2021) Blood–brain barrier dysfunction amplifies the development of neuroinflammation: understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front Cell Neurosci 13:15
Marchetti L, Engelhardt B (2020) Immune cell trafficking across the blood-brain barrier in the absence and presence of neuroinflammation. Vasc Biol 2(1):H1-18
López-Posadas R, Stürzl M, Atreya I, Neurath MF, Britzen-Laurent N (2017) Interplay of GTPases and cytoskeleton in cellular barrier defects during gut inflammation. Front Immunol 5:8
Sutton NR, Baek A, Pinsky DJ. Endothelial cells and inflammation. In: Encyclopedia of medical immunology. Springer, New York, NY; 2014. p. 367–81.
Zhang Y, Yang W-X (2016) Tight junction between endothelial cells: the interaction between nanoparticles and blood vessels. Beilstein J Nanotechnol 6(7):675–684
Zhao B, Yin Q, Fei Y, Zhu J, Qiu Y, Fang W et al (2022) Research progress of mechanisms for tight junction damage on blood–brain barrier inflammation. Arch Physiol Biochem 128(6):1579–1590
Chalimeswamy A, Thanuja MY, Ranganath SH, Pandya K, Kompella UB, Srinivas SP (2022) Oxidative stress induces a breakdown of the cytoskeleton and tight junctions of the corneal endothelial cells. J Ocul Pharmacol Ther 38(1):74–84
Engelhardt B (2010) T cell migration into the central nervous system during health and disease: different molecular keys allow access to different central nervous system compartments. Clin Exp Neuroimmunol 1(2):79–93
Rodriguez-Mogeda C, Rodríguez-Lorenzo S, Attia J, van Horssen J, Witte ME, de Vries HE (2022) Breaching brain barriers: B cell migration in multiple sclerosis. Biomolecules 12(6):800
Yang X, Chen X (2022) The crosstalk between the blood–brain barrier dysfunction and neuroinflammation after general anaesthesia. Curr Issues Mol Biol 44(11):5700–5717
Ní Chasaide C, Lynch MA (2020) The role of the immune system in driving neuroinflammation. Brain Neurosci Adv 29(4):239821281990108
Dando SJ, Mackay-Sim A, Norton R, Currie BJ, St. John JA, Ekberg JAK et al (2014) Pathogens penetrating the central nervous system: infection pathways and the cellular and molecular mechanisms of invasion. Clin Microbiol Rev. 27(4):691–726
Chen YZ, Dai ZZ, Shen ZW, Lin GS, Zhuang CY, Li HJ et al (2016) Magnetic resonance imaging of glutamate in neuroinflammation. Radiol Infect Dis 3(2):92–97
Haroon E, Miller AH, Sanacora G (2017) Inflammation, glutamate, and glia: a trio of trouble in mood disorders. Neuropsychopharmacology 42(1):193–215
Ganor Y, Levite M (2014) The neurotransmitter glutamate and human T cells: glutamate receptors and glutamate-induced direct and potent effects on normal human T cells, cancerous human leukemia and lymphoma T cells, and autoimmune human T cells. J Neural Transm 121(8):983–1006
Currais A, Fischer W, Maher P, Schubert D (2017) Intraneuronal protein aggregation as a trigger for inflammation and neurodegeneration in the aging brain. FASEB J 31(1):5–10
Muzio L, Viotti A, Martino G (2021) Microglia in neuroinflammation and neurodegeneration: from understanding to therapy. Front Neurosci. 15:742065
Forloni G (2023) Alpha synuclein: neurodegeneration and inflammation. Int J Mol Sci 24(6):5914
Sengupta U, Kayed R (2022) Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog Neurobiol 214:102270
Miranda M, Morici JF, Zanoni MB, Bekinschtein P (2019) Brain-derived neurotrophic factor: a key molecule for memory in the healthy and the pathological brain. Front Cell Neurosci 13:363
Colucci-D’Amato L, Speranza L, Volpicelli F (2020) Neurotrophic factor BDNF physiological functions and therapeutic potential in depression, neurodegeneration and brain cancer. Int J Mol Sci. 21(20):7777
Lima Giacobbo B, Doorduin J, Klein HC, Dierckx RAJO, Bromberg E, de Vries EFJ (2019) Brain-derived neurotrophic factor in brain disorders: focus on neuroinflammation. Mol Neurobiol 56(5):3295–3312
Sibly RM, Brown JH, Kodric-Brown A (2012) Metabolic ecology. Wiley, New York
Coleman FC, Williams SL (2002) Overexploiting marine ecosystem engineers: potential consequences for biodiversity. Trends Ecol Evol 17(1):40–44
Boudreau S, Worm B (2012) Ecological role of large benthic decapods in marine ecosystems: a review. Mar Ecol Prog Ser 469:195–213
Verdes A, Holford M. Beach to bench to bedside: marine invertebrate biochemical adaptations and their applications in biotechnology and biomedicine. 2018. p. 359–76.
