Abstract
Background
Hepatic energy metabolism is intricately linked with the metabolic changes associated with HCV infection and hepatocellular carcinoma (HCC). This study investigated the potential of carnitine and acylcarnitines as markers for monitoring the development and progression of HCC in the context of HCV-induced liver cirrhosis. Blood samples from patients with chronic liver cirrhosis due to HCV (n = 55) and from those with HCC (n = 90) were analyzed, alongside samples from healthy control subjects (n = 25), utilizing liquid chromatography coupled with mass spectrometry.
Results
Principal component analysis revealed distinct metabolic clustering patterns, segregating HCC and HCV patients into separate groups. Pairwise comparisons showed significantly elevated acylcarnitine levels in both HCV and HCC patients when compared to the control group (p = 0.001). Importantly, there was no significant difference between HCV and HCC groups, although linear trend analysis indicated an increasing linear trend that reached its peak in the HCC group, signifying a notable relationship with disease severity (p = 0.001).
Conclusion
The observed carnitine and acylcarnitine levels in HCV and HCC patients offer crucial clinical insights, suggesting their potential as valuable diagnostic and prognostic markers.
Similar content being viewed by others
Introduction
Carnitine plays a vital role in fatty acid oxidation, facilitating the transport of long-chain fatty acids into the mitochondria. This process involves the conversion of long-chain fatty acids and carnitine into acylcarnitine, which is then transported into the mitochondria [1, 2]. Inside the mitochondria, acylcarnitines are converted into acyl-CoA at the inner mitochondrial membrane, serving as a substrate for β-oxidation. Additionally, this process helps maintain the balance between coenzyme A (CoA) and acyl-CoA [3]. Blood carnitine levels are influenced by various metabolic conditions, including liver cirrhosis, end-stage renal failure, and type 2 diabetes [4]. In clinical practice, L-carnitine administration is used to alleviate symptoms associated with cirrhosis, such as muscle cramps and hepatic encephalopathy [5]. Carnitine exists in the bloodstream in two forms: free carnitine and acylcarnitine fractions. The acylcarnitine fraction can be further broken down into various compounds, and advances in tandem mass spectrometry (MS/MS) have enabled the detailed analysis of these acylcarnitines in blood [6]. Given the liver’s central role in energy metabolism, disruptions in these processes are commonly observed in patients with HCV infection and hepatocellular carcinoma (HCC) [3, 7, 8]. These conditions are characterized by severe hepatic injury and disturbances in energy production, especially related to fatty acid oxidation and the increased utilization of fatty acids by rapidly dividing tumor cells [9, 10]. Moreover, recent research has explored the metabolic profiling of acylcarnitines, revealing their potential as biomarkers for various cancers, including renal, prostatic, colorectal, thyroid, and lung cancer, as well as HCC associated with steatohepatitis and breast cancer. These studies have demonstrated that specific acylcarnitine profiles are linked to different cancer statuses, stages, and risks of disease progression [11,12,13,14,15,16,17,18].
This study aimed to explore the potential of carnitine and acylcarnitines as markers for HCC development and progression within the context of HCV-induced liver cirrhosis. Blood levels of free carnitine and acylcarnitines (AC2 to AC18) were quantified using liquid chromatography coupled with mass spectrometry (LC/MS) in patients with chronic liver cirrhosis due to HCV and in patients with HCC. The comprehensive metabolic profile was collectively assessed to discern its significance.
Patients and methods
This prospective cohort study was conducted from March 2018 to June 2020 to investigate the clinical characteristics and outcomes of patients with chronic liver disease. The study protocol was approved by the Institutional Review Board (NLI IRB 00565) of the National Liver Institute, and all participants provided written informed consent prior to enrollment.
Patient selection
Three distinct patient groups were recruited for this study: (1) a post-hepatitis C virus (HCV) liver disease group (n = 55), (2) a post-HCV liver cirrhosis group complicated with HCC (n = 90), and (3) a control group consisting of healthy subjects (n = 25). The control group was rigorously matched for age and gender with the other patient groups, showing no clinical, laboratory, or imaging signs of liver cirrhosis or focal hepatic lesions. Control subjects were also free from cancers and systemic diseases (e.g., diabetes mellitus, obesity, chronic cholecystitis) and refrained from drug abuse and alcohol consumption.
Inclusion criteria
All enrolled patients had no history of alcohol intake or illicit drug abuse. Demographic, clinical, and laboratory data, including age, gender, liver function tests, AFP levels, imaging findings, and histological reports, were used to select and classify the patients group.
