Abstract
A prevalent liver condition called non-alcoholic fatty liver disease (NAFLD) may progress into non-alcoholic steatohepatitis (NASH) and cause life-threatening complications like cirrhosis and liver cancer. The development and progression of NAFLD has been linked to the make-up and functioning of the gut microflora. This article reviews the clinical studies reported to investigate the connection between changes in the gut microbiota and metabolic markers in NAFLD patients. According to the study findings, dysbiosis of the gut microflora were observed in NAFLD patients, which are manifested by variations in the proportions of particular bacterial species. These changes are linked to fibrosis, liver inflammation, and metabolic abnormalities. The article also discusses various treatments targeting the gut microbiota, including dietary modifications, exercise, prebiotics, probiotics, synbiotics, antibiotics, and fecal microbiota transplantation. These therapies are intended to enhance NAFLD outcomes and reestablish the healthy gut microflora. While some studies have shown promising results, further research is needed to establish the optimal approaches, long-term safety, and efficacy of these treatments for NAFLD.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is characterized by the accumulation of fat in liver cells, unrelated to alcohol consumption. When the fat level in the liver exceeds 5–10% of its weight, inflammation occurs. If NAFLD progresses, it can lead to non-alcoholic steatohepatitis (NASH), which increases the risk of liver cancer, fibrosis, cirrhosis, and liver failure. Approximately one in five individuals with NAFLD eventually develops NASH, and if left untreated, NAFLD can increase the risk of liver cancer [1, 2]. The etiology of NAFLD is complex and influenced by biological, environmental, and lifestyle factors. Recent research has highlighted the crucial role of gut dysbiosis, an imbalance in the gut microbiota composition and function, in the development of NAFLD. The disruption of the symbiotic relationship between the host and gut microorganisms contributes to NAFLD pathogenesis, emphasizing the significance of the gut–liver axis [3,4,5].
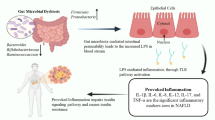
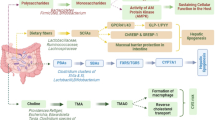
Gut dysbiosis is characterized by a variation in the makeup and functioning of the gut microflora. Dysbiosis increases the formation of microbial metabolites, changes the way by which gut barrier works, and stirs up immunological responses, all of which have been linked to inflammation associated with NAFLD (Fig. 1). Dysbiosis-induced inflammation can cause the liver to produce reactive oxygen species (ROS), which can harm the liver cells through oxidative stress. This oxidative stress exacerbates liver inflammation further and hastens NAFLD progression [6,7,8].
In our prior review, we studied the complex connection between gut dysbiosis and NAFLD [9]. We explored the underlying mechanisms that intricately link the liver, gut, and systemic metabolism. These mechanisms encompass alterations in gut permeability, inflammation, immunological dysregulation, changes in bile acid metabolism, and the impact of microbiota-derived metabolites. Through a comprehensive analysis of the existing research and experimental studies documented in various literature databases, we elucidated the pathways connecting gut dysbiosis to the initiation and progression of NAFLD. Further in this article, we provide a comprehensive review of clinical investigations that have examined the relationship between alterations in the gut microflora and metabolic indicators in individuals diagnosed with NAFLD. Additionally, the paper offers an in-depth discussion of therapeutic interventions aimed at manipulating the gut microbiota, encompassing dietary adjustments, physical activity, prebiotics, probiotics, synbiotics, antibiotic treatments, and fecal microbiota transplantation.
Search strategy
A comprehensive search was conducted across five electronic databases: PubMed, Web of Science, Embase, ScienceDirect, and Google Scholar, covering the period from 2013 to August 2023. To investigate gut microbiota changes in relation to NAFLD, our search incorporated various keywords including “Cirrhosis,” “NAFLD,” “Liver Disease,” “NASH,” “Gut Microflora,” “Fecal Microbiome,” “Gut Dysbiosis,” “Steatohepatitis,” and “Simple Steatosis.” Our primary research emphasis focused on human studies concerning the diagnostic utility of fecal microbiota analysis in identifying NAFLD. To ensure the robustness of the data collected, we have included studies that employed analytical techniques like 16S rRNA gene sequencing, 16S rDNA sequencing, shotgun metagenomics sequencing, and quantitative real-time PCR.
In order to investigate therapeutic interventions, a comprehensive search strategy was employed, involving a diverse range of keywords such as “Probiotics,” “Lactobacillus,” “Bifidobacterium,” “Cirrhosis,” “NAFLD,” “Liver Disease,” “NASH,” and “Gut Microflora,” with a primary focus on human studies, particularly those exploring probiotic usage in NAFLD treatment.
Clinical studies on gut microbiota in NAFLD
Studies on humans have uncovered crucial information on the changes in gut microflora composition and functions related to NAFLD. Table 1 summarizes the clinical studies that have been documented correlating the emergence of variations in gut microflora among NAFLD patients.
