Abstract
Background
The human gut microbiota (GM) is a diverse ecosystem crucial for health, impacting physiological processes across the host's body. This review highlights the GM's involvement in Non-Alcoholic Fatty Liver Disease (NAFLD) and explores its diagnosis, treatment, and management.
Main Text
The GM influences gut functionality, digestion, immunity, and more. Short-chain fatty acids (SCFAs), produced by microbial fermentation, regulate metabolism, inflammation, and immune responses. Bile acids (BAs) modulate the microbiome and liver functions, affecting NAFLD progression. Dysbiosis and increased gut permeability contribute to NAFLD through bacterial components and metabolites reaching the liver, causing inflammation and oxidative stress. The microbiome's impact on immune cells further exacerbates liver damage. Symptoms of NAFLD can be subtle or absent, making diagnosis challenging. Imaging techniques assist in diagnosing and staging NAFLD, but liver biopsy remains vital for accurate assessment. Promising treatments include FXR agonists, GLP-1 agonists, and FGF19 and FGF21 mimetics, targeting various pathways associated with NAFLD pathogenesis. Fecal Microbiota Transplantation (FMT) emerges as a potential therapeutic avenue to restore gut microbiota diversity and alleviate NAFLD. Lifestyle interventions, such as dietary modifications, exercise, and probiotics, also play a pivotal role in managing NAFLD and restoring gut health.
Conclusion
Despite significant progress, the complex interplay between the gut microbiome, NAFLD, and potential treatments necessitates further research to unravel underlying mechanisms and develop effective therapeutic strategies.
Similar content being viewed by others
Introduction
The human gut microbiota (GM) is a complex and diverse ecosystem essential to human health and overall well-being. It comprises an immense number of microorganisms, including bacteria, fungi, archaea, viruses, and helminths [1]. Collectively, these microorganisms are referred to as the gut microbiota, and their combined genetic material is termed the gut microbiome [2]. These microorganisms consist of up to 5000 different species and weigh approximately 1% of an adult human's body mass [3]. Indeed, the GM plays a vital role in supporting various physiological processes of the host. Its most significant contribution lies in supporting the intestine, which ensures optimal gut functionality across multiple aspects. These include aiding in digestion, harvesting energy from nutrients, enhancing mucosal immunity, maintaining the integrity of the intestinal barrier, defending against pathogens, and producing essential vitamins, neurotransmitters (NT), and potentially beneficial bioactive compounds, such as short-chain fatty acids (SCFAs), which are valuable molecules for the host [4,5,6,7,8]. The human gut microbiome closely interacts with different organs within the host body, such as the gut responsible for food digestion, the liver for processing after absorption, and adipose tissue for storage. This significant level of integration has led numerous researchers to assume the human GM to a microbial organ of human body [9]. The gut microbiota consists mainly of four primary categories of microorganisms: Firmicutes, Bacteroides, Actinomycetes, Verrucomicrobia, and Proteus [10] Among these, the ratio of Firmicutes to Bacteroidetes is commonly used as a critical parameter in identifying potential gut health disorders [11]. In recent years, the field of gut microbiota research has experienced significant progress, driven by advancements in molecular biology, genomics, bioinformatics analysis technology, and high-throughput sequencing technology. This review elaborates on the involvement of the gut microbiome in chronic diseases like non-alcoholic fatty liver disease (NAFLD) and explores how it can be diagnosed, treated, and managed for prevention.
Gut microbiota dysbiosis in non-alcoholic fatty liver disease
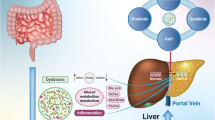
In gut, the microbiota community plays a crucial role in various physiological processes within the human digestive system. Importantly, this community significantly contributes to functions like digestion, metabolism, and protective mechanisms. Several studies illustrate that different pathway of the gut microbiome are involved in the progression of NAFLD [12, 13]. The gut microbiota’s utilization of enzymes is vital for the efficient conversion of undigested polysaccharides into monosaccharides and the conversion of dietary fibers into short-chain fatty acids (SCFAs). This process is crucial as it provides essential energy support to the host cells. These SCFAs form a group of organic acids produced through bacterial fermentation of dietary fibers within the colon (Fig. 1). They have attracted considerable interest due to their potential health benefits, particularly in regulating metabolism, immune responses, the absorption of electrolytes and nutrients, as well as exhibiting anti-inflammatory and antitumor characteristics [14]. The daily production of SCFAs in the colon varies based on the intake of dietary fiber, usually falling within the range of 500 to 600 mmol. Among these SCFAs, acetate, propionate, and butyrate are notable for being the most abundant within the intestinal tract [15].
The involvement of gut microbiota and their resulting substances in the progression of NAFLD. The products generated by gut microorganisms, encompassing monosaccharides, short-chain fatty acids (SCFAs), bile acids (BAs), and trimethylamine oxide (TMAO), assume crucial roles not only in the liver’s energy metabolism and the cellular lining of the intestines but also exert a direct influence on the production of liver fat and overall systemic inflammation. A range of molecular elements come into play, including adenosine monophosphate-dependent protein kinase (AMPK), carbohydrate-responsive element-binding protein (ChREBP), Cytochrome P450 7A1 (CYP7A1), farnesoid X receptor (FXR), glucagon-like peptide-1 (GLP-1), G protein-coupled receptor 41/43 (GPCR41/43), peptide YY (PYY), sterol regulatory element-binding protein 1 (SREBP-1), and Takeda G protein receptor 5 (TGR5). Collectively, these elements contribute to these effects
Acetic acid plays a significant role as a vital energy source for the body, contributing roughly 10% of the daily energy requirement. In contrast, butyric acid assumes a crucial function in providing energy to support epithelial cells, thereby playing a vital role in upholding the integrity of the intestinal barrier. Furthermore, it acts as the primary metabolic substance for the gastrointestinal microbiota, meeting at least 60–70% of their energy demands for growth and differentiation [16]. Moreover, butyric acid has the ability to hinder the activation of Carbohydrate response element binding protein (ChREBP) and sterol regulatory element binding protein 1 (SREBP-1), then, suppress the process of lipogenesis [17]. Propionic acid primarily undergoes catabolism within the liver, participating in the conversion of pyruvate to glucose. Furthermore, it has demonstrated the capacity to diminish lipid buildup in individuals dealing with excess weight and adiposity [18]. Unlike butyric and acetic acids, which serve as energy sources for host cells, propionic acid serves as a precursor for adipogenesis and gluconeogenesis. These latter processes hold greater significance in the development of NAFLD [19]. Dietary fiber has gained recognition for its multitude of health advantages, especially in enhancing digestive well-being and overall vitality. The consumption of dietary fiber, irrespective of its origin, can exert favorable impacts on the intestinal tract, particularly benefiting individuals with insufficient dietary fiber intake [20].