Diagnosis of chronic HCV infection is typically confirmed through positive results for anti-HCV antibody and HCV-RNA PCR tests. Liver function tests play a crucial role in identifying abnormalities consistent with chronic HCV infection, including elevated levels of serum alanine transaminase (ALT) and aspartate transaminase (AST) above the upper limit of normal (ULN). For men, the ULN for ALT and AST is typically around 40 IU/L, while for women, it is approximately 30 IU/L. Similarly, alkaline phosphatase (ALP) has a ULN of around 100 IU/L. Total bilirubin levels are also evaluated, with a ULN of approximately 1.2 mg/dL, where elevated levels may indicate liver dysfunction or biliary obstruction. Additionally, abnormal liver synthetic function tests, such as decreased serum albumin and prolonged prothrombin time, are assessed. Cutoffs for abnormal liver function tests include ALT and AST levels greater than 1.5 times the ULN, serum albumin levels below 3.5 g/dL, and prothrombin time prolongation beyond 3 s of the control value, along with elevated INR, where a normal range typically falls between 0.8 and 1.2. Liver ultrasound examination is often utilized to confirm characteristic echogenic patterns suggestive of chronic liver disease. For the diagnosis of liver cirrhosis, patients with evidence of cirrhosis on imaging studies, such as nodular liver surface, splenomegaly, and signs of portal hypertension, are included in the cirrhosis group [19,20,21]. The severity of liver cirrhosis is further assessed using the Child–Pugh classification system [20].
Diagnosis of HCC according to the diagnostic criteria established by the American Association for the Study of Liver Diseases (AASLD), including imaging criteria showing single or multiple focal hepatic lesions, elevated serum AFP levels exceeding 400 ng/mL, and/or histological examination confirming HCC. The study employed the Barcelona Clinic Liver Cancer (BCLC) staging system for HCC staging [22,23,24].
Exclusion criteria
Comorbidities with other diseases such as hepatitis B (HBV) infection, chronic cholestasis, extrahepatic obstructive gall bladder diseases, severe renal or systemic diseases, cardiovascular conditions, diabetes mellitus, and obesity.
Serum sample collection and acylcarnitine analysis
Blood samples were aseptically collected from both patients and control subjects following an overnight fast. The collected samples were subjected to a battery of tests, including CBC, INR, prothrombin time, blood chemistry, and acylcarnitine determination. In immediate succession to collection, 300 µL of each blood sample was dispensed onto three filter cards. These filter cards were then left to air-dry for 2–3 h in a dark room at room temperature. Each set of cards from individual samples was securely enclosed in an envelope, labeled with unique identifying codes, and preserved at − 20 °C until required for the assay. Moreover, comprehensive assessments, encompassing liver function, renal function, serum cholesterol, and triglycerides, were performed using an automated biochemical analyzer (Bachman Ltd., London, UK).
The liquid chromatographic tandem mass spectrometric (LC–MS/MS) analyses were conducted on an Agilent Series system from Agilent Technologies (CA, USA), which was coupled with a 4000 Q-TRAP instrument from AB SCIEX (Foster City, CA, USA). The chromatographic separation was executed using a reverse-phase approach with mobile phases comprised of 0.1% (v/v) formic acid in bi-distilled water and 0.1% (v/v) formic acid in acetonitrile. The elution was carried out in a gradient mode following three different elution schemes as described in [25]. Acylcarnitines, including acetylcarnitine (AC2), propionylcarnitine (AC3), and others, were analyzed using standardized protocols for non-derivatized methods employing the NeoBase kit from PerkinElmer (MA, USA). In brief, a single 5-mm DBS punch was placed in each well of a 96-well assay plate, and 100 μL of an extraction solution containing internal standards for acylcarnitines was added to each well. The plate was incubated at 45 °C for 45 min with a shaker set at 700 rpm. Subsequently, the supernatant was transferred to a new plate, followed by centrifugation at 1000 rpm for 5 min. Each well received 1 μL for injection into a C18 column (1.7 µm, 100 mm × 2.1 mm internal dimensions) of an ultra-performance liquid chromatography system with the column temperature maintained at 50 °C (Waters ACQUITY, Milford, MA, USA). The individual acylcarnitines were eluted using a gradient at a flow rate of 0.5 mL/min. This involved a 2-min run with 80% mobile phase A (0.001 formic acid in water) and 20% mobile phase B (acetonitrile), followed by a 5-min linear gradient of mobile phase B (20 to 30%), and concluding with 8 min of mobile phase B at 80%. The mass spectrometer featured an electrospray source that operated in the negative ion mode using the multiple reactions monitoring (MRM). The raw UPLC-MS data obtained in MRM mode were analyzed utilizing TargetLynx Application Manager version 4.1 from Waters Corp. (Milford, MA, USA) to derive the calibration equations and the quantitative concentration of each acylcarnitine in the samples.