Olivotto I, Planas M, Simões N, Holt GJ, Avella MA, Calado R (2011) Advances in breeding and rearing marine ornamentals. J World Aquac Soc 42(2):135–166
Lenz M, da Gama BAP, Gerner NV, Gobin J, Gröner F, Harry A et al (2011) Non-native marine invertebrates are more tolerant towards environmental stress than taxonomically related native species: results from a globally replicated study. Environ Res 111(7):943–952
Chiarelli R, Roccheri MC (2014) Marine invertebrates as bioindicators of heavy metal pollution. Open J Met 04(04):93–106
Guillard RRL. Culture of phytoplankton for feeding marine invertebrates. In: Culture of marine invertebrate animals. Springer, Boston; 1975. p. 29–60.
Kerrison PD, Stanley MS, Edwards MD, Black KD, Hughes AD (2015) The cultivation of European kelp for bioenergy: SITE and species selection. Biomass Bioenerg 80:229–242
Murray JM, Watson GJ (2014) A critical assessment of marine aquarist biodiversity data and commercial aquaculture: identifying gaps in culture initiatives to inform local fisheries managers. PLoS ONE. 9(9):e105982
Talmage SC, Gobler CJ (2009) The effects of elevated carbon dioxide concentrations on the metamorphosis, size, and survival of larval hard clams (Mercenaria mercenaria), bay scallops (Argopecten irradians), and Eastern oysters (Crassostrea virginica). Limnol Oceanogr 54(6):2072–2080
Pereira MA, Nunes MM, Nuernberg L, Schulz D, Batista CRV (2006) Microbiological quality of oysters (Crassostrea gigas) produced and commercialized in the coastal region of Florianópolis—Brazil. Braz J Microbiol 37(2):159–163
Chávez-Villalba J, Reynaga-Franco F, Hoyos-Chairez F (2022) Worldwide overview of reproduction, juvenile collection, spat production and cultivation of pen shells. Rev Aquac. 14(3):1371–88
Unuma T, Sakai Y, Agatsuma Y, Kayaba T. Sea urchin aquaculture in Japan. In: Echinoderm aquaculture. Wiley, Hoboken, NJ, 2015. p. 75–126.
Manore AJW, Harper SL, Sargeant JM, Weese JS, Cunsolo A, Bunce A et al (2020) Cryptosporidium and Giardia in locally harvested clams in Iqaluit. Nunavut Zoonoses Public Health 67(4):352–361
Davidson K, Dudas SE (2016) Microplastic ingestion by wild and cultured manila clams (Venerupis philippinarum) from Baynes Sound. British Columbia Arch Environ Contam Toxicol 71(2):147–156
Hondula K, Pace M (2014) Macroalgal support of cultured hard clams in a low nitrogen coastal lagoon. Mar Ecol Prog Ser 498:187–201
Bannister RCA, Addison JT (1998) Enhancing lobster stocks: a review of recent European methods, results, and future prospects. Bull Mar Sci 62(2):369–387
Baechler BR, Stienbarger CD, Horn DA, Joseph J, Taylor AR, Granek EF et al (2020) Microplastic occurrence and effects in commercially harvested North American finfish and shellfish: current knowledge and future directions. Limnol Oceanogr Lett 5(1):113–136
Hinchcliffe J, Agnalt A, Daniels CL, Drengstig A, Lund I, McMinn J et al (2022) European lobster Homarus gammarus aquaculture: technical developments, opportunities and requirements. Rev Aquac 14(2):919–937
Bordbar S, Anwar F, Saari N (2011) High-value components and bioactives from sea cucumbers for functional foods—a review. Mar Drugs 9(10):1761–1805
Pangestuti R, Arifin Z (2018) Medicinal and health benefit effects of functional sea cucumbers. J Tradit Complement Med 8(3):341–351
Caplan SL, Zheng B, Dawson-Scully K, White CA, West LM (2016) Pseudopterosin a: protection of synaptic function and potential as a neuromodulatory agent. Mar Drugs 14(3):1–14
Chen CH, Chen NF, Feng CW, Cheng SY, Hung HC, Tsui KH et al (2016) A coral-derived compound improves functional recovery after spinal cord injury through its antiapoptotic and anti-inflammatory effects. Mar Drugs 14(9):1–19
Tammam MA, Rárová L, Kvasnicová M, Gonzalez G, Emam AM, Mahdy A et al (2020) Bioactive steroids from the red sea soft coral Sinularia polydactyla. Mar Drugs 18(12):1–16
Soft S. Uncommon capnosane diterpenes with neuroprotective. 2022.