HP-NASH histology proven non-alcoholic steatohepatitis, HC healthy controls, SS simple steatosis, PCR polymerase chain reaction, ALT alanine transaminase, AST aspartate transaminase, BP biopsy-proven, LC liver cirrhosis, LC-MS liquid chromatography-mass spectrometry, BMI body mass index
A series of studies investigated the role of gut microbiota and related factors in various liver conditions. Wong et al. [10] found that fecal dysbiosis significantly impacts individuals with NASH and that improvements in liver fat accumulation are associated with changes in the gut microflora. However, the study was limited to a relatively small sample population that included exclusively participants of Chinese origin, thereby limiting generalizability to a wider population. Mouzaki and coworkers [11] observed an inverse relationship between the occurrence of NASH and the quantity of Bacteroidetes in stool samples, suggesting the potential role of this microbiota in NAFLD progression. However, the study did not consider age as a confounding factor and used quantitative PCR, which may not identify new microbial species effectively.
Jiang et al. [12] analyzed 53 patients with NAFLD, reporting decreased levels of CD8+ and CD4+ T cells, elevated pro-inflammatory cytokines, and increased liver enzyme levels in these individuals. The homogenous focus of this study on Chinese volunteers however limits its correlation to a larger demographics. Boursier et al. [13] associated dysbiosis and metabolic changes in the gut microbiota with NAFLD severity, with specific microbial species linked to NASH and severe fibrosis, however again restricted to a small sample size, 57 NAFLD patients. Elevated plasma ALT levels and reduced abundance of several bacterial genera was linked by Wang et al. [14]. They also identified a negative correlation between Lactobacillus and various NAFLD markers. However, the study relied on ultrasound for NAFLD diagnosis, limiting disease severity assessment.
Shen et al. [15] suggested that decreased Prevotella levels could be detrimental in NAFLD, while higher levels of Blautia and E. Shigella might indicate NAFLD progression. However, the study’s limited BMI diversity requires further investigation of the impact of BMI on gut microbiota in NAFLD. Del Chierico and coworkers [16] in their study identified specific microbial markers for the onset and progression of NAFLD but emphasized the need for well-defined study groups for model validation. Hoyles et al. [17] connected microbiome changes and serum biomarkers to NAFLD, but their study exclusively included female volunteers, thereby raising concerns over its reproducibility in male counterparts. In the study carried out by Caussy et al. [18], the metabolites associated with hepatic fibrosis were identified, but exploration of the role of bile acid changes in NAFLD progression were not addressed. Li et al. [19] identified specific microbial taxa linked to obesity and NAFLD through metastatic analysis. However, the cross-sectional design of the study and lack of liver biopsy driven interpretation, limits the conclusions drawn. The association of changes in gut microbiota function with NAFLD were well studied by Schwimmer et al. [20]. However, this study failed to assess impact of dietary factors on disease progression.
Tsai et al. [21] suggested that shifts in bacterial composition impacts the development of NAFLD/NASH but did not correlate the LPS levels in NASH. Further, the study in specific ethnic population (Taiwan) is the major limitation. Daud and colleagues [22] reported reduced gut microbial diversity in NAFLD but involve a small sample population and lacked histopathological confirmation. Li et al. [23] proposed using specific microbial markers and metabolites for HCC diagnosis. However, this study again focused on a small sample population and did not consider disease prognosis.
In all the abovementioned studies among patients with NAFLD, a reduced diversity of gut bacteria is observed, linked to disease severity and progression. This decrease in microbial species is accompanied by an increase in potentially harmful bacteria, especially from the Proteobacteria family. This includes species like Escherichia coli, Lactobacillus, and Streptococcus, contributing to inflammation and increased gut permeability via endotoxin production. Beneficial anti-inflammatory bacteria like Bifidobacterium and F. prausnitzii are often diminished in NAFLD patients. The Firmicutes to Bacteroidetes (F/B) ratio, a biomarker of gut dysbiosis, tends to be higher in NAFLD, indicating an imbalance between these phyla. These shifts in gut microflora suggest a transition toward a more pro-inflammatory and less diverse microbial community in NAFLD, though specific profiles may vary among individuals and populations, highlighting the complexity of gut dysbiosis in the disease.
Limitations and challenges in studying impact of gut microbiota in NAFLD
Studying the correlation between gut dysbiosis and NAFLD underlines considerable challenges. The extensive diversity and individual variability of the gut microbiota hinder the identification of specific microbial patterns linked to NAFLD, influenced by genetics, lifestyle, and diet. Understanding whether changes in the microbiota cause NAFLD or if NAFLD leads to changes in the microbiota is complex due to the fact that there are interactions occurring in both directions. This complexity necessitates longitudinal studies, which track changes over time, in order to establish causal relationships. Often, studies investigating gut dysbiosis in NAFLD suffer from limited sample sizes and cohort diversity, with varied disease stages, ethnical, geographical, and cultural variations, posing obstacles to data interpretation and generalization. Research predominantly conducted in animal models may not fully mirror human NAFLD complexity, with translation hurdles arising from species differences and multifactorial disease nature. Inconsistent methodologies for sample collection, DNA extraction, and data analysis yield result variability, underlining the importance of reproducibility across research groups. The intricate interactions between microbial communities and host physiology supporting gut dysbiosis’ effect on NAFLD remain only partly understood, complicating the elucidation of precise mechanisms. Addressing these limitations necessitates well-designed, expansive studies featuring standardized approaches, integration of multi-omics data, and interdisciplinary collaborations, contributing to a more nuanced understanding of the gut-liver axis' role in NAFLD onset and progression [24].