Statistical analysis
The data analysis was conducted using SPSS 23 (SPSS Inc., CA, USA). To identify significant differences among the various study groups, nonparametric pairwise comparisons were performed using the Kruskal–Wallis test followed by the Mann–Whitney test. Principal component analysis (PCA) was conducted using GraphPad Software (CA, USA) to assess the global clustering patterns of metabolic profiles among patients with HCC, individuals with HCV infection, and normal control (NC) subjects. Linear contrast analysis, with adjustments for heteroscedasticity and estimation of effect size, was applied in the context of an ANOVA test to evaluate linear trends in metabolic profiles across these three groups. Additionally, a multivariate analysis of variance (MANOVA) was employed to explore potential associations between various clinicopathological aspects of HCC and acylcarnitine profiles. Key statistical measures, such as Pillai’s trace, were utilized to assess the overall multivariate effects. This analysis aimed to determine whether factors such as gender, portal vein status, child class, BCLC score, and the number of focal lesions were linked to variations in acylcarnitine profiles. Significance levels were employed to evaluate the statistical significance of these associations. All statistical tests were conducted using a significance level of p < 0.05, indicating that results with p-values less than 0.05 were considered statistically significant.
Results
The demographic and clinical parameters of study groups
Table 1 displays demographic and clinical characteristics of the enrolled groups. HCC individuals exhibit a significantly higher mean age (58.8 years) compared to both HCV (50.9 years) and NC (51.7 years) groups (p < 0.001). Gender distribution differs significantly (p = 0.001), with HCC having a higher percentage of males (86.7%) than HCV (41.8%) and NC (48%). No significant differences in BMI exist between the groups. Patients with either chronic liver disease or HCC had a history of previous HCV infection with compensated liver function. In the HCV liver disease group, the liver enlarged in 30.9% of cases, with evidence of cirrhosis observed in 87.3% of cases. In the HCC group, the liver enlarged in 13% of cases, and all cases developed on top of existing liver cirrhosis. Among the HCV group, patients were classified as Child–Pugh A in 72.7% of cases, Child–Pugh B in 20%, and Child–Pugh C in 7.3%. Among the HCC group, patients either had a single focal lesion in 42.2% of cases or multiple focal lesions in 57.8% of cases. The HCC patients were classified as Child–Pugh A in 71.1% of cases, Child–Pugh B in 23.3%, and Child–Pugh C in 5.6%. According to the Barcelona staging system, the HCC group was distributed across stages A in 27.8% of cases, B in 53.3%, C in 13.3%, and D in 5.6%. Various clinical parameters, including hemoglobin (Hb), platelet count, PT%, INR, ALT, AST, albumin, total bilirubin (T.Bil), direct bilirubin (D.Bil), creatinine, and alpha-fetoprotein (AFP), are also presented. Statistically significant differences were observed in these parameters between both the HCC and HCV groups compared to the control group (p < 0.05).
Metabolic profiling and cluster analysis of carnitine and its derivatives across the groups
Principal component analysis was employed using carnitine and acylcarnitine concentrations as variables to create a predictive model for identifying global clustering patterns in the metabolic profiles, differentiating between the control, HCC, and HCV groups.
Table 2 shows a comprehensive summary of the principal component analysis outcomes, highlighting the substantial influence of the first principal component (PC1) in explaining 36.04% of the overall variance. The subsequent principal components, PC2 and PC3, elucidate additional portions of the variance at 14.11% and 9.60%, respectively. The cumulative variance of 59.75% underscores the collective explanatory power of the three principal components in the dataset.