Lee SR, Pronto JRD, Sarankhuu BE, Ko KS, Rhee BD, Kim N et al (2014) Acetylcholinesterase inhibitory activity of pigment echinochrome A from sea urchin Scaphechinus mirabilis. Mar Drugs 12(6):3560–3573
Zhu YZ, Liu JW, Wang X, Jeong IH, Ahn YJ, Zhang CJ (2018) Anti-BACE1 and antimicrobial activities of steroidal compounds isolated from marine urechis unicinctus. Mar Drugs. 16(3):94
Wu FJ, Xue Y, Liu XF, Xue CH, Wang JF, Du L et al (2014) The protective effect of eicosapentaenoic acid-enriched phospholipids from sea cucumber Cucumaria frondosa on oxidative stress in PC12 cells and SAMP8 mice. Neurochem Int 64(1):9–17
Che H, Du L, Cong P, Tao S, Ding N, Wu F et al (2017) Cerebrosides from sea cucumber protect against oxidative stress in SAMP8 mice and PC12 cells. J Med Food 20(4):392–402
Li Q, Che H, Wang C, Zhang L, Ding L, Xue C, et al. Cerebrosides from sea cucumber improved A β 1–42‐induced cognitive deficiency in a rat model of Alzheimer’s disease. Mol Nutr Food Res. 2018;1800707.
Wu FJ, Xue Y, Tang QJ, Xu J, Du L, Xue CH et al (2013) The protective effects of cerebrosides from sea cucumber and starfish on the oxidative damage in PC12 cells. J Oleo Sci 62(9):717–727
Chalorak P, Sanguanphun T, Limboonreung T, Meemon K (2021) Neurorescue effects of frondoside a and ginsenoside rg3 in c. Elegans Model of Parkinson’s Disease Mol 26(16):1–16
Chalorak P, Sornkaew N, Manohong P, Niamnont N, Malaiwong N, Limboonreung T, et al. Corrigendum to “Diterpene glycosides from Holothuria scabra exert the α-synuclein degradation and neuroprotection from α-synuclein-mediated neurodegeneration in C. elegans model” [J. Ethnopharmacol. 279 (2021) 114347](S0378874121005766)(https://doi.org/10.1016/j.jep.2021. J Ethnopharmacol. 2023;301.
Tangrodchanapong T, Sornkaew N, Yurasakpong L, Niamnont N, Nantasenamat C, Sobhon P et al (2021) Beneficial effects of cyclic ether 2-butoxytetrahydrofuran from sea cucumber Holothuria scabra against Aβ aggregate toxicity in transgenic Caenorhabditis elegans and potential chemical interaction. Molecules 26(8):2195
Prebble DW, Er S, Hlushchuk I, Domanskyi A, Airavaara M, Ekins MG et al (2022) α-Synuclein binding activity of the plant growth promoter asterubine. Bioorganic Med Chem Lett 64:128677
Leirós M, Sánchez JA, Alonso E, Rateb ME, Houssen WE, Ebel R et al (2014) Spongionella secondary metabolites protect mitochondrial function in cortical neurons against oxidative stress. Mar Drugs 12(2):700–718
Zhou X, Lu Y, Lin X, Yang B, Yang X, Liu Y (2011) Brominated aliphatic hydrocarbons and sterols from the sponge Xestospongia testudinaria with their bioactivities. Chem Phys Lipids 164(7):703–706
Frau J, Flores-Holguín N, Glossman-Mitnik D (2018) Chemical reactivity properties, PKA values, ages inhibitor abilities and bioactivity scores of the mirabamides A-H peptides of marine origin studied by means of conceptual DFT. Mar Drugs. 16(9):302
Kolesnikova SA, Lyakhova EG, Kalinovsky AI, Popov RS, Yurchenko EA, Stonik VA (2018) Oxysterols from a marine sponge Inflatella sp. and their action in 6-hydroxydopamine-induced cell model of Parkinson’s disease. Mar Drugs. 16(11):458
Feng CW, Chen NF, Wen ZH, Yang WY, Kuo HM, Sung PJ et al (2019) In Vitro and in vivo neuroprotective e-ects of stellettin b through anti-apoptosis and the nrf2/ho-1 pathway. Mar Drugs. 17(6):1888
Miguel-Gordo M, Gegunde S, Calabro K, Jennings LK, Alfonso A, Genta-Jouve G et al (2019) Bromotryptamine and bromotyramine derivatives from the tropical southwestern pacific sponge Narrabeena nigra. Mar Drugs. 17(6):319