NAFLD treatments focusing on the gut microbiota
NAFLD has turned out to be one among the major contributors to hepatic damage in recent years. If NAFLD worsens, it can lead to a condition known as nonalcoholic steatohepatitis or NASH. NASH can cause liver cancer, fibrosis (excessive accumulation of scar tissue or fibrous tissue in the liver), cirrhosis (advanced stage of liver disease characterized by widespread fibrosis and the formation of regenerative nodules in the liver tissue), and finally liver failure. Thus, a significant risk factor for liver cancer is NAFLD. Primarily dietary changes and lifestyle modifications are currently available treatments for NAFLD, which are conceptually straightforward but extremely challenging to follow. Other pharmacological therapies now in use are aimed at relieving the symptoms of NAFLD rather than addressing the disease’s pathophysiology. Therefore, it is crucial to focus on the underlying mechanisms causing NAFLD development and to pinpoint molecular targets for more effective therapy methods [25]. An attention has been drawn to therapies that target the gut microbiota as possible therapeutic approaches for NAFLD. Here are a few instances of interventions that try to alter the gut flora and how they affect NAFLD [26, 27].
Diet
Fat-rich diets and cholesterol have been linked with liver fibrosis, inflammation, and hepatic steatosis. A 5% weight fall may help with steatosis, but a 7–10% loss is essential to reduce inflammation and fibrosis. It is typically advised to eat a low-fat, low-carb diet with a reduction in calorie intake (500–1000 kcal/day loss to result in a weight loss of 0.5–1.0 kg/week) [28].
Chronic high fat diet treatment to mice was found to be associated with comparatively greater Firmicutes levels and lower Bacterioidetes levels, which causes an elevated F/B ratio. As opposed to that, a diet rich in fiber connected with an elevated level of Akkermansia muciniphila and has been reported to be favorable for reduction of hepatic inflammation [29].
It has been shown that caffeine intake can prevent both the onset of NAFLD and its development to the liver fibrosis stage [30]. Additionally, it has been demonstrated that in a dose-dependent fashion, consumption of coffee is related with improved hepatic enzymes, a lower consequence of cirrhosis and HCC, and fatalities [31]. Additionally, greater coffee consumption has a connection to modifications in the makeup of the gut microflora; in actual, elevated numbers of Prevotella, Bacteroides, and Porphyromonas have been observed in individuals with high coffee intake [32, 33]. Improvements in various obesity markers have been linked to the use of green tea extract, likely as a result of the Bacteroides-to-Prevotella ratio and the F/B ratio returning to normal [34]. It is interesting that after 12 weeks of dosing, people with NAFLD who received a combination of extract of green tea and 2.5% caffeine reported notable changes in their liver enzymes. Additionally, drinking green tea in fluid form has been shown to change the gut flora in numerous analyses [35].
Exercise
Workout, including both cardio and resistance workouts, has been reported to prevent or sometimes reverse NAFLD, making it one of the more promising recommended lifestyle changes. Inactive lifestyle has been linked to the advancement of the condition; hence, it is indicated that exercise is beneficial due to weight loss as well as additional metabolic effects that come along with exercising [36]. Additionally, modifications in the gut microbiota composition as an outcome of workout have been observed. More precisely, exercise has been linked to lower levels of Parabacteroides, Flavobacterium, and Alkaliphilus in animal studies [37].
Further, exercise has been linked to a relative drop in the F/B ratio and a rise in the levels of Verrucomicrobia and Proteobacteria in overweight women [38]. The interaction between the gut and liver may be able to throw light on some of the strong outcomes of exercise in NAFLD patients. For instance, it has been demonstrated that exercise increases the amount of SCFAs, particularly butyrate. Diet alone has less positive impact than exercise. Significantly, exercise has been found to improve sensitivity to insulin and leads to better LDL drop in animal models with high fat diet, primarily because of changes that exercise induces in the microbiome [39]. Interestingly, Barton et al. [40] found that athletes with active workout regimens had higher levels of Akkermansia spp. than those who were more sedentary. Also, Allen et al. [41] found that exercise increased the amount of Faecalibacterium spp. in lean people in comparison with those suffering from obesity. Faecalibacterium and Akkermansia are both evident for having favorable impacts on health.
Prebiotics
Prebiotics are nondigestible fibers that supply nutrition to the good bacteria in the gut. They promote a healthy gut microflora by specifically promoting the growth and function of particular bacterial populations. Prebiotics aid in gut dysbiosis regulation by promoting beneficial bacteria like Bifidobacteria and Lactobacilli, increasing SCFA production for anti-inflammatory effects, strengthening the gut barrier, and modulating the immune response, fostering defense and reducing inflammation [42, 43].
Akbarzadeh et al. [44] performed a placebo-controlled, double-blind, randomized clinical investigation on 80 obese NAFLD individuals, age ranging from 18 to 77 years to investigate the effect of psyllium and ground wheat on physical parameters and liver enzymes. After 10 weeks of treatment, prebiotic supplementation considerably decreased liver levels of ALT and AST as well as BMI compared to placebo.
Probiotics
Probiotics, according to the WHO, are “live microbes that, when given in sufficient quantities, impart beneficial effects on host’s health”. While the usefulness of probiotics is still been proven in a few conditions, their intake is gaining more popularity as a clinical strategy of disease prevention and improved well-being [45].