The SCFAs trigger the activation of G protein-coupled receptors, namely GPR41 and GPR43, located within the intestinal and adipose tissues [21]. The stimulation of GPR41 leads to enhance the secretion of glucagon-like peptide 1 and peptide YY (PYY) from enteroendocrine cells. This process, in turn, causes a reduction in intestinal motility while concurrently enhancing the absorption of nutrients. On the other hand, the activation of GPR43 hinders the differentiation of adipocytes and amplifies hepatic lipogenesis, thus fostering the progression of NAFLD [22]. Bile acids constitute the principal elements of bile and can be categorized into primary and secondary forms. Hepatocytes manufacture primary bile acids (PBAs), which encompass cholic acid (CA) and chenodeoxycholic acid (CDCA), through the conversion of cholesterol. Subsequently, these PBAs are secreted into the bile duct. Bacteria located in the small intestine then facilitate the conversion of primary bile acids into secondary bile acids (SBAs), which comprise lithocholic acid (LCA), deoxycholic acid (DCA), ursodeoxycholic acid (UDCA), and corresponding isoforms like isolithocholic acid. Primarily, bile acids play a significant role in regulating the balance of intestinal microbiota by directly preventing the proliferation of harmful bacteria, but they also function as natural activators of the intestinal farnesoid X receptor receptor (FXR). This activation sequence, prioritized as CDCA, DCA, LCA, and CA, triggers the activation of protective genes in the mucosal lining of the ileum. As a result, this helps safeguard the intestinal epithelial cells from the corrosive effects of bacteria and other microorganisms, contributing to overall gut health [23]. Furthermore, FXR decreases the expression of liver X receptor (LXR) and sterol regulatory element binding protein-1c (SREBP-1c), leading to a reduction in the synthesis of fatty acids and triglycerides within the liver. This, in turn, mitigates the processes of steatogenesis and gluconeogenesis. Additionally, FXR enhances hepatic glycogen synthesis by activating fibroblast growth factor (FGF) 15/19, PPARg, GLUT-4, and GLP-1, thereby enhancing insulin sensitivity [24].
The bile acid G-protein-coupled membrane receptor-5 (TGR5) represents another conventional BA receptor, primarily triggered by SBAs (TGR5 activation sequence: LCA > DCA > CDCA > CA). Insufficient SBAs result in decreased FXR activity but heightened inflammation within the body, whereas excessive SBAs have the potential to induce harm to cellular DNA through the generation of reactive oxygen species (ROS), subsequently leading to the emergence of hepatocellular carcinoma (HCC). Research has demonstrated that the activation of TGR5 through SBAs initiates the transcription of the type 2 iodothyronine deiodinase (Dio2) gene. This, in turn, facilitates the conversion of thyroid hormone (T4) to the more potent triiodothyronine (T3), consequently elevating basal metabolism and fostering energy utilization in the brown adipose tissue and muscle of mice fed a high-fat diet (HFD) [25]. Stimulation of TGR5 within cells responsible for intestinal secretion through SBAs triggers the upregulation of GLP1 expression. This, in turn, enhances insulin production and release, leading to safeguarding against apoptosis of islet b-cells and enhancement of blood glucose regulation. Nevertheless, changes in gut microbiota and liver function directly impact the eventual compositions and quantities of BAs. As a result, distinct expression patterns of BA receptors (such as FXR and TGR5) emerge at various stages of the NAFLD progression. In any case, both FXR and TGR5, the two BA receptors, have emerged as potential targets for addressing obesity and NAFLD. Trimethylamine (TMA) and trimethylamine N-oxide (TMAO) are metabolites of choline and its derivatives synthesized by gut microbiota. These compounds not only contribute to the onset of atherosclerosis, but also have a significant effect on the metabolism of cholesterol and triglycerides [26]. Clinical trials have demonstrated that a decrease in choline levels can result in the accumulation of lipids in the liver. This occurs due to a reduction in the production and release of very low-density lipoproteins (VLDL) in liver cells, which ultimately leads to the development of steatohepatitis. This outcome is commonly observed in rodents that are fed a diet deficient in methionine and choline (MCD). It has been established that foods containing dietary methylamine, choline, phosphatidylcholine, and carnitine undergo breakdown into various metabolites, including trimethylamine (TMA), through the degradation of trimethylamine lytic enzymes in bacteria of the Proteobacteria and Firmicutes phyla. TMA is transported to the liver through the portal vein. Within the liver, it undergoes conversion by flavin-containing monooxygenases, resulting in the formation of TMAO. This compound plays a pivotal role in fostering the buildup of activated leukocytes within human endothelial cells. Consequently, this process leads to impaired functioning of endothelial cells, thereby substantially amplifying the susceptibility to atherosclerosis and cardiovascular diseases [27, 28].
Influence of gut microbiota on intestinal and hepatic immune function
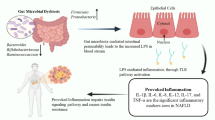
Research indicates that the advancement of NAFLD is associated with the decline in the integrity of the intestinal barrier [29, 30]. During the development and advancement of NAFLD, significant amounts of metabolites originating from gut bacteria, along with bacterial components and other potential hazards, enter the liver through the portal vein. This occurs due to the disruption of the intestinal mucosal barrier caused by various factors, leading to an increased permeability of the intestines (Fig. 2). These intrusions have the capability to accelerate liver damage and fibrosis by amplifying inflammation, oxidative stress, and the accumulation of lipids [31]. The investigation through in situ hybridization discovered the presence of bacterial metabolites and DNA fragments from the gut in the livers of mice fed a high-fat diet (HFD). However, it remains unclear whether bacteria are present in the livers of patients with non-alcoholic steatohepatitis (NASH). In comparison to individuals with normal health, individuals with obesity or non-alcoholic fatty liver disease (NAFLD) exhibited a significant increase in the number of enteric bacteria, particularly Gram-negative types, which led to noticeable endotoxemia [32].