Yurchenko EA, Kolesnikova SA, Lyakhova EG, Menchinskaya ES, Pislyagin EA, Chingizova EA et al (2020) Lanostane triterpenoid metabolites from a Penares sp. marine sponge protect neuro-2a cells against paraquat neurotoxicity. Molecules. 25(22):1–14
Esposito G, Mai LH, Longeon A, Mangoni A, Durieu E, Meijer L et al (2019) A collection of bioactive nitrogen-containing molecules from the marine sponge acanthostrongylophora ingens. Mar Drugs 17(8):1–13
Sun Q, Liu F, Sang J, Lin M, Ma J, Xiao X et al (2019) 9-Methylfascaplysin is a more potent Aβ aggregation inhibitor than the marine-derived alkaloid, fascaplysin, and produces nanomolar neuroprotective effects in SH-SY5Y cells. Mar Drugs 17(2):1–15
Jennings LK, Robertson LP, Rudolph KE, Munn AL, Carroll AR (2019) Anti-prion butenolides and diphenylpropanones from the Australian Ascidian Polycarpa procera. J Nat Prod 82(9):2620–2626
Fadhila N, Sriwidodo S, Chaerunisaa A (2022) Instant granules of mangosteen peel (Garcinia mangostana L.) ethanol extract as antioxidants. Sci Pharm. 1(1):1–7
Umar AK, Zothantluanga JH (2021) Structure-based virtual screening and molecular dynamics of quercetin and its natural derivatives as potent oxidative stress modulators in ROS-induced cancer. Indones J Pharm 3(2):60
Umar AK, Kelutur FJ, Zothantluanga JH (2021) Flavonoid compounds of Buah Merah (Pandanus conoideus Lamk) as a potent oxidative stress modulator in ROS-induced cancer. In Silico Approach Maj Obat Tradis 26(3):221
Slamenova D, Kozics K, Hunakova L, Melusova M, Navarova J, Horvathova E (2013) Comparison of biological processes induced in HepG2 cells by tert-butyl hydroperoxide (t-BHP) and hydroperoxide (H2O2): the influence of carvacrol. Mutat Res Toxicol Environ Mutagen 757(1):15–22
Cui Y, Liu B, Xie J, Xu P, Habte-Tsion H-M, Zhang Y (2014) The effect of emodin on cytotoxicity, apoptosis and antioxidant capacity in the hepatic cells of grass carp (Ctenopharyngodon idellus). Fish Shellfish Immunol 38(1):74–79
Tassa B, Sahu N, Barman N, Sahu G (2023) Antihyperlipidemic and antioxidant activities of ethanolic extract of Paederia foetida Leaves (EEPFL) in Albino rats. Sci Pharm 2(1):22–30
Hybertson BM, Gao B, Bose SK, McCord JM (2011) Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Mol Aspects Med 32(4–6):234–246
Jiang W-D, Liu Y, Hu K, Jiang J, Li S-H, Feng L et al (2014) Copper exposure induces oxidative injury, disturbs the antioxidant system and changes the Nrf2/ARE (CuZnSOD) signaling in the fish brain: protective effects of myo-inositol. Aquat Toxicol 155:301–313
Sarkar S, Mukherjee S, Chattopadhyay A, Bhattacharya S (2014) Low dose of arsenic trioxide triggers oxidative stress in zebrafish brain: Expression of antioxidant genes. Ecotoxicol Environ Saf 107:1–8
Sandau KB, Brüne B (2000) Up-regulation of Bcl-2 by redox signals in glomerular mesangial cells. Cell Death Differ 7(1):118–125
Korsmeyer SJ, Wei MC, Saito M, Weiler S, Oh KJ, Schlesinger PH (2000) Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ 7(12):1166–1173
Guerrero AD, Chen M, Wang J (2008) Delineation of the caspase-9 signaling cascade. Apoptosis 13(1):177–186
Xi H, Wu R, Liu J, Zhang L, Li Z (2015) Role of acetylcholinesterase in lung cancer. Thorac Cancer 6(4):390–398
Ferreira-Vieira TH, Guimaraes IM, Silva FR, Ribeiro FM (2016) Alzheimer’s disease: targeting the cholinergic system. Curr Neuropharmacol. 14(1):101–15
Francis PT, Palmer AM, Snape M, Wilcock GK (1999) The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol Neurosurg Psychiatry 66(2):137–147