Typically, probiotics are types of bacteria or yeast that exist naturally in the gut or have favorable impacts on gut health. Probiotics have been proven to help NAFLD patients with their lipid profiles and liver function tests, although the research on liver histologic abnormalities is yet preliminary. Probiotic delivery studies have been challenged by dietary variables that affect the gut flora’s composition as well as by varied formulas and dosages. Probiotics combat intestinal dysbiosis by restoring microbial balance, strengthening the gut barrier, regulating the immune system, and influencing metabolic processes. They colonize the gut, outcompete pathogens, and produce antimicrobial substances, collectively aiding in gut health and reducing inflammation associated with dysbiosis [46, 47].
Although many probiotic strains were investigated for the treatment of NAFLD, many of these treatments involved combination of Bifidobacteria and Lactobacilli. However, next-generation probiotics with promising outcomes have been found, including Faecalibacterium prausnitzii, Akkermansia muciniphila, and Clostridia strains [48].
Numerous findings have stated the impact of probiotics in NAFLD individuals up to this point (Table 2). Improvements in a number of biochemical markers, such as ALT, AST, and TNF, have been noted in the research.
n sample size, DB-RCT double-blind randomized controlled trial, OL-RCT open label randomized controlled trial, CC HCV-related cirrhosis, RCT randomized controlled trial, DB-PCT double-blind, placebo-controlled trial, NB-RCT non-blinded randomized clinical trial, AC alcohol-related cirrhosis, BP-NAFLD biopsy-proven NAFLD, HP-NASH histology-proven NASH, CH chronic hepatitis linked to HCV.
Synbiotic
Synbiotics, which is a mixture of prebiotics and probiotics, can be consumed to restore a healthy gut flora. Synbiotics have drawn interest as a possible treatment method for the control of NAFLD due to their advantageous effects of encouraging the growth and activity of beneficial bacteria [64].
Synbiotics offer synergistic benefits in treating NAFLD. They reduce hepatic steatosis by improving gut barrier function, control inflammation, and enhance metabolic parameters like insulin resistance and lipid profiles. These effects are attributed to their influence on the gut microbiota and production of beneficial metabolites, including SCFAs, ultimately reducing inflammation in the liver [24].
Scorletti et al. executed double-blind, placebo-controlled phase 2 trial on 104 individuals with NAFLD to observe the impact of synbiotic treatment on hepatic fat content, liver fibrosis-biomarker scores, and gut microbiota composition. The study evaluated the synbiotic comprising of fructo-oligosaccharides and B. animalis subsp. lactis BB-12 against a placebo during a 12-month period. The constitution of the gut microbiota changed as a consequence of the synbiotic therapy, though neither the liver fat content nor the markers of liver fibrosis in NAFLD patients significantly improved. The report emphasizes the need for additional research to investigate alternate NAFLD management techniques [65].
Neyrinck et al. in a study examined the consequences of the symbiotic fructo-oligosaccharides (FOS) and B. animalis combination in middle-aged individuals for a period of 30 days. Comparing the synbiotic therapy to a placebo, it was found that the consistency and frequency of stool was improved while reducing abdominal discomfort. The synbiotic treatment markedly reduced plasma levels of proinflammatory cytokines while having no effect on mood dimensions or the overall composition of the gut microflora. These results indicate that the synbiotic approach may be useful in lowering inflammation and enhancing gut-related symptoms in middle-aged people [66].
Antibiotics
Research and debate about the effectiveness of antibiotics therapy for NAFLD are going on. Antibiotics are often used to treat bacterial infections, but they may also be used to treat NAFLD because it is thought that modifications in the gut microflora may influence disease’s onset and development. Antibiotics can alter the gut microflora by lowering the population of particular bacterial species. Many studies have examined how antibiotics affect NAFLD both in humans and in animals. Reports on animals have demonstrated that taking antibiotics can reduce inflammation and hepatic steatosis (the buildup of fat in the liver). It is challenging to draw firm findings because different research utilizes different antibiotics and treatment lengths [67].
Neomycin, metronidazole, rifaximin, and polymyxin B have been widely employed in the treatment of cirrhosis. Furthermore, the concurrent administration of polymyxin B and neomycin has shown promise in preventing liver lipid accumulation through modulation of the gut microbiota. It is significant to highlight that there are currently no defined recommendations of antibiotics therapy for NAFLD and that their use is still regarded as experimental. The ideal antibiotic regimen, duration, and patient selection criteria must be determined through additional research. Additionally, it is vital to thoroughly assess the possible hazards and long-term implications of antibiotic therapy in NAFLD [68].
Fecal microbiota transplantation (FMT)
Fecal microbiota transplantation (FMT) consists of relocating the fecal microbiota of a healthy donor into the digestive system of a receiver. It has drawn interest as a possible cure for a number of ailments, including NAFLD [69].
Vrieze et al. in their study, investigated how FMT from efficient donors affected metabolic markers in people with metabolic disorder, including NAFLD. The findings demonstrated that FMT with lean donors enhanced insulin sensitivity and decreased liver fat in the receivers. This shows that altering the gut microflora by FMT may be beneficial for treating NAFLD. Individuals with mild hepatic encephalopathy (MHE), a liver disease consequence that may be connected to NAFLD, were estimated for the impacts of FMT on hepatic and cognitive outcomes by Bajaj et al. According to the findings, FMT from a healthy donor significantly improved cognitive function and liver function in recipients in comparison to the placebo group. These findings suggest that FMT may have therapeutic potential for liver-related diseases like NAFLD [70].