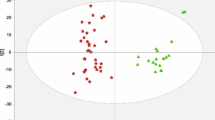
PCA score plot revealed the first principal component (PC1) explains a substantial 34.1% of the total variance and reveals a clear separation between the control clusters, situated on the negative side of PC1, and the HCC and HCV clusters, which are distributed on both positive and negative sides of PC1. This separation suggests distinct data patterns among the groups, especially between controls and patients. The fact that HCC and HCV clusters are found on both sides of PC1 indicates some degree of a close association within these patient groups as depicted in the score plot of Fig. 1. Table 3 presents the results of statistical pairwise comparisons of carnitine and acylcarnitine levels across different groups. Both the HCC and HCV groups demonstrated significantly higher levels of acylcarnitines, with most acylcarnitine levels yielding p-values < 0.01, clearly distinguishing them from the control group. However, no statistically significant difference was observed between the HCV and HCC groups (all p > 0.05), except for specific acylcarnitines, namely C2 and C5, where the p-values were lower (p < 0.05), signifying significant differences in acylcarnitine levels between HCC and HCV. Nonetheless, there is an observable trend suggesting higher acylcarnitine levels in HCC compared to HCV patients. This trend, while not statistically significant, was supported by linear contrast analysis, as detailed in Table 3 and illustrated in Fig. 2 which demonstrated a significant linear trend towards higher levels in HCC for FC0, AC2, AC3, AC4, AC10-1, AC8, AC12, AC16, and AC18, all with p-values < 0.001. Importantly, this trend held consistently regardless of whether equal variances were assumed or not, underlining the robustness of these observed linear trends. On the other hand, AC3DC, AC5, AC5DC, AC6, AC10, and AC18-1 did not display significant linear trends under either assumption (p > 0.05).
PCA scores plot of NC subjects, HCV, and HCC patients. The PCA scores plot visually represents the distribution of control subjects, HCV, and HCC patients based on their profiles. The first principal component (PC1) accounts for 34.1% of the total variance, with control clusters predominantly located on the negative side of PC1. HCC and HCV clusters are observed on both positive and negative sides of PC1. The second principal component (PC2) explains 12.42% of the variance but was not utilized for group clustering in this analysis. HCC, hepatocellular carcinoma; HCV, hepatitis C virus; Con, normal control
Linear trend of blood levels of carnitine and its acylcarnitine derivatives across the groups. C0, free carnitine; AC2, acetylcarnitine; AC3, propionylcarnitine; AC4, butyrylcarnitine; AC5, isovalerylcarnitine; AC5DC, glutarylcarnitine; AC6, hexanoylcarnitine; AC8, octanoylcarnitine; AC8:1, octanoylcarnitine; AC10, decanoylcarnitine; AC10:1, decanoylcarnitine; AC12, dodecanoylcarnitine; AC14, myristoylcarnitine; AC14:1, myristoleylcarnitine; AC16, palmitoylcarnitine; AC16:1, octapalmitoylcarnitine; AC18, octadecanoylcarnitine; AC18:1, oleoylcarnitine
The relationship between acylcarnitines and clinicopathological aspects of HCC
Multivariate analysis of variance (MANOVA) was conducted to investigate the relationship between different clinicopathological aspects of HCC, including gender, portal vein status, Child Class, BCLC score, and the number of focal lesions, and carnitine compounds. The results, as displayed in Table 4, revealed significant multivariate effects (Pillai’s trace = 0.420, F = 2.184, p = 0.025). Breaking down the analysis into individual variables, it was found that the main effect of “gender” was not statistically significant (Pillai’s trace = 0.274, F = 1.177, p = 0.315). Similarly, the portal vein status, whether patent or thrombosed, did not exhibit a significant main effect (Pillai’s trace = 0.148, F = 0.542, p = 0.917). Likewise, “child class,” “BCLC score,” and the number of focal lesions also did not yield statistically significant main effects (child class: Pillai’s trace = 0.322, F = 1.481, p = 0.138; BCLC score: Pillai’s trace = 0.292, F = 1.285, p = 0.238; FL number: Pillai’s trace = 0.237, F = 0.969, p = 0.505).
Furthermore, interactions between “gender” and PVT, child class, BCLC score, or the number of HFL were not statistically significant (p > 0.05 for all). Higher-order interactions involving three or more independent variables also did not exhibit statistical significance. These results suggest that the main and interaction effects of carnitine and its acylcarnitine derivatives, whether short, intermediate, or long, may not have a substantial impact on the clinical aspects of HCC under investigation (Table 5).