Abreu-Villaça Y, Filgueiras CC, Manhães AC (2011) Developmental aspects of the cholinergic system. Behav Brain Res 221(2):367–378
Bu X-L, Xiang Y, Jin W-S, Wang J, Shen L-L, Huang Z-L et al (2018) Blood-derived amyloid-β protein induces Alzheimer’s disease pathologies. Mol Psychiatry 23(9):1948–1956
Tan JZA, Gleeson PA (2019) The role of membrane trafficking in the processing of amyloid precursor protein and production of amyloid peptides in Alzheimer’s disease. Biochim Biophys Acta Biomembr 1861(4):697–712
Vassar R (2016) BACE1 inhibition as a therapeutic strategy for Alzheimer’s disease. J Sport Heal Sci 5(4):388–390
Ahmad A, Ali T, Park HY, Badshah H, Rehman SU, Kim MO (2017) Neuroprotective effect of fisetin against amyloid-beta-induced cognitive/synaptic dysfunction, neuroinflammation, and neurodegeneration in adult mice. Mol Neurobiol 54(3):2269–2285
Hoppe JB, Haag M, Whalley BJ, Salbego CG, Cimarosti H (2013) Curcumin protects organotypic hippocampal slice cultures from Aβ1–42-induced synaptic toxicity. Toxicol Vitr 27(8):2325–2330
Wang H, Jiang T, Li W, Gao N, Zhang T (2018) Resveratrol attenuates oxidative damage through activating mitophagy in an in vitro model of Alzheimer’s disease. Toxicol Lett 282:100–108
Mor DE, Daniels MJ, Ischiropoulos H (2019) The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord 34(2):167–179
Mahul-Mellier A-L, Burtscher J, Maharjan N, Weerens L, Croisier M, Kuttler F et al (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci 117(9):4971–4982
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J et al (2017) Parkinson disease. Nat Rev Dis Prim 3(1):17013
Potdar C, Kaushal A, Raj A, Mallick R, Datta I (2022) Reduction of phosphorylated α-synuclein through downregulation of casein kinase 2α alleviates dopaminergic-neuronal function. Biochem Biophys Res Commun 615:43–48
Gaugler MN, Genc O, Bobela W, Mohanna S, Ardah MT, El-Agnaf OM et al (2012) Nigrostriatal overabundance of α-synuclein leads to decreased vesicle density and deficits in dopamine release that correlate with reduced motor activity. Acta Neuropathol 123(5):653–669
Gao J, Perera G, Bhadbhade M, Halliday GM, Dzamko N (2019) Autophagy activation promotes clearance of α-synuclein inclusions in fibril-seeded human neural cells. J Biol Chem 294(39):14241–14256
Zhang Z, Shen Y, Luo H, Zhang F, Peng D, Jing L et al (2018) MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp Neurol 308:59–71
Soto C, Satani N (2011) The intricate mechanisms of neurodegeneration in prion diseases. Trends Mol Med 17(1):14–24
Corsaro A, Thellung S, Villa V, Nizzari M, Florio T (2012) Role of prion protein aggregation in neurotoxicity. Int J Mol Sci 13(7):8648–8669
Mallucci GR, White MD, Farmer M, Dickinson A, Khatun H, Powell AD et al (2007) Targeting cellular prion protein reverses early cognitive deficits and neurophysiological dysfunction in prion-infected mice. Neuron 53(3):325–335
Zhou Z, Xiao G (2013) Conformational conversion of prion protein in prion diseases. Acta Biochim Biophys Sin 45(6):465–476
Hutti CR, Welle KA, Hryhorenko JR, Ghaemmaghami S (2020) Global analysis of protein degradation in prion infected cells. Sci Rep 10(1):10800
Barron RM, King D, Jeffrey M, McGovern G, Agarwal S, Gill AC et al (2016) PrP aggregation can be seeded by pre-formed recombinant PrP amyloid fibrils without the replication of infectious prions. Acta Neuropathol 132(4):611–624
Gough KC, Maddison BC (2010) Prion transmission. Prion 4(4):275–282
Chen C, Dong X (2021) Therapeutic implications of prion diseases. Biosaf Heal 3(2):92–100