Despite the fact that FMT has promise as a viable treatment for NAFLD, further research is necessary to establish its ideal procedures, long-term security, and efficiency. To assess its effectiveness, identify any potential side effects, and develop recommendations for its use in the management of NAFLD, extensive clinical trials are required.
Difficulties in developing NAFLD treatments based on gut microbiota
Customizing treatments based on individual microbial profiles proves challenging and may necessitate personalized strategies. Establishing a clear cause-and-effect relationship between microbiota changes and NAFLD improvement is challenging, necessitating extensive research to unravel the exact mechanisms through which altered microbiota impact liver health. Clinical trials focused on gut microbiota treatments for NAFLD are still in their early stages, lacking robust, large-scale studies that could definitively determine treatment efficacy and safety. The diversity of potential interventions, including prebiotics, probiotics, and fecal microbiota transplantation, demands standardized protocols for dosages, administration, and durations to ensure consistent outcomes.
Navigating complex regulatory pathways for novel microbiota-focused treatments poses challenges in demonstrating safety, efficacy, and regulatory compliance. Successful implementation of microbiota-based interventions often hinges on changes in dietary habits or lifestyles, presenting difficulties in ensuring patient adherence that can influence treatment outcomes. Responses to microbiota-focused therapies can vary significantly among individuals, with some experiencing significant improvements while others see limited or no benefits. The potent influence of placebo effects, especially in studies involving subjective health measures like gastrointestinal symptoms, can confound the interpretation of treatment results. Developing and deploying microbiota-focused treatments can be resource-intensive, potentially limiting accessibility to a broader patient population. Ethical considerations surround interventions like fecal microbiota transplantation, spanning donor screening, informed consent, and potential risks to both donors and recipients [24].
Strategic foresight in studying gut dysbiosis and NAFLD
Future research is likely to focus on developing personalized approaches for diagnosing and treating NAFLD based on an individual’s gut microbiome profile. The development of microbiome-based therapies, including FMT, will be a significant area of research. Identifying specific microbial biomarkers for NAFLD could enable early detection and intervention. Forthcoming studies may also focus on developing non-invasive tests that rely on gut microbiota composition for diagnosis in early stage of the disease. Pharmaceutical companies are likely to invest in the development of drugs that target the gut microbiome to manage NAFLD. Lifestyle modifications, including diet and exercise, will continue to play a crucial role in managing the disease. Futuristic strategies may refine dietary guidelines and exercise recommendations tailored to an individual’s microbiome. However, prospective studies will be essential in assessing the long-term effects of gut dysbiosis on NAFLD.
Public health initiatives such as community education, promoting dietary diversity, and optimizing early-life microbiome development should be incorporated to prevent NAFLD and obesity. Monitoring patients over extended periods will provide insights into the progression of the disease and the efficacy of microbiome-based interventions. Integrating gut microbiome data into the framework of precision medicine will allow for more comprehensive, patient-centered approaches for its management. Genomic, metabolic, and microbial information can be combined to provide a holistic view of an individual’s health. Microalgae extracts may have hepatoprotective properties, which could be explored for its potential in managing NAFLD. Sayuti et al. explored the potential benefits of fucoxanthin, a marine carotenoid, in combating NAFLD by examining its hepatoprotective, anti-obesity, anti-tumor, anti-diabetes, antioxidant, and anti-inflammatory properties. Through analysis of human clinical trials, animal experiments, and in vitro studies, fucoxanthin demonstrated positive effects on lipid metabolism, lipogenesis, fatty acid oxidation, adipogenesis, and oxidative stress, highlighting its therapeutic potential for NAFLD [71]. The coming decade will witness the investigation of the synergistic effects of microalgae in combination with other dietary interventions, probiotics, or medications to address both gut dysbiosis and NAFLD simultaneously.
As researchers continue to unravel the intricate interplay between the gut microbiome and NAFLD, we can anticipate innovative diagnostic methods, personalized treatment strategies, and novel therapeutic interventions that will significantly impact the prevention and management of NAFLD. However, it is important to remain vigilant and ensure that these advancements are ethically and responsibly applied in clinical practice.
Conclusion
This article provides valuable insights into the connection between gut dysbiosis and NAFLD, shedding light on the role of specific bacterial species in NAFLD progression and its associated complications. It reviews clinical studies that investigate this relationship, offering a comprehensive overview of the current state of research. These findings serve as a foundation for future research in the field of gut dysbiosis and NAFLD. Researchers can use this information to design more targeted studies, exploring the mechanisms behind gut microbiota alterations and their role in NAFLD progression. Moreover, the review of various treatments targeting the gut microbiota offers a comprehensive overview of potential interventions, paving the way for further investigations into their long-term safety and efficacy. Ultimately, this article contributes to the growing body of knowledge on gut dysbiosis and NAFLD, guiding researchers toward developing more effective strategies for managing and treating this prevalent liver condition in the future. To optimize the therapy for the treatment of NAFLD, however, future research should concentrate on precision medicine, long-term sustainability of interventions, personalized dietary regimens, and interdisciplinary collaboration.
Availability of data and materials
The data that supports the findings of this study are openly available in the dataset like PubMed, Web of Science, Embase, ScienceDirect, and Google Scholar.