Discussion
The present study investigates metabolic alterations and variations in blood-free L-carnitine (C0) and its acylcarnitine derivatives in patients with chronic HCV infection complicated by liver cirrhosis, with or without HCC. The findings revealed a substantial increase in blood acylcarnitine levels in both the HCV and HCC groups when compared to the healthy control group and demonstrated a linear increasing trend towards HCC. This finding aligns with the study conducted by Sharif et al., which showed higher carnitine levels in HCC compared to liver cirrhosis, highlighting the pivotal role of excessive carnitine production in supporting mitochondrial activity and promoting rapid HCC growth [26]. Fujiwara et al. also reported elevated acylcarnitine levels in steatohepatitis associated with HCC, proposing that high acylcarnitine levels could serve as a potential early diagnostic marker for HCC in steatohepatitis [27]. However, Lu et al. presented contrasting findings, with decreased levels of LCACs, SCACs, and MCACs in patients with hepatitis virus-related HCC [28]. Zhou et al., in a study comparing the serum metabolic profile of HCC patients to those with chronic liver diseases, observed LCAC accumulation and free carnitine, MCAC, and SCAC deficiencies and linked these metabolic shifts to the energy expenditure mechanisms of HCC cells as the buildup of LCAC might stimulate citrate synthase activity and inhibit citrate lyase activity, ultimately upregulating cytosolic citrate production and contributing to energy supplementation for proliferating HCC [6, 13]. Conversely, Miyaaki demonstrated no significant association between LCACs and liver cirrhosis or HCC [29]. The discrepancies among these studies might arise due to the frequency of carnitine palmitoyltransferase-2 downregulation, which occurs more frequently in HCC than in other liver diseases such as steatohepatitis and HCV infection [30, 31].
Xias et al. observed reduced levels of several carnitine species, including LCACs, oleoyl carnitine, palmitoyl carnitine, and linoelaidyl carnitine; in patients with HCC compared to cirrhotic controls, their findings suggest that the downregulation of oleoyl carnitine in HCC may contribute to alterations in cancer cell energy metabolism, favoring glycolysis while inhibiting the TCA cycle [32]. Moreover, LCACs play a crucial role in FFA oxidation, with recent studies revealing progressive FFA accumulation from controls to cirrhosis and HCC. Disruptions in carnitine metabolism in chronic liver disease may stem from decreased liver expression of biosynthesis enzymes. Notably, Zhou et al. highlighted the varied carnitine metabolism in HCC and cirrhosis, suggesting reversed acylcarnitine accumulation patterns with SCACs and LCACs could show reverse accumulation in cirrhosis and HCC; thus, some types of acylcarnitines might increase in cirrhosis but decrease in HCC and vice versa [6]. Fitian et al. performed HPLC analysis of serum metabolites in patients with HCV-associated HCC and HCV-associated cirrhosis, and normal healthy control reveals significant alterations in acylcarnitine metabolism. MCACs, including octanoylcarnitine, decanoylcarnitine, and lauryl carnitine, exhibit substantial increases in fold change in both HCV-cirrhosis disease and HCC comparisons, indicating metabolic dysregulation associated with HCC development. Conversely, isobutyrylcarnitine shows a significant decrease in fold change, suggesting a distinct metabolic profile in HCC patients. While LCACs, such as oleoylcarnitine and palmitoylcarnitine, display modest changes, they do not reach statistical significance. These findings underscore the potential of acylcarnitines as metabolic biomarkers for HCC and provide insights into the metabolic alterations associated with HCC progression [7].
The main and interaction effects of carnitine and its acylcarnitine derivatives, whether short, intermediate, or long, may not have a substantial impact on the clinical aspects of HCC; notably, none of the acylcarnitines showed significant correlations with clinicopathological features of HCC, including AFP level, tumor volume, focal lesion count, Child–Pugh score, and BCLC HCC stage. This is in line with Shiraki et al.’s findings, which indicated no correlation between the Child–Pugh score and total carnitine, free carnitine, or acylcarnitines in cirrhotic patients [26]. Conversely, Miyaaki et al. reported that most carnitine fractions in the blood exhibited a significant correlation with the Child–Pugh score [29]. Notably, Lu et al. showed that acylcarnitine displayed a robust correlation with tumor grade and exhibited excellent discriminatory power in distinguishing HCC from matched normal tissues [33]. Zhang et al. investigated blood acylcarnitines in HCC and liver cirrhosis patients and identified carnitine and AC3 as risk factors for HCC, and C3DC, C10, and C18:1 were risk factors for liver cirrhosis [34]. Lu et al. reported a decrease in AC2 levels in patients with HCC compared to those without HCC, and this decrease correlated with tumor stage, suggesting that low AC2 levels could signal HCC development [33]. AC2 significantly downregulated the expression of vascular endothelial growth factor (VEGF) and VEGF receptor 2 (VEGFR-2), implying that AC2 possesses antiangiogenic properties via the VEGF pathway [35]. Various studies have indicated that LCACs are linked to poorer clinical outcomes, whereas SCACs and MCACs are associated with positive effects [36, 37].