McCarthy JM, Franke M, Resenberger UK, Waldron S, Simpson JC, Tatzelt J et al (2013) Anti-Prion Drug mPPIg5 Inhibits PrPC Conversion to PrPSc. PLoS ONE 8(1):e55282
Jossé L, Marchante R, Zenthon J, von der Haar T, Tuite MF (2012) Probing the role of structural features of mouse PrP in yeast by expression as Sup35-PrP fusions. Prion 6(3):201–210
Chaudhuri J, Bains Y, Guha S, Kahn A, Hall D, Bose N et al (2018) The role of advanced glycation end products in aging and metabolic diseases: bridging association and causality. Cell Metab 28(3):337–352
Shen C-Y, Lu C-H, Wu C-H, Li K-J, Kuo Y-M, Hsieh S-C et al (2020) The development of maillard reaction, and advanced glycation end product (AGE)-receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules 25(23):5591
Rungratanawanich W, Qu Y, Wang X, Essa MM, Song B-J (2021) Advanced glycation end products (AGEs) and other adducts in aging-related diseases and alcohol-mediated tissue injury. Exp Mol Med 53(2):168–188
D’Cunha NM, Sergi D, Lane MM, Naumovski N, Gamage E, Rajendran A et al (2022) The effects of dietary advanced glycation end-products on neurocognitive and mental disorders. Nutrients 14(12):2421
Vitek MP, Bhattacharya K, Glendening JM, Stopa E, Vlassara H, Bucala R et al (1994) Advanced glycation end products contribute to amyloidosis in Alzheimer disease. Proc Natl Acad Sci 91(11):4766–4770
Origlia N, Bonadonna C, Rosellini A, Leznik E, Arancio O, Yan SS et al (2010) Microglial receptor for advanced glycation end product-dependent signal pathway drives β-amyloid-induced synaptic depression and long-term depression impairment in entorhinal cortex. J Neurosci 30(34):11414–11425
Münch G, Lüth HJ, Wong A, Arendt T, Hirsch E, Ravid R et al (2000) Crosslinking of α-synuclein by advanced glycation endproducts—an early pathophysiological step in Lewy body formation? J Chem Neuroanat 20(3–4):253–257
Tobon-Velasco J, Cuevas E, Torres-Ramos M (2014) Receptor for AGEs (RAGE) as mediator of NF-kB pathway activation in neuroinflammation and oxidative stress. CNS Neurol Disord Drug Targets 13(9):1615–1626
Geerlings P, De Proft F, Langenaeker W (2003) Conceptual density functional theory. Chem Rev 103(5):1793–1874
Rahman A, Ali MT, Shawan MMAK, Sarwar MG, Khan MAK, Halim MA (2016) Halogen-directed drug design for Alzheimer’s disease: a combined density functional and molecular docking study. Springerplus 5(1):1346
Berry C, La Vecchia C, Nicotera P (2010) Paraquat and Parkinson’s disease. Cell Death Differ 17(7):1115–1125
Colle D, Farina M, Ceccatelli S, Raciti M (2018) Paraquat and maneb exposure alters rat neural stem cell proliferation by inducing oxidative stress: new insights on pesticide-induced neurodevelopmental toxicity. Neurotox Res 34(4):820–833
Zhao F, Wang W, Wang C, Siedlak SL, Fujioka H, Tang B et al (2017) Mfn2 protects dopaminergic neurons exposed to paraquat both in vitro and in vivo : Implications for idiopathic Parkinson’s disease. Biochim Biophys Acta - Mol Basis Dis 1863(6):1359–1370
Arsac J-N, Sedru M, Dartiguelongue M, Vulin J, Davoust N, Baron T et al (2021) Chronic exposure to paraquat induces alpha-synuclein pathogenic modifications in drosophila. Int J Mol Sci 22(21):11613
Sun Y, Zheng J, Xu Y, Zhang X (2018) Paraquat-induced inflammatory response of microglia through HSP60/TLR4 signaling. Hum Exp Toxicol 37(11):1161–1168
Buneeva OA, Medvedev AE (2021) DJ-1 protein and its role in the development of Parkinson’s disease: studies on experimental models. Biochemistry 86(6):627–640