Gut dysbiosis disrupts the integrity of the intestinal barrier, enabling the passage of bacterial endotoxins into the liver. This, in turn, amplifies the inflammatory processes and the accumulation of fat, contributing to the development of NAFLD. Unhealthy lifestyle choices, such as a high-fat, low-fiber diet, alter the composition of the gut microbiota. This alteration increases the permeability of the gut, leading to the production of various proinflammatory molecules, including LPS, TMAO, SBAs, and bacterial 16sDNA. These proinflammatory molecules further exacerbate liver inflammation and fibrosis, potentially accelerating the progression of NAFLD. Various interventions, such as treatment with FXR/TGR5 agonists, and probiotics play a crucial role in strengthening the tight junctions within the intestinal barrier. They also regulate glucose and lipid metabolism by activating FXR and TGR5 signaling pathways, while simultaneously inhibiting the TLR4/NF-kB and JAK1/STAT6 pathways
Abundant of lipopolysaccharides (LPS) trigger the activation of adenylate cyclase in the intestinal mucosa, which subsequently harms mitochondria and lysosomes within epithelial cells. This harmful cascade ultimately results in the necrosis of apical cells on intestinal villi and the autolysis of epithelial cells. Additionally, the inflammation of the liver and its ongoing damage are primarily driven by gut-derived LPS. These LPS molecules play a crucial role in initiating signaling through LPS-dependent pattern recognition receptors, contributing to the inflammatory process [33]. LPS triggers the activation of TLR4 within endothelial cells and TLR9 within dendritic cells. This activation leads to the secretion of a significant array of pro-inflammatory cytokines like TNF-a, IL-1b, and IL-6 and chemokines such as CCL2, CXCL2, CXCL10, and CXCL16. These molecules collectively drive inflammation and pathological harm to the liver [34, 35]. Consequently, LPS initiates inflammation and substantial metabolic alterations in the body, including increased fat utilization, enhanced circulation of free fatty acids (FFA), and elevated triglyceride (TG) levels. This accumulation of FFA in the liver could also incite inflammation and insulin resistance (IR), thereby further promoting the progression of NAFLD [36, 37]. Conversely, the gut microbiota has the capacity to modify the equilibrium between proinflammatory and anti-inflammatory cytokines produced by M1 and M2 macrophages. This modulation occurs through the influence on the metabolism of short-chain fatty acids (SBAs), ultimately impacting the immune function of the liver [38]. Small amounts of SBAs produced by the gut microbiota can have the capability to decrease FXR activity and enhance inflammation in the body. On the other hand, high levels of SBAs can result in the production of a considerable number of ROS, leading to damage to the DNA of cells and promoting the development of HCC [39]. Additionally, PBAs can prompt an increased expression of CXCL16 in hepatic vascular endothelial cells. Subsequently, this process triggers the attraction of NKT cells, which are capable of eliminating tumor cells in a manner that relies on CD1d [40].
Symptoms of NAFLD
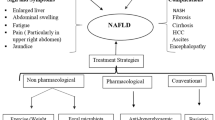
NAFLD often presents either mild or vague symptoms during its initial stages [41]. Some individuals may not perceive any noticeable symptoms at all. This lack of prominent indicators can create difficulties in identifying the condition without specific medical assessments. Certain individuals might not show any observable signs. The absence of notable symptoms can hinder the detection of the condition without particular medical examinations. Fatigue, discomfort in the upper right abdomen, enlarged liver (hepatomegaly), a skin condition with dark and thickened patches, (acanthosis nigricans), and accumulation of excess fat (lipomatosis) might be experienced by some individuals. In certain cases, NAFLD can progress to more severe liver disorders, ultimately leading to cirrhosis. Patients with cirrhosis may exhibit symptoms that are characteristic of end-stage liver disease.
Diagnosis of NAFLD
Different imaging techniques can be used in NAFLD to support the diagnosis. However, these imaging modalities are not routinely employed to differentiate between the histological subtypes of NAFLD. Following are some diagnostic tools utilized for detecting hepatic steatosis.
Ultrasound
Ultrasound emerges as the primary imaging modality for evaluating patients diagnosed or suspected with NAFLD. B-Mode ultrasound images show the potential to improve diagnostic accuracy for detecting and grading hepatic steatosis. It proves to be an exceptional tool for identifying moderate to severe steatosis among NAFLD patients [42].
Computed tomography (CT)
Clinical CT shows significant promise in detecting incidental steatosis and aiding in understanding the typical course of NAFLD. Dual-energy CT (DECT), utilizing different energy levels, offers potential to distinguish diverse tissue compositions, such as fat, thereby providing improved diagnostic accuracy for hepatic steatosis compared to traditional single-energy CT [43].
Magnetic resonance imaging
MRI technology, particularly multiparametric methods like PDFF, T2 and T1 mapping, and MR elastography, is continuously being integrated into clinical practice. This comprehensive approach to liver imaging shows potential in managing NAFLD by accurately measuring fat content, iron levels, and fibrosis, essential features of the disease [44].
Transient elastography (FibroScan)
assist in diagnosing liver diseases and provide information about the fat presence and liver stiffness, they cannot substitute for a liver biopsy when it comes to accurately determining the histological subtypes and to guide appropriate clinical management and monitoring [45].
Vibration-controlled transient elastography (VCTE)
VCTE is a non-invasive medical imaging technique used to assess the stiffness or elasticity of the liver [46, 47]. This quick and painless procedure offers valuable information on liver fibrosis and cirrhosis. VCTE also aids in guiding treatment decisions and monitoring disease progression. However, it does not identify the underlying cause of liver disease.
Liver biopsy
Liver biopsy plays an important role as a diagnostic tool in differentiating between simple fatty liver and NASH and in assessing the state of fibrosis in NAFLD patients. This enables assessment of the degree of fibrosis, which provides valuable prognostic information and improves the clinical management of NAFLD [48,49,50]. However, it carries some risks and discomfort for the patient. Therefore, non-invasive methods are also employed to assess liver health and fibrosis in NAFLD patients. These non-invasive techniques include imaging studies such as transient elastography and blood tests (Fibrosis-4 Index and enhanced liver fibrosis tests) [51]. These methods offer valuable information about liver stiffness and fibrosis levels without the need for a liver biopsy, and they play a significant role in the clinical evaluation of NAFLD patients, providing an alternative or complementary approach to liver biopsy when appropriate.