References
Blencowe M, Karunanayake T, Wier J, Hsu N, Yang X (2019) Network modeling approaches and applications to unravelling non-alcoholic fatty liver disease. Genes (Basel) 10(12):966
Farzaneh Z, Vosough M, Agarwal T, Farzaneh M (2021) Critical signaling pathways governing hepatocellular carcinoma behavior; small molecule-based approaches. Cancer Cell Int 21(1):208
Kutlu O, Kaleli HN, Ozer E (2018) Molecular pathogenesis of non-alcoholic steatohepatitis- (NASH-) related hepatocellular carcinoma. Can J Gastroenterol Hepatol 8543763
Dimri M, Satyanarayana A (2020) Molecular signaling pathways and therapeutic targets in hepatocellular carcinoma. Cancers (Basel) 12(2):491
Younossi ZM (2019) Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol 70(3):531–544
Bessone F, Razori MV, Roma MG (2019) Molecular pathways of non-alcoholic fatty liver disease development and progression. Cell Mol Life Sci 76(1):99–128
Afzaal M, Saeed F, Shah YA, Hussain M, Rabail R, Socol CT et al (2022) Human gut microbiota in health and disease: unveiling the relationship. Front Microbiol 13:999001
Gasmi A, Mujawdiya PK, Pivina L, Doşa A, Semenova Y, Benahmed AG et al (2021) Relationship between gut microbiota, gut hyperpermeability and obesity. Curr Med Chem 28(4):827–839
Jadhav PA, Thomas AB, Nanda RK, Chitlange SS (2023) Unveiling the role of gut dysbiosis in non-alcoholic fatty liver disease. Eur J Gastroenterol Hepatol 35(12):1324–1333
Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC et al (2013) Molecular characterization of the fecal microbiota in patients with non-alcoholic steatohepatitis-a longitudinal study. PLoS One 8(4):e62885
Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE et al (2013) Intestinal microbiota in patients with non-alcoholic fatty liver disease. Hepatology 58(1):120–127
Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y (2015) Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 5:8096
Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F et al (2016) The severity of non-alcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63(3):764–775
Wang B, Jiang X, Cao M, Ge J, Bao Q, Tang L et al (2016) Altered fecal microbiota correlates with liver biochemistry in nonobese patients with non-alcoholic fatty liver disease. Sci Rep 6:32002
Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG (2017) Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int 16(4):375–381
Del Chierico F, Nobili V, Vernocchi P, Russo A, De Stefanis C, Gnani D et al (2017) Gut microbiota profiling of pediatric non-alcoholic fatty liver disease and obese patients unveiled by an integrated meta-omics-based approach. Hepatology 65(2):451–464
Hoyles L, Fernández-Real JM, Federici M, Serino M, Abbott J, Charpentier J et al (2018) Molecular phenomics and metagenomics of hepatic steatosis in non-diabetic obese women. Nat Med 24(7):1070–1080
Caussy C, Hsu C, Singh S, Bassirian S, Kolar J, Faulkner C et al (2019) Serum bile acid patterns are associated with the presence of NAFLD in twins, and dose-dependent changes with increase in fibrosis stage in patients with biopsy-proven NAFLD. Aliment Pharmacol Ther 49(2):183–193
Li F, Sun G, Wang Z, Wu W, Guo H, Peng L et al (2018) Characteristics of fecal microbiota in non-alcoholic fatty liver disease patients. Sci China Life Sci 61(7):770–778
Schwimmer JB, Johnson JS, Angeles JE, Behling C, Belt PH, Borecki I et al (2019) Microbiome signatures associated with steatohepatitis and moderate to severe fibrosis in children with nonalcoholic fatty liver disease. Gastroenterology 157(4):1109–1122
Tsai MC, Liu YY, Lin CC, Wang CC, Wu YJ, Yong CC et al (2020) Gut microbiota dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: a cross-sectional study in Taiwan. Nutrients 12(3):820
Daud NA, Akram NH, Hidayah N, Jayanti S, Handayani I, Massi MN (2022) Gut microbiome profiling in nonalcoholic fatty liver disease and healthy individuals in Indonesian population. J Med Sci 42:166–174
Li X, Yi Y, Wu T, Chen N, Gu X, Xiang L et al (2023) Integrated microbiome and metabolome analysis reveals the interaction between intestinal flora and serum metabolites as potential biomarkers in hepatocellular carcinoma patients. Front Cell Infect Microbiol 13:1170748
Sharpton SR, Schnabl B, Knight R, Loomba R (2021) Current concepts, opportunities, and challenges of gut microbiome-based personalized medicine in nonalcoholic fatty liver disease. Cell Metab 33(1):21–32
Hrncir T, Hrncirova L, Kverka M, Hromadka R, Machova V, Trckova E et al (2021) Gut microbiota and nafld: pathogenetic mechanisms, microbiota signatures, and therapeutic interventions. Microorganisms 9(5):957
Vallianou N, Christodoulatos GS, Karampela I, Tsilingiris D, Magkos F, Stratigou T et al (2021) Understanding the role of the gut microbiome and microbial metabolites in non-alcoholic fatty liver disease: current evidence and perspectives. Biomolecules 12(1):56
Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M et al (2018) The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 67(1):328–357
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI (2008) Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3(4):213–223
Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F et al (2010) Composition and energy harvesting capacity of the gut microbiota: relationship to diet, obesity and time in mouse models. Gut 59(12):1635–1642
Molloy JW, Calcagno CJ, Williams CD, Jones FJ, Torres DM, Harrison SA (2012) Association of coffee and caffeine consumption with fatty liver disease, non-alcoholic steatohepatitis, and degree of hepatic fibrosis. Hepatology 55(2):429–436
Vitaglione P, Morisco F, Mazzone G, Amoruso DC, Ribecco MT, Romano A et al (2010) Coffee reduces liver damage in a rat model of steatohepatitis: the underlying mechanisms and the role of polyphenols and melanoidins. Hepatology 52(5):1652–1661
Kennedy OJ, Roderick P, Buchanan R, Fallowfield JA, Hayes PC, Parkes J (2016) Systematic review with meta-analysis: coffee consumption and the risk of cirrhosis. Aliment Pharmacol Ther 43(5):562–574
Hodge A, Lim S, Goh E, Wong O, Marsh P, Knight V et al (2017) Coffee intake is associated with a lower liver stiffness in patients with non-alcoholic fatty liver disease, Hepatitis C, and Hepatitis B. Nutrients 9(1):56
Seo DB, Jeong HW, Cho D, Lee BJ, Lee JH, Choi JY et al (2015) Fermented green tea extract alleviates obesity and related complications and alters gut microbiota composition in diet-induced obese mice. J Med Food 18(5):549–556
Pezeshki A, Safi S, Feizi A, Askari G, Karami F (2016) The effect of green tea extract supplementation on liver enzymes in patients with non-alcoholic fatty liver disease. Int J Prev Med 7:28
Baker CJ, Martinez-Huenchullan SF, D'Souza M, Xu Y, Li M, Bi Y et al (2021) Effect of exercise on hepatic steatosis: are benefits seen without dietary intervention? A systematic review and meta-analysis. J Diabetes 13(1):63–77
Ortiz-Alvarez L, Xu H, Martinez-Tellez B (2020) Influence of exercise on the human gut microbiota of healthy adults: a systematic review. Clin Transl Gastroenterol 11(2):e00126
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R (2020) The Firmicutes/Bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients 12(5):1474
Welly RJ, Liu TW, Zidon TM, Rowles JL 3rd, Park YM, Smith TN et al (2016) Comparison of diet versus exercise on metabolic function and gut microbiota in obese rats. Med Sci Sports Exerc 48(9):1688–1698
Barton W, Penney NC, Cronin O, Garcia-Perez I, Molloy MG, Holmes E et al (2018) The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 67(4):625–633
Allen JM, Mailing LJ, Niemiro GM, Moore R, Cook MD, White BA et al (2018) Exercise alters gut microbiota composition and function in lean and obese humans. Med Sci Sports Exerc 50(4):747–757
Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B et al (2014) Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol 11(8):506–514
Lambert JE, Parnell JA, Eksteen B, Raman M, Bomhof MR, Rioux KP et al (2015) Gut microbiota manipulation with prebiotics in patients with non-alcoholic fatty liver disease: a randomized controlled trial protocol. BMC Gastroenterol 15169
Akbarzadeh M, Nourian M, Askari G, Maracy MR, Rafiei R (2015) The effect of psyllium on anthropometric measurements and liver enzymes in overweight or obese adults with nonalcoholic fatty liver disease (NAFLD). Int J Adv Biotechnol Res 33:1771–1783
Hemarajata P, Versalovic J (2013) Effects of probiotics on gut microbiota: mechanisms of intestinal immunomodulation and neuromodulation. Ther Adv Gastroenterol 6(1):39–51
Zhao C, Men X, Dang Y, Zhou Y, Ren Y (2023) Probiotics mediate intestinal microbiome and microbiota-derived metabolites regulating the growth and immunity of rainbow trout (oncorhynchus mykiss). Microbiol Spectr 11(2):e0398022
Hoffmann DE, Fraser CM, Palumbo F, Ravel J, Rowthorn V, Schwartz J (2014) Probiotics: achieving a better regulatory fit. Food Drug Law J 69(2):237–2ii
Govender M, Choonara YE, Kumar P, du Toit LC, van Vuuren S, Pillay V (2014) A review of the advancements in probiotic delivery: conventional vs. non-conventional formulations for intestinal flora supplementation. AAPS Pharm Sci Tech 15(1):29–43
Aller R, De Luis DA, Izaola O, Conde R, Gonzalez Sagrado M, Primo D et al (2011) Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 15(9):1090–1095
Shavakhi A, Minakari M, Firouzian H, Assali R, Hekmatdoost A, Ferns G (2013) Effect of a probiotic and metformin on liver aminotransferases in non-alcoholic steatohepatitis: a double blind randomized clinical trial. J Prev Med 4(5):531–537
Nabavi S, Rafraf M, Somi MH, Homayouni-Rad A, Asghari-Jafarabadi M (2014) Effects of probiotic yogurt consumption on metabolic factors in individuals with nonalcoholic fatty liver disease. J Dairy Sci 97(12):7386–7393
Sepideh A, Karim P, Hossein A, Leila R, Hamdollah M, Mohammad EG et al (2016) Effects of multistrain probiotic supplementation on glycemic and inflammatory indices in patients with nonalcoholic fatty liver disease: a double-blind randomized clinical trial. J Am Coll Nutr 35(6):500–505