Gallinat A, Rakovic A, Klein C, Badimon L (2022) DJ-1 regulates mitochondrial gene expression during ischemia and reperfusion. Free Radic Biol Med 193:430–436
Zhang X-L, Wang Z-Z, Shao Q-H, Zhang Z, Li L, Guo Z-Y et al (2019) RNAi-mediated knockdown of DJ-1 leads to mitochondrial dysfunction via Akt/GSK-3ß and JNK signaling pathways in dopaminergic neuron-like cells. Brain Res Bull 146:228–236
Zhang Y, Gong X-G, Wang Z-Z, Sun H-M, Guo Z-Y, Hu J-H et al (2016) Overexpression of DJ-1/PARK7, the Parkinson’s disease-related protein, improves mitochondrial function via Akt phosphorylation on threonine 308 in dopaminergic neuron-like cells. Eur J Neurosci. 43(10):1379–88
Vasseur S, Afzal S, Tardivel-Lacombe J, Park DS, Iovanna JL, Mak TW (2009) DJ-1/PARK7 is an important mediator of hypoxia-induced cellular responses. Proc Natl Acad Sci 106(4):1111–1116
De Zoysa M. Medicinal benefits of marine invertebrates. 2012. p. 153–69.
Haggag Y, Abd Elrahman A, Ulber R, Zayed A (2023) Fucoidan in pharmaceutical formulations: a comprehensive review for smart drug delivery systems. Mar Drugs 21(2):112
Umar AK, Sriwidodo S, Maksum IP, Wathoni N (2021) Film-forming spray of water-soluble chitosan containing liposome-coated human epidermal growth factor for wound healing. Molecules 26(17):5326
Umar AK, Butarbutar M, Sriwidodo S, Wathoni N. Film-forming sprays for topical drug delivery. Drug Des Devel Ther. 2020.
Umar AK. Stem cell’s secretome delivery systems. Adv Pharm Bull. 2022.
Sriwidodo S, Umar AK, Wathoni N, Zothantluanga JH, Das S, Luckanagul JA (2022) Liposome-polymer complex for drug delivery system and vaccine stabilization. Heliyon 8(2):e08934
Sriwidodo ST, Maksum IP, Subarnas A, Kesumawardhany B, Lestari DD et al (2020) Preparation and optimization of Chitosan-hEGF nanoparticle using ionic gelation method stabilized by polyethylene glycol (PEG) for wound healing therapy. Int J Res Pharm Sci. 11(1):1220–30
Umar AK, Luckanagul JA, Zothantluanga JH, Sriwidodo S (2022) Complexed polymer film-forming spray: An optimal delivery system for secretome of mesenchymal stem cell as diabetic wound dressing? Pharmaceuticals 15(7):867
Illastria-Rosalina A, Sagita E, Iskandarsyah I (2023) Novasome: combining ufasome and niosome for excellent vesicular drug delivery system. Sci Pharm. 2(1):35–49
Sriwidodo S, Pratama R, Umar AK, Chaerunisa AY, Ambarwati AT, Wathoni N (2022) Preparation of mangosteen peel extract microcapsules by fluidized bed spray-drying for tableting: improving the solubility and antioxidant stability. Antioxidants 11(7):1331
Acknowledgements
The authors thank the Chulalongkorn University’s Graduate Scholarship Programme (ASEAN and Non-ASEAN scholarship) for funding their Master’s (BR) and PhD (AKU) study in Thailand.
Funding
This work received no funding.
Author information
Authors and Affiliations
Contributions
BR and AKU contributed to conceptualization, resources, writing—original draft, and writing—review and editing; BR was involved in data curation and investigation; and AKU contributed to formal analysis, supervision, and methodology.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no conflicting interest. The scholarship funder had no role in the design of the study; in the collection, analysis, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rivai, B., Umar, A.K. Neuroprotective compounds from marine invertebrates. Beni-Suef Univ J Basic Appl Sci 12, 71 (2023). https://doi.org/10.1186/s43088-023-00407-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-023-00407-3