Limitations of the study
Although acylcarnitines show potential as metabolic biomarkers in HCC, their interpretation is limited by various factors, including the influence of patients’ diets and daily activities. Sample variability due to age, sex, diet, and genetic differences can also impact acylcarnitine levels. Standardizing sample handling and analytical procedures is crucial to ensure accurate and reliable measurements. Matrix effects and the presence of other compounds in blood samples can interfere with acylcarnitine measurements, emphasizing the need for rigorous sample preparation techniques. Additionally, the lack of specific reference ranges tailored to HCC poses challenges in interpreting acylcarnitine level changes, necessitating further research. Establishing direct correlations between acylcarnitine changes and clinical parameters in HCC patients remains a complex challenge, demanding meticulous study design and interdisciplinary collaboration among researchers and clinicians to advance our understanding of their potential in HCC diagnosis and management [6, 26, 38].
The findings of the current research on metabolic alterations in patients with chronic HCV infection complicated by liver cirrhosis, with or without HCC, hold significant implications for patient care. Elevated levels of blood acylcarnitines observed in both HCV and HCC groups compared to healthy controls, along with their linear increasing trend towards HCC, highlight the potential of acylcarnitines as diagnostic markers for HCC development. These results are consistent with previous studies, indicating the importance of excessive carnitine production in supporting mitochondrial activity and promoting rapid HCC growth. However, discrepancies in carnitine metabolism among different liver diseases underscore the complexity of these metabolic pathways and the need for further investigation. While acylcarnitines show promise as biomarkers, their interpretation is limited by various factors, including diet, sample variability, and the lack of specific reference ranges tailored to HCC. Standardizing sample handling and analytical procedures, as well as establishing direct correlations with clinical parameters, are crucial steps in advancing our understanding of acylcarnitines’ role in HCC diagnosis and management. Overall, these findings contribute to identifying potential metabolic shifts in liver cells and pave the way for improved disease management strategies.
In conclusion, the observed changes in acylcarnitine levels in HCV and HCC patients offer crucial clinical insights, indicating potential metabolic shifts in liver cells. The increasing trend of acylcarnitine levels from healthy controls to HCV and HCC patients suggests their promise as valuable diagnostic and prognostic markers, encouraging further research for improved disease management.
Availability of data and materials
The datasets used and/or analyzed during the current study are partially available from the corresponding author on reasonable request.
Change history
06 July 2024
A Correction to this paper has been published: https://doi.org/10.1186/s43066-024-00347-2
Abbreviations
- AASLD:
-
American Association for the Study of Liver Diseases
- BCLC:
-
Barcelona Clinic Liver Cancer
- BMI:
-
Body mass index
- DBS:
-
Dried blood spot
- DM:
-
Diabetes mellitus
- HCC:
-
Hepatocellular carcinoma
- HCV:
-
Hepatitis C virus
- LC:
-
L-Carnitine
- LCAC:
-
Long-chain acylcarnitines
- MCAC:
-
Medium-chain acylcarnitines
- MRM:
-
Multiple reactions monitoring
- PCA:
-
Principal component analysis
- ROC:
-
Receiver operating characteristic
- SCAC:
-
Short-chain acylcarnitines
- VEGF:
-
Vascular endothelial growth factor
References
Bremer J (1983) Carnitine–metabolism and functions. Physiol Rev 63(4):1420–1480
Jacob C, Belleville F (1992) L-carnitine: metabolism, functions and value in pathology. Pathol Biol (Paris). 40(9):910–919
Hoppel C (2003) The role of carnitine in normal and altered fatty acid metabolism. Am J Kidney Dis 41(4 Suppl 4):S4–12
Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledoand FG, DeLany JP (2010) Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18(9):1695–1700