Loomba etal. investigated the potential correlation between the gut microbiome and liver disease associated with obesity [52]. To explore this connection, Loomba analyzed two distinct sets of patients. The initial group encompassed 86 patients who were diagnosed with non-alcoholic fatty liver disease (NAFLD) via biopsy. Among them, 72 had mild to moderate NAFLD, while 14 had advanced-stage disease. The team employed sequencing techniques to scrutinize the microbial genes derived from stool samples provided by each participant. This allowed them to pinpoint the species present and their relative proportions. Noteworthy findings emerged, as they identified 37 bacterial species that could differentiate between mild/moderate NAFLD and advanced-stage disease. Remarkably, this differentiation accurately predicted advanced-stage disease in patients with an impressive 93.6% accuracy. To validate this discovery, a subsequent study involving 16 patients with advanced NAFLD and 33 healthy individuals as a control group was conducted. This phase revealed nine bacterial species that set apart NAFLD patients from the healthy volunteers, achieving an accuracy rate of 88%. Notably, seven of these bacterial species aligned with the previously discovered 37. The study demonstrated that patients with advanced NAFLD exhibited a higher prevalence of Proteobacteria and a lower presence of Firmicutes in their stool compared to those with early-stage NAFLD. At a more specific level, the abundance of E. coli was notably three times higher in patients with advanced NAFLD compared to those in the early stages of the disease.
Treatment
In recent years, a multitude of trials have investigated diverse medications with varying mechanisms of action in the context of NAFLD/NASH, yielding encouraging results. Within this specific context, we aim to provide a comprehensive overview of key clinical findings, alongside stratified pharmacological mechanisms designed to specifically target NAFLD. Effective drugs like vitamin E and Pioglitazone exist for treating and preventing NAFLD [53]. Pioglitazone has shown efficacy in cases involving advanced NASH patients with type 2 diabetes; However, this underscores the lack of reliable clinical data to fully support its use in this particular context. Vitamins E and D show certain effectiveness although uncertainty remains regarding their long-term safety and therapeutic efficacy. On the other hand, Statins can lower serum LDL levels and mitigating cardiovascular problems, but they do not address the progression of liver disease. Currently, there is no FDA-approved treatment for NAFLD. However, targeted therapies are in different phases of clinical trials. Table 1 outlines a number of encouraging drug contenders at various stages of clinical development for NAFLD.
Fecal microbiota transplantation (FMT)
FMT presents a novel approach for restoring and rebalancing the diversity of the gut’s microorganisms, aiming to address various diseases, including Clostridioides difficile infection [79]. Additionally, FMT has shown promise in treating metabolic diseases, tumors, autoimmune disorders, and hepatic encephalopathy [80,81,82]. Studies on animals have indicated that FMT can effectively improve the manifestations of NAFLD by addressing gut microbiota dysbiosis [83,84,85]. As a result, FMT has become an appealing option for NAFLD patients. However, there have been only a limited number of studies exploring the clinical efficacy of FMT in NAFLD treatment. One randomized control trial revealed that FMT has the potential to reduce small intestinal permeability in NAFLD patients [86, 87]. Moreover, FMT from healthy donors has been found to impact hepatic gene expression and plasma metabolites related to inflammation and lipid metabolism, demonstrating the significant interplay between gut microbiota composition and NAFLD.
Management of gut dysbiosis
Patients with NAFLD often follow high-calorie diets rich in carbohydrates and fats, contributing to obesity. Mitigating NAFLD risk involves replacing saturated and trans fats with healthier unsaturated fats, particularly omega-3 fatty acids. Opting for low-glycemic index foods such as fruits, vegetables, and whole grains is recommended as they have a milder effect on blood glucose compared to high-glycemic index foods like white bread and potatoes. Sugary beverages, notably high in sucrose and fructose, are linked to NAFLD and should be avoided [88, 89]. NAFLD’s connection to obesity emphasizes the need for gradual weight loss through balanced eating and exercise. Shedding 7 to 10% of body weight through diet and physical activity notably improves NAFLD and its more severe form, NASH, decreasing liver fat content and addressing fibrosis.
Regular physical activity, aiming for 150 min of moderate-intensity or 75 min of vigorous-intensity exercise weekly, positively impacts gut microbiome and liver health [90]. Emerging research indicates the significance of probiotics, prebiotics, and synbiotics in gastrointestinal health. These therapies target disrupted gut microbiota, which plays a pivotal role in NAFLD development. Probiotic and prebiotic supplementation have shown promise in reducing liver enzymes AST and ALT in damaged liver patients [91,92,93,94]. The intricate gut microbiota is essential for digestion, vitamin synthesis, immune training, and pathogen prevention. Antibiotic use, particularly fluoroquinolones, can disrupt this ecosystem, leading to reduced diversity and opportunistic infections.
Conclusion
The human gut microbiota plays a crucial role in maintaining various physiological processes and overall health. The intricate interactions between the gut microbiome, liver function, and immune responses have significant implications for the development and progression of non-alcoholic fatty liver disease (NAFLD). The dysbiosis of gut microbiota, characterized by alterations in microbial composition and metabolic activity, has been associated with NAFLD through its influence on digestion, energy metabolism, inflammation, and immune function. This review article has highlighted the role of short-chain fatty acids (SCFAs), bile acids, and gut-derived endotoxins in the development of NAFLD. SCFAs, produced by the fermentation of dietary fibers, have been shown to influence energy homeostasis, lipid metabolism, and inflammation. Bile acids, beyond their role in digestion, regulate various aspects of liver health, including lipid metabolism and inflammation. Dysbiosis-related alterations in SCFAs and bile acids contribute to liver inflammation and lipid accumulation, pivotal factors in NAFLD progression. Moreover, the disruption of the intestinal barrier integrity allows the translocation of bacterial products, including lipopolysaccharides (LPS), into the liver. This initiates an inflammatory response and oxidative stress, further promoting liver damage. The interplay between gut microbiota and the immune system has been shown to impact the progression of NAFLD, with dysbiosis promoting inflammation through the activation of pattern recognition receptors.
Diagnosis and management of NAFLD have also seen advancements in recent years. Non-invasive techniques such as transient elastography and blood tests have emerged as alternatives to liver biopsy for assessing liver fibrosis. Targeted therapies, including FXR agonists and antagonists, GLP-1 agonists, and thyroid hormone receptor agonists, are being investigated for their potential to address NAFLD at the molecular level. Furthermore, the potential of fecal microbiota transplantation (FMT) has garnered attention as a novel approach to restoring gut microbiota balance in NAFLD patients. Promising results from animal studies and limited clinical trials suggest that FMT could influence gut-liver crosstalk and potentially mitigate NAFLD-associated conditions. Lifestyle interventions remain crucial for managing NAFLD. Dietary modifications, physical activity, and weight loss continue to be cornerstones of NAFLD management, as they can positively impact gut microbiota composition and diversity. Probiotics, prebiotics, and synbiotics also hold promise in improving gut health and mitigating NAFLD risk.