Behrouz V, Jazayeri S, Aryaeian N, Zahedi MJ, Hosseini F (2017) Effects of probiotic and prebiotic supplementation on leptin, adiponectin, and glycemic parameters in non-alcoholic fatty liver disease: a randomized clinical trial. Middle East J Dig Dis 9(3):150–157
Abdel Monem SM (2017) Probiotic therapy in patients with nonalcoholic steatohepatitis in zagazig university hospitals. Euroasian J Hepatogastroenterol 7(1):101–106
Ahn SB, Jun DW, Kang BK, Lim JH, Lim S, Chung MJ (2019) Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep 9(1):5688
Duseja A, Acharya SK, Mehta M, Chhabra S, Shalimar Rana S, Das A et al (2019) High potency multistrain probiotic improves liver histology in non-alcoholic fatty liver disease (NAFLD): a randomised, double-blind, proof of concept study. BMJ Open Gastroenterol 6(1):e000315
Chong PL, Laight D, Aspinall RJ, Higginson A, Cummings MH (2021) A randomised placebo-controlled trial of VSL#3® probiotic on biomarkers of cardiovascular risk and liver injury in non-alcoholic fatty liver disease. BMC Gastroenterol 21(1):144
Mohamad Nor MH, Ayob N, Mokhtar NM, Raja Ali RA, Tan GC, Wong Z et al (2021) The effect of probiotics (MCP® BCMC® strains) on hepatic steatosis, small intestinal mucosal immune function, and intestinal barrier in patients with non-alcoholic fatty liver disease. Nutrients 13(9):3192
Ayob N, Muhammad Nawawi KN, Mohamad Nor MH, Raja Ali RA, Ahmad HF, Oon SF et al (2023) The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines 11(2):640
Vajro P, Claudia M, Licenziati M, Franzese A, Vitale D, Lenta S et al (2011) Effects of Lactobacillus rhamnosus Strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr 52(6):740–743
Alisi A, Bedogni G, Baviera G, Giorgio V, Porro E, Paris C et al (2014) Randomised clinical trial: the beneficial effects of VSL#3 in obese children with non-alcoholic steatohepatitis. Aliment PharmacolTher 39(11):1276–1285
Miccheli A, Capuani G, Marini F, Tomassini A, Praticò G, Ceccarelli S et al (2015) Urinary (1)H-NMR-based metabolic profiling of children with NAFLD undergoing VSL#3 treatment. Int J Obes 39(7):1118–1125
Rodrigo T, Dulani S, Nimali Seneviratne S, De Silva AP, Fernando J, De Silva HJ et al (2022) Effects of probiotics combined with dietary and lifestyle modification on clinical, biochemical, and radiological parameters in obese children with nonalcoholic fatty liver disease/nonalcoholic steatohepatitis: a randomized clinical trial. Clin Exp Pediatr 65(6):304–311
Vallianou N, Stratigou T, Christodoulatos GS, Tsigalou C, Dalamaga M (2020) Probiotics, prebiotics, synbiotics, postbiotics, and obesity: current evidence, controversies, and perspectives. Curr Obes Rep 9(3):179–192
Scorletti E, Afolabi PR, Miles EA, Smith DE, Almehmadi A, Alshathry A et al (2020) Synbiotics alter fecal microbiomes, but not liver fat or fibrosis, in a randomized trial of patients with nonalcoholic fatty liver disease. Gastroenterology 158(6):1597–1610.e7
Neyrinck AM, Rodriguez J, Taminiau B, Amadieu C, Herpin F, Allaert FA et al (2021) Improvement of gastrointestinal discomfort and inflammatory status by a synbiotic in middle-aged adults: a double-blind randomized placebo-controlled trial. Sci Rep 11(1):2627
Reijnders D, Goossens GH, Hermes GD, Neis EP, van der Beek CM, Most J et al (2016) Effects of gut microbiota manipulation by antibiotics on host metabolism in obese humans: a randomized double-blind placebo-controlled trial. Cell Metab 24(1):63–74
Gangarapu V, Ince AT, Baysal B, Kayar Y, Kılıç U, Gök Ö, Uysal Ö, Şenturk H (2015) Efficacy of rifaximin on circulating endotoxins and cytokines in patients with nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol 27(7):840–845
Van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM et al (2013) Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 368(5):407–415
Kelly CR, Kim AM, Laine L, Wu GD (2017) The AGA's fecal microbiota transplantation national registry: an important step toward understanding risks and benefits of microbiota therapeutics. Gastroenterology 152(4):681–684
Sayuti NH, Muhammad Nawawi KN, Goon JA, Mokhtar NM, Makpol S (1954) Tan JK (2023) A Review of the effects of fucoxanthin on NAFLD. Nutrients 15(8)
Acknowledgements
The authors would like to thank Dr. D. Y. Patil Institute of Pharmaceutical Sciences and Research, Pimpri, Pune, India, for providing the necessary infrastructural facilities to carry out the work.
Funding
None
Author information
Authors and Affiliations
Contributions
Idea: Asha Thomas; study design: Pranali Jadhav and Asha Thomas; data acquisition: Asha Thomas and Pranali Jadhav; data analysis: Pranali Jadhav and Sohan Chitlange; data interpretation: Asha Thomas and Rabindra Nanda; drafting the manuscript: Pranali Jadhav and Asha Thomas; manuscript revision: Asha Thomas, Rabindra Nanda, and Sohan Chitlange
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
None.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jadhav, P.A., Thomas, A.B., Nanda, R.K. et al. Correlation of non-alcoholic fatty liver disease and gut microflora: clinical reports and treatment options. Egypt Liver Journal 14, 21 (2024). https://doi.org/10.1186/s43066-024-00327-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00327-6