Nakanishi H, Kurosaki M, Tsuchiya K, Nakakuki N, Takada H, Matsuda S, Izumi N (2015) L-carnitine reduces muscle cramps in patients with cirrhosis. Clin Gastroenterol Hepatol 13(8):1540–3
Zhou L, Wang Q, Yin P, Xing W, Wu Z, Chen S, Xu G (2012) Serum metabolomics reveals the deregulation of fatty acids metabolism in hepatocellular carcinoma and chronic liver diseases. Anal Bioanal Chem 403(1):203–13
Fitian AI, DR Nelson, C Liu, Y Xu, M Araratand R Cabrera (2014) Integrated metabolomic profiling of hepatocellular carcinoma in hepatitis C cirrhosis through GC/MS and UPLC/MS-MS. Liver Int 34(9):1428–1444
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144(5):646–74
Koppenol WH, PL Boundsand CV Dang (2011) Otto Warburg’s contributions to current concepts of cancer metabolism. Nat Rev Cancer 11(5):325–337
Longo N, M Frigeniand, M Pasquali (2016) Carnitine transport and fatty acid oxidation. Biochim Biophys Acta 1863(10):2422–2435
Ganti S, Taylor SL, Kim K, Hoppel CL, Guo L, Yang J, RH Weiss (2012) Urinary acylcarnitines are altered in human kidney cancer. Int J Cancer 130(12):2791–800
Zoni E, Minoli M, Bovet C, Wehrhan A, Piscuoglio S, Ng CKY, Kruithof-de Julio M (2019) Preoperative plasma fatty acid metabolites inform risk of prostate cancer progression and may be used for personalized patient stratification. BMC Cancer 19(1):1216
Ren S, Shao Y, Zhao X, Hong CS, Wang F, Lu X, Sun Y (2016) Integration of metabolomics and transcriptomics reveals major metabolic pathways and potential biomarker involved in prostate cancer. Mol Cell Proteomics 15(1):154–63
Farshidfar F, Kopciuk KA, Hilsden R, McGregor SE, Mazurak VC, Buie WD, Bathe OF (2018) A quantitative multimodal metabolomic assay for colorectal cancer. BMC Cancer 18(1):26
Violante S, L Ijlst, H Te Brinke, I Tavares de Almeida, RJ Wanders, FV Ventura, SM Houten (2013) Carnitine palmitoyltransferase 2 and carnitine/acylcarnitine translocase are involved in the mitochondrial synthesis and export of acylcarnitines. FASEB J 27(5):2039–2044
Yao Z, Yin P, Su D, Peng Z, Zhou L, Ma L, Jiao B (2011) Serum metabolic profiling and features of papillary thyroid carcinoma and nodular goiter. Mol Biosyst 7(9):2608–14
Ni J, L Xu, W Li, C Zhengand L Wu (2019) Targeted metabolomics for serum amino acids and acylcarnitines in patients with lung cancer. Exp Ther Med 18(1):188–198
Sun C, F Wang, Y Zhang, J Yuand X Wang (2020) Mass spectrometry imaging-based metabolomics to visualize the spatially resolved reprogramming of carnitine metabolism in breast cancer. Theranostics 10(16):7070–7082
Tapper EB, Parikh ND (2023) Diagnosis and management of cirrhosis and its complications a review. JAMA 329(18):1589–1602
Pugh RN, IM Murray-Lyon, JL Dawson, MC Pietroniand R Williams (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60(8):646–649
Smith A, K Baumgartnerand C Bositis (2019) Cirrhosis: diagnosis and management. Am Fam Physician 100(12):759–770
Heimbach JK (2017) Overview of the updated AASLD guidelines for the management of HCC. Gastroenterol Hepatol (N Y) 13(12):751–753
de Freitas LBR, L Longo, D Santos, I Grivicichand MR Alvares-da-Silva (2019) Hepatocellular carcinoma staging systems: Hong Kong liver cancer vs Barcelona Clinic Liver Cancer in a Western population. World J Hepatol 11(9):678–688
Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, Heimbach JK (2018) Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology 68(2):723–750
Moretti M, Freni F, Valentini B, Vignali C, Groppi A, Visona SD, Morini L (2019) Determination of antidepressants and antipsychotics in dried blood spots (DBSs) collected from post-mortem samples and evaluation of the stability over a three-month period. Molecules. 24(20):3636
Shariff MI, Gomaa AI, Cox IJ, Patel M, Williams HR, Crossey MM, Taylor-Robinson SD (2011) Urinary metabolic biomarkers of hepatocellular carcinoma in an Egyptian population: a validation study. J Proteome Res 10(4):1828–36
Fujiwara N, Nakagawa H, Enooku K, Kudo Y, Hayata Y, Nakatsuka T, Koike K (2018) CPT2 downregulation adapts HCC to lipid-rich environment and promotes carcinogenesis via acylcarnitine accumulation in obesity. Gut 67(8):1493–1504