In summary, the intricate relationship between gut microbiota, liver health, and NAFLD underscores the importance of understanding these interactions for the development of targeted therapeutic strategies. Advances in diagnostic techniques, treatment options, and lifestyle interventions are paving the way for a comprehensive approach to managing NAFLD by addressing gut microbiota dysbiosis and its implications for overall health. Further research in this field holds the potential to revolutionize our approach to preventing and treating NAFLD, a growing global health concern.
Availability of data and materials
Not applicable.
Abbreviations
- GM:
-
Gut microbiota
- NAFLD:
-
Non-alcoholic fatty liver disease
- BAs:
-
Bile acids
- SCFAs:
-
Short-chain fatty acids
- CA:
-
Cholic acid
- CDCA:
-
Chenodeoxycholic acid
- LCA:
-
Lithocholic acid
- DCA:
-
Deoxycholic acid
- UDCA:
-
Ursodeoxycholic acid
- HCC:
-
Hepatocellular carcinoma
- TMA:
-
Trimethylamine
- TMAO:
-
Trimethylamine N-oxide (TMAO)
- VLDL:
-
Very low-density lipoproteins
- HFD:
-
High-fat diet
- FMT:
-
Fecal microbiota transplantation
References
Chen Y, Zhou J, Wang L (2021) Role and mechanism of gut microbiota in human disease. Front Cell Infect Microbiol 17(11):86
Vemuri R, Shankar EM, Chieppa M, Eri R, Kavanagh K (2020) Beyond just bacteria: functional biomes in the gut ecosystem including virome, mycobiome, archaeome and helminths. Microorganisms 8(4):483
Riedl RA, Burnett CM, Pearson NA, Reho JJ, Mokadem M, Edwards RA, Kindel TL, Kirby JR, Grobe JL (2021) Gut microbiota represent a major thermogenic biomass. Function 2(3):zqab019
Riccio P, Rossano R (2020) The human gut microbiota is neither an organ nor a commensal. FEBS Lett 594(20):3262–3271
Paone P, Cani PD (2020) Mucus barrier, mucins and gut microbiota: the expected slimy partners? Gut 69(12):2232–2243
Wahlström A, Sayin SI, Marschall HU, Bäckhed F (2016) Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab 24(1):41–50
Lindsay EC, Metcalfe NB, Llewellyn MS (2020) The potential role of the gut microbiota in shaping host energetics and metabolic rate. J Anim Ecol 89(11):2415–2426
Van de Wouw M, Boehme M, Lyte JM, Wiley N, Strain C, O’Sullivan O, Clarke G, Stanton C, Dinan TG, Cryan JF (2018) Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain–gut axis alterations. J Physiol 596(20):4923–4944
Byndloss MX and B€aumler AJ (2018) The germ-organ theory of non-communicable diseases. Nat Rev Microbiol 16, 103–110
Rinninella E, Raoul P, Cintoni M, Franceschi F, Miggiano GAD, Gasbarrini A, Mele MC (2019) What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 7(1):14. https://doi.org/10.3390/microorganisms7010014
Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, Balamurugan R (2020) The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients 12(5):1474. https://doi.org/10.3390/nu12051474
Stražar M, Temba GS, Vlamakis H, Kullaya VI, Lyamuya F, Mmbaga BT et al (2021) Gut microbiome-mediated metabolism effects on immunity in rural and urban African populations. Nat Commun 12(1):4845. https://doi.org/10.1038/s41467-021-25213-2
Zheng Y, Shi H, Zhou Y, Wang A, Kang D, Kang L (2022) Effects of endoplasmic reticulum stress, liver function, insulin resistance and vascular endothelial function in patients with nonalcoholic fatty liver disease. Cell Mol. Biol (Noisy-le-grand) 67(5):210–217. https://doi.org/10.14715/cmb/2021.67.5.29
Fang J, Yu CH, Li XJ, Yao JM, Fang ZY, Yoon SH, Yu WY (2022) Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front Cell Infect Microbiol 8(12):997018
Rauf A, Khalil AA, Rahman UU, Khalid A, Naz S, Shariati MA et al (2022) Recent advances in the therapeutic application of short-chain fatty acids (SCFAs): An updated review. Crit Rev Food Sci Nutr 62(22):6034–6054. https://doi.org/10.1080/10408398.2021.1895064
Zhou D, Chen YW, Zhao ZH, Yang RX, Xin FZ, Liu XL et al (2018) Sodium butyrate reduces high-fat diet-induced non-alcoholic steatohepatitis through upregulation of hepatic GLP-1R expression. Exp Mol Med 50(12):1–12. https://doi.org/10.1038/s12276-018-0183-1
Park JH, Kotani T, Konno T, Setiawan J, Kitamura Y, Imada S et al (2016) Promotion of intestinal epithelial cell turnover by commensal bacteria: Role of shortchain fatty acids. PLoS ONE 11(5):e0156334. https://doi.org/10.1371/journal
Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM et al (2017) Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci Rep 7(1):2360. https://doi.org/10.1038/s41598-017-02546-x
Liu W, Luo X, Tang J, Mo Q, Zhong H, Zhang H et al (2021) A bridge for short-chain fatty acids to affect inflammatory bowel disease, type 1 diabetes, and non-alcoholic fatty liver disease positively: by changing gut barrier. Eur J Nutr 60(5):2317–2330. https://doi.org/10.1007/s00394-020-02431-w
Letourneau J, Holmes ZC, Dallow EP, Durand HK, Jiang S, Carrion VM et al (2022) Ecological memory of prior nutrient exposure in the human gut microbiome. ISME J 10:114. https://doi.org/10.1038/S41396-022-01292-X
Lu Y, Fan C, Li P, Lu Y, Chang X, Qi K (2016) Short chain fatty acids prevent high-fat-diet-induced obesity in mice by regulating G protein-coupled receptors and gut microbiota. Sci Rep 6:37589. https://doi.org/10.1038/srep37589
Kimura T, Pydi SP, Pham J, Tanaka N (2020) Metabolic functions of G protein-coupled receptors in hepatocytes-potential applications for diabetes and NAFLD. Biomolecules 10(10):1445. https://doi.org/10.3390/biom10101445
Fiorucci S, Distrutti E (2019) The pharmacology of bile acids and their receptors. Handb Exp Pharmacol 256:3–18. https://doi.org/10.1007/164_2019_238
Han X, Cui ZY, Song J, Piao HQ, Lian LH, Hou LS et al (2019) Acanthoic acid modulates lipogenesis in nonalcoholic fatty liver disease via FXR/ LXRs-dependent manner. Chem Biol Interact 311:108794. https://doi.org/10.1016/j.cbi.2019.108794
Zietak M, Kozak LP (2016) Bile acids induce uncoupling protein 1- dependent thermogenesis and stimulate energy expenditure at thermoneutrality in mice. Am J Physiol Endocrinol Metab 310(5):E346–E354. https://doi.org/10.1152/ajpendo.00485.2015
Li X, Su C, Jiang Z, Yang Y, Zhang Y, Yang M et al (2021) Berberine attenuates choline-induced atherosclerosis by inhibiting trimethylamine and trimethylamine-n-oxide production via manipulating the gut microbiome. NPJ Biofilms Microbiomes 7(1):36. https://doi.org/10.1038/s41522-021-00205-8
Tan X, Liu Y, Long J, Chen S, Liao G, Wu S et al (2019) Trimethylamine n-oxide aggravates liver steatosis through modulation of bile acid metabolism and inhibition of farnesoid X receptor signaling in nonalcoholic fatty liver disease. Mol Nutr Food Res 63(17):e1900257. https://doi.org/10.1002/mnfr.201900257
Shi C, Pei M, Wang Y, Chen Q, Cao P, Zhang L et al (2022) Changes of flavin-containing monooxygenases and trimethylamine-n-oxide may be involved in the promotion of non-alcoholic fatty liver disease by intestinal microbiota metabolite trimethylamine. Biochem Biophys Res Commun 594:1–7. https://doi.org/10.1016/J.BBRC.2022.01.060
Leng, J., Tian, H. J., Fang, Y., Hu, Y. Y., and Peng, J. H. (2022). Amelioration of non-alcoholic steatohepatitis by atractylodes macrocephala polysaccharide, chlorogenic acid, and geniposide combination is associated with reducing endotoxin gut leakage. Front. Cell Infect. Microbiol. 12. 10.3389/ FCIMB.2022.827516
Kaushal K, Agarwal S, Sharma S, Goswami P, Singh N, Sachdev V et al (2022) Demonstration of gut-barrier dysfunction in early stages of non-alcoholic fatty liver disease: A proof-Of-Concept study. Clin Exp Hepatol 12(4):1102–1113. https://doi.org/10.1016/J.JCEH.2022.01.006
Lechner S, Yee M, Limketkai BN, Pham EA (2020) Fecal microbiota transplantation for chronic liver diseases: Current understanding and future direction. Dig Dis Sci 65(3):897–905. https://doi.org/10.1007/s10620-020-06100-0
Li Q, Rempel JD, Yang J, Minuk GY (2022) The effects of pathogen associated molecular patterns on peripheral blood monocytes in patients with non-alcoholic fatty liver disease. Clin Exp Hepatol 12(3):808–817. https://doi.org/10.1016/j.jceh.2021.11.011
An L, Wirth U, Koch D, Schirren M, Drefs M, Koliogiannis D et al (2022) The role of gut-derived lipopolysaccharides and the intestinal barrier in fatty liver diseases. J Gastrointest Surg 26(3):671–683. https://doi.org/10.1007/S11605-021-05188-7
Arelaki S, Koletsa T, Sinakos E et al (2022) Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch 481:455–465. https://doi.org/10.1007/s00428-022-03330-7
Tiegs G, Horst AK (2022) TNF in the liver: targeting a central player in inflammation. Semin Immunopathol 44:445–459. https://doi.org/10.1007/s00281-022-00910-2
Rennert C, Heil T, Schicht G, Stilkerich A, Seidemann L, Kegel-Hübner V et al (2020) Prolonged lipid accumulation in cultured primary human hepatocytes rather leads to ER stress than oxidative stress. Int J Mol Sci 21:7097. https://doi.org/10.3390/ijms21197097
Zhang SL, Han B, Mao YQ, Zhang ZY, Li ZM, Kong CY et al (2022) Lacticaseibacillus paracasei sh2020 induced antitumor immunity and synergized with anti-programmed cell death 1 to reduce tumor burden in mice. Gut Microbes 14(1):2046246. https://doi.org/10.1080/19490976.2022.2046246
Yang ZH, Liu F, Zhu XR, Suo FY, Jia ZJ, Yao SK (2021) Altered profiles of fecal bile acids correlate with gut microbiota and inflammatory responses in patients with ulcerative colitis. World J Gastroenterol 27(24):3609
Sun Y, Zhu M, Zhao H, Ni X, Chang R, Su J et al (2020) Serum fibroblast growth factor 19 and total bile acid concentrations are potential biomarkers of hepatocellular carcinoma in patients with type 2 diabetes mellitus. BioMed Res Int 2020:1751989. https://doi.org/10.1155/2020/1751989
Ma, C., Han, M., Heinrich, B., Fu, Q., Zhang, Q., Sandhu, M., et al. (2018). Gut microbiome-mediated bile acid metabolism regulates liver cancer via NKT cells. Science 360 (6391), eaan5931. doi: https://doi.org/10.1126/science. aan5931
Francque SM, Marchesini G, Kautz A, Walmsley M, Dorner R, Lazarus JV, Zelber-Sagi S, Hallsworth K, Busetto L, Frühbeck G, Dicker D (2021) Non-alcoholic fatty liver disease: a patient guideline. JHEP Reports 3(5):100322
Petzold G (2022) Role of ultrasound methods for the assessment of NAFLD. J Clin Med 11(15):4581. https://doi.org/10.3390/jcm11154581
Gasim GI, Elshehri FM, Kheidr M, Alshubaily FK, ElZaki EM, Musa IR (2017) The use of computed tomography in the diagnosis of fatty liver and abdominal fat distribution among a Saudi population. Open Access Maced J Med Sci 5(6):762–765. https://doi.org/10.3889/oamjms.2017.187
Schaapman JJ, Tushuizen ME, Coenraad MJ, Lamb HJ (2021) Multiparametric MRI in patients with nonalcoholic fatty liver disease. J Magn Reson Imaging 53(6):1623–1631. https://doi.org/10.1002/jmri.27292. (Epub 2020 Aug 21)
Shrestha R, Kc S, Thapa P, Pokharel A, Karki N, Jaishi B (2021) Estimation of liver fat by fibroscan in patients with nonalcoholic fatty liver disease. Cureus 13(7):e16414. https://doi.org/10.7759/cureus.16414
Wong GL, Wong VW (2015) Fat and fiber: how the controlled attenuation parameter complements noninvasive assessment of liver fibrosis. Dig Dis Sci 60(1):9–12
Shi KQ, Tang JZ, Zhu XL, Ying L, Li DW, Gao J et al (2014) Controlled attenuation parameter for the detection of steatosis severity in chronic liver disease: a meta-analysis of diagnostic accuracy. J Gastroenterol Hepatol 29(6):1149–1158
Saadeh S, Younossi ZM, Remer EM et al (2002) The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology 123(3):745–750
Ong JP, Younossi ZM (2005) Approach to the diagnosis and treatment of nonalcoholic fatty liver disease. Clin Liver Dis 9(4):617–634
Adams LA, Angulo P (2007) Role of liver biopsy and serum markers of liver fibrosis in non-alcoholic fatty liver disease. Clin Liver Dis 11(1):25–35
Kjaergaard M, Lindvig KP, Thorhauge KH, Andersen P, Hansen JK, Kastrup N, Jensen JM, Hansen CD, Johansen S, Israelsen M, Torp N. Using the ELF test, FIB-4 and NAFLD fibrosis score to screen the population for liver disease. Journal of hepatology. 2023 Apr 21.
Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, Dulai PS, Caussy C, Bettencourt R, Highlander SK, Jones MB (2017) Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 25(5):1054–1062
Trauner M, Fuchs CD (2022) Novel therapeutic targets for cholestatic and fatty liver disease. Gut 71(1):194–209
Lazarević S, Đanić M, Goločorbin-Kon S et al (2019) Semisynthetic bile acids: a new therapeutic option for metabolic syndrome. Pharmacol Res 146:104333. https://doi.org/10.1016/j.phrs.2019.104333
Li C, Li Y, Gai Z (2019) Bile acids and farnesoid X receptor: novel target for the treatment of diabetic cardiomyopathy. Curr Protein Pept Sci 20:976–983. https://doi.org/10.2174/1389203720666190726152847
Li H, Shen J, Wu T et al (2019) Irisin is controlled by farnesoid X receptor and regulates cholesterol homeostasis. Front Pharmacol 10:548. https://doi.org/10.3389/fphar.2019.00548
Traussnigg S, Halilbasic E, Hofer H, Munda P, Stojakovic T, Fauler G, Kashofer K, Krssak M, Wolzt M, Trauner M (2021) Open-label phase II study evaluating safety and efficacy of the non-steroidal farnesoid X receptor agonist PX-104 in non-alcoholic fatty liver disease. Wien Klin Wochenschr 133(9–10):441–451. https://doi.org/10.1007/s00508-020-01735-5
Chianelli D, Rucker PV, Roland J, Tully DC, Nelson J, Liu X, Bursulaya B, Hernandez ED, Wu J, Prashad M, Schlama T (2020) Nidufexor (LMB763), a novel FXR modulator for the treatment of nonalcoholic steatohepatitis. J Med Chem 63(8):3868–3880
Schwabl P, Hambruch E, Budas GR, et al. (9 Jan 2021). The non-steroidal FXR agonist cilofexor improves portal hypertension and reduces hepatic fibrosis in a rat NASH model. Biomedicines. 9 (1): 60. doi:https://doi.org/10.3390/biomedicines9010060
Patel K, Harrison SA, Elkhashab M, Trotter JF, Herring R, Rojter SE et al (2020) Cilofexor, a nonsteroidal FXR agonist, in patients with noncirrhotic NASH: A phase 2 randomized controlled trial. Hepatology 72(1):58–71. https://doi.org/10.1002/hep.31205
Tully DC, Rucker PV, Chianelli D, Williams J, Vidal A, Alper PB et al (2017) Discovery of Tropifexor (LJN452), a highly potent non-bile acid FXR agonist for the treatment of cholestatic liver diseases and nonalcoholic steatohepatitis (NASH). J Med Chem 60(24):9960–9973. https://doi.org/10.1021/acs.jmedchem.7b00907
Ratziu V, Rinella ME, Neuschwander-Tetri BA, Lawitz E, Denham D, Kayali Z, Sheikh A, Kowdley KV, Desta T, Elkhashab M, DeGrauw J (2022) EDP-305 in patients with NASH: A phase II double-blind placebo-controlled dose-ranging study. J Hepatol 76(3):506–517
Panzitt K, Zollner G, Marschall HU, Wagner M (2022) Recent advances on FXR-targeting therapeutics. Mol Cell Endocrinol 552:111678. https://doi.org/10.1016/j.mce.2022.111678
Lin X, Mai M, He T, Huang H, Zhang P, Xia E, Guo H (2022) Efficiency of ursodeoxycholic acid for the treatment of nonalcoholic steatohepatitis: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol 16(6):537–545. https://doi.org/10.1080/17474124.2022.2083605
Wang X, Xia J, Jiang C (2019) Role of gut microbiota in the development of non-alcoholic fatty liver disease. Liver Research 3(1):25–30
Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, Sanyal AJ, Sejling AS, Harrison SA (2021) A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med 384(12):1113–1124
Sanyal AJ, Ling L, Beuers U, DePaoli AM, Lieu HD, Harrison SA, Hirschfield GM (2021) Potent suppression of hydrophobic bile acids by aldafermin, an FGF19 analogue, across metabolic and cholestatic liver diseases. JHEP Rep 3(3):100255. https://doi.org/10.1016/j.jhepr.2021.100255
Luo Y, Decato BE, Charles ED, Shevell DE, McNaney C, Shipkova P, Apfel A, Tirucherai GS, Sanyal AJ (2022) Pegbelfermin selectively reduces secondary bile acid concentrations in patients with non-alcoholic steatohepatitis. JHEP Reports 4(1):100392
Harrison SA, Ruane PJ, Freilich B, Neff G, Patil R, Behling C, Hu C, Shringarpure R, de Temple B, Fong E, Tillman EJ, Rolph T, Cheng A, Yale K (2022) A randomized, double-blind, placebo-controlled phase IIa trial of efruxifermin for patients with compensated NASH cirrhosis. JHEP Rep 5(1):100563. https://doi.org/10.1016/j.jhepr.2022.100563
Haczeyni F, Wang H, Barn V, Mridha AR, Yeh MM, Haigh WG (2017) The selective peroxisome proliferator-activated receptor-delta agonist seladelpar reverses nonalcoholic steatohepatitis pathology by abrogating lipotoxicity in diabetic obese mice. Hepatol Commun 1:663–674
Kaul U, Parmar D, Manjunath K, Shah M, Parmar K, Patil KP (2019) New dual peroxisome proliferator activated receptor agonist-Saroglitazar in diabetic dyslipidemia and non-alcoholic fatty liver disease: Integrated analysis of the real world evidence. Cardiovasc Diabetol 18:80
Kumar DP, Caffrey R, Marioneaux J, Santhekadur PK, Bhat M, Alonso C (2020) The PPAR α/γ agonist saroglitazar improves insulin resistance and steatohepatitis in a diet induced animal model of nonalcoholic fatty liver disease. Sci Rep 10:9330
Esler WP, Bence KK (2019) Metabolic targets in nonalcoholic fatty liver disease. Cell Mol Gastroenterol Hepatol 8:247–267
Prikhodko VA, Bezborodkina NN, Okovityi SV (2022) Pharmacotherapy for non-alcoholic fatty liver disease: emerging targets and drug candidates. Biomedicines 10(2):274. https://doi.org/10.3390/biomedicines10020274