Lu X, Zhang X, Zhang Y, Zhang K, Zhan C, Shi X, Li Y (2019) Metabolic profiling analysis upon acylcarnitines in tissues of hepatocellular carcinoma revealed the inhibited carnitine shuttle system caused by the downregulated carnitine palmitoyltransferase 2. Mol Carcinog. 58(5):749–759
Miyaaki H, H Kobayashi, S Miuma, M Fukusima, R Sasaki, M Haraguchiand K Nakao (2020) Blood carnitine profiling on tandem mass spectrometry in liver cirrhotic patients. BMC Gastroenterol 20(1):41
Shibahara J S Ando, Y Sakamoto, N Kokudoand M Fukayama (2014) Hepatocellular carcinoma with steatohepatitic features: a clinicopathological study of Japanese patients. Histopathology 64(7):951–962
Enooku K, Nakagawa H, Fujiwara N, Kondo M, Minami T, Hoshida Y, Koike K (2019) Altered serum acylcarnitine profile is associated with the status of nonalcoholic fatty liver disease (NAFLD) and NAFLD-related hepatocellular carcinoma. Sci Rep 9(1):10663
Xiao JF, Varghese RS, Zhou B, Nezami Ranjbar MR, Zhao Y, Tsai TH, Ressom HW (2012) LC-MS based serum metabolomics for identification of hepatocellular carcinoma biomarkers in Egyptian cohort. J Proteome Res 11(12):5914–23
Lu Y, Li N, Gao L, Xu YJ, Huang C, Yu K, Ong CN (2016) Acetylcarnitine is a candidate diagnostic and prognostic biomarker of hepatocellular carcinoma. Cancer Res 76(10):2912–20
Zhang Y, N Ding, Y Cao, Z Zhuand P Gao (2018) Differential diagnosis between hepatocellular carcinoma and cirrhosis by serum amino acids and acylcarnitines. Int J Clin Exp Pathol 11(3):1763–1769
Baci D, Bruno A, Bassani B, Tramacere M, Mortara L, Albiniand A, Noonan DM (2018) Acetyl-l-carnitine is an anti-angiogenic agent targeting the VEGFR2 and CXCR4 pathways. Cancer Lett 429:100–116
Lum H, Huffman KM, Kraus VB, Thompson DK, Kraus WE, Morey MC (2011) Plasma acylcarnitines are associated with physical performance in elderly men. J Gerontol A Biol Sci Med Sci 66(5):548–53
Redman LM, Huffman KM, Landerman LR, Pieper CF, Bain JR, Muehlbauer MJ, Ravussin E (2011) Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J Clin Endocrinol Metab 96(2):E312–21
Li S, Gaoand D, Jiang Y (2019) Function, detection and alteration of acylcarnitine metabolism in hepatocellular carcinoma. Metabolites 9(2):36
Acknowledgements
We extend our sincere appreciation and gratitude to Dr. Azza Elsheshaei and Dr. Samah Elsheaty from the HPLC Unit in the Department of Clinical Biochemistry and Molecular Diagnostics for their invaluable and indispensable assistance.
Funding
The authors declare they do not have any financial disclosure.
Author information
Authors and Affiliations
Contributions
AAb and AEF developed the methodology, designed the experiments, and collected the UPLC/MS/MS data. MO, contributed to the study concept and design. EA, MA, and AI were involved in patient recruitment, evaluation, and the collection of clinical data, as well as obtaining ethical approval. AK, corresponding author, conducted statistical data analysis, and wrote and edited the manuscript. All authors reviewed and edited the manuscript and approved the final version.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the protocols applied in this study were conducted following the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and approved by the ethical committees of the National Liver Institute, Menoufia University (IRB-00003469), and all participants enrolled in the study provided informed consent.
Consent for publication
Obtained from all participants.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: the authors corrected the following errors (1) Ashraf Abbass has been corrected to Ashraf Abbass Basuni (2) Mohammed of Mohamed Abdel‑Samiee has been corrected to Mohamed (3) Rectification of the reference section (Incorrect placement of the publication year) (4) Adjustment to Table 1: The 7th column labeled “MSD” has been moved two spaces to the left next to the 4th column Med (IQR).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abbass Basuni, A., Sheashaey, A.E., Fert, A.E. et al. Exploring the prognostic significance of blood carnitine and acylcarnitines in hepatitis C virus-induced hepatocellular carcinoma. Egypt Liver Journal 14, 19 (2024). https://doi.org/10.1186/s43066-024-00322-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-024-00322-x