Terns Pharmaceuticals. Terns achieves primary endpoint and all secondary endpoints in phase 2a duet trial of Thr-Β agonist tern-501 in nash. Press release. Published August 8, 2023. https://ir.ternspharma.com/news-releases/news-release-details/terns-achieves-primary-endpoint-and-all-secondary-endpoints
Harrison SA, Bashir MR, Guy CD, Zhou R, Moylan CA, Frias JP et al (2019) Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394:2012–2024. https://doi.org/10.1016/S0140-6736(19)32517-6
Surawicz, CM, Brandt LJ, Binion DG, Ananthakrishnan AN, Curry SR, Gilligan PH. et al (2013). Guidelines for diagnosis, treatment, and prevention of clostridium difficile infections. Am. J. Gastroenterol. 108 (4)478-498 https://doi.org/10.1038/ajg.2013.4
de Groot PF, Frissen MN, de Clercq NC, Nieuwdorp M (2017) Fecal microbiota transplantation in metabolic syndrome: history, present and future. Gut Microbes 8(3):253–267. https://doi.org/10.1080/19490976.2017.1293224
Vaughn BP, Rank KM, Khoruts A (2019) Fecal microbiota transplantation: current status in treatment of GI and liver disease. Clin Gastroenterol Hepatol 17(2):353–361. https://doi.org/10.1016/j.cgh.2018.07.026
Meighani A, Alimirah M, Ramesh M, Salgia R (2020) Fecal microbiota transplantation for clostridioides difficile infection in patients with chronic liver disease. Int J Hepatol 2020:1874570. https://doi.org/10.1155/2020/1874570
Zhou D, Pan Q, Shen F, Cao HX, Ding WJ, Chen YW et al (2017) Total fecal microbiota transplantation alleviates high-fat diet-induced steatohepatitis in mice via beneficial regulation of gut microbiota. Sci Rep 7(1):1529. https://doi.org/10.1038/s41598-017-01751-y
Soderborg TK, Clark SE, Mulligan CE, Janssen RC, Babcock L, Ir D et al (2018) The gut microbiota in infants of obese mothers increases inflammation and susceptibility to NAFLD. Nat Commun 9(1):4462. https://doi.org/10.1038/s41467-018-06929-0
García-Lezana T, Raurell I, Bravo M, Torres-Arauz M, Salcedo MT, Santiago A et al (2018) Restoration of a healthy intestinal microbiota normalizes portal hypertension in a rat model of nonalcoholic steatohepatitis. Hepatology (Baltimore, MD) 67(4):1485–1498. https://doi.org/10.1002/hep.29646
Craven L, Rahman A, Nair Parvathy S, Beaton M, Silverman J, Qumosani K et al (2020) Allogenic fecal microbiota transplantation in patients with nonalcoholic fatty liver disease improves abnormal small intestinal permeability: a randomized control trial. Am J Gastroenterol 115(7):1055–1065. https://doi.org/10.14309/ajg.0000000000000661
Witjes JJ, Smits LP, Pekmez CT, Prodan A, Meijnikman AS, Troelstra MA et al (2020) Donor fecal microbiota transplantation alters gut microbiota and metabolites in obese individuals with steatohepatitis. Hepatol Commun 4(11):1578–1590. https://doi.org/10.1002/hep4.1601
Abid A, Taha O, Nseir W, Farah R, Grosovski M, Assy N (2009) Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J Hepatol 51:918–924
Assy N, Nasser G, Kamayse I, Nseir W, Beniashvili Z, Djibre A, Grosovski M (2008) Soft drink consumption linked with fatty liver in the absence of traditional risk factors. Can J Gastroenterol 22:811–816
https://www.news-medical.net/news/20230209/Exercise-can-be-used-as-a treatment-for-nonalcoholic-fatty-liver-disease.
Kobyliak N, Abenavoli L, Mykhalchyshyn G, Kononenko L, Boccuto L, Kyriienko D, Dynnyk O (2018) A multi-strain probiotic reduces the fatty liver index, cytokines and aminotransferase levels in NAFLD patients: evidence from a randomized clinical trial. J Gastrointest Liver Dis 27:41–49. https://doi.org/10.15403/jgld.2014.1121.271.kby
Javadi L., Ghavami M., Khoshbaten M., Safaiyan A., Barzegari A., Gargari B.P. The effect of probiotic and/or prebiotic on liver function tests in patients with nonalcoholic fatty liver disease: A double blind randomized clinical trial. Iran. Red Crescent Med. J. 2017:e46017. https://doi.org/10.5812/ircmj.46017.
Ekhlasi G, Mohammadi RK, Agah S, Zarrati M, Hosseini AF, Arabshahi SSS, Shidfar F (2016) Do symbiotic and vitamin E supplementation have favorite effects in nonalcoholic fatty liver disease? A randomized, double-blind, placebo-controlled trial. J Res Med Sci 21:106. https://doi.org/10.4103/1735-1995.193178
Bakhshimoghaddam F, Shateri K, Sina M, Hashemian M, Alizadeh M (2018) Daily consumption of synbiotic yogurt decreases liver steatosis in patients with nonalcoholic fatty liver disease: a randomized controlled clinical trial. J Nutr 148:1276–1284. https://doi.org/10.1093/jn/nxy088
Acknowledgements
Not applicable
Funding
No funding was received for this article.
Author information
Authors and Affiliations
Contributions
DMS, SD, and VVSRP wrote and edited the manuscript. JN and SP drew the images. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Swamikkannu, D.M., Dasarapu, S., Siva, R.V. et al. The gut-liver nexus: exploring gut microbiota dysbiosis in non-alcoholic fatty liver disease and its therapeutic implications. Egypt Liver Journal 14, 28 (2024). https://doi.org/10.1186/s43066-024-00331-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00331-w