Abstract
Background
The management of mitral valve disease in young children is challenging. Mechanical mitral valves could provide long-term durability; however, the need for anticoagulation increases the risk profile of mechanical valves. We report our experience in mechanical mitral valve replacement (MVR) in children under 2 years of age and evaluate factors affecting the outcomes.
The study included all patients younger than 2 years who underwent mechanical MVR between 2000 and 2023. The study outcomes were mitral valve reoperation, bleeding, valve-related thrombosis, and survival.
Results
Twenty-three patients were included, with a mean age of 10.2 ± 5.9 months. The mitral valve sizes ranged from 16 to 25 mm, and 6 (26%) were placed in the supra-annular position. Complete heart block occurred in seven patients (30%), and operative mortality occurred in three patients (13%). Postoperative warfarin was used in 17 patients (74%). After discharge, bleeding occurred in five patients (22%), four were managed conservatively, and one had intracranial hemorrhage treated with craniotomy. Nine patients (39%) had valve-related thrombosis; two underwent reoperation, while seven were treated with alteplase in 26 patients. Valve-related thrombosis was more common in patients with supra-annular valves (p < 0.001) and in those who were not on warfarin (p < 0.001). A total of seven patients (30%) underwent redo MVR, and redo was more common in young patients (p = 0.029) and in patients with supra-annular valves (p < 0.001). Survival of the whole cohort was 73% at 5 years. Among the annular position group, 5-year survival was 88%, while among the supra-annular position group, survival was 50% after 3 months and 25% after 14 months (p = 0.009).
Conclusions
Mechanical MVR in children younger than 2 years is associated with high complication rates, including thrombosis and bleeding. The supra-annular valve position appears to be a risk factor for thrombosis and reoperation. Anticoagulation with warfarin remains challenging. However, further studies evaluating alternative options are needed.
Graphical Abstract

Similar content being viewed by others
Background
Mitral valve replacement (MVR) in children is a rare and challenging procedure with reported high complication rates [1, 2]. The surgical challenge of MVR in children arises because of the small annular diameter, the associated lesions that may affect valvular morphology and annular growth, and the unavailability of suitable mitral valve prostheses [3,4,5]. Mitral valve repair in children offers a durable option, with long-term freedom from mitral valve reinterventions; therefore, MVR is performed when the repair cannot be performed or is inadequate [6]. The availability of small prosthetic mitral valves and advances in cardiopulmonary bypass techniques in children have led to an increase in the number of mechanical mitral valves in young children [7,8,9]. The use of a small mitral valve prosthesis in children may increase the risk of heart block and valve-related thrombosis. Furthermore, it can affect long-term survival, including the need for redo surgery to upsize the mitral valve [1, 10, 11]. Research is ongoing to develop dynamic valves that expand with annular growth [12]; however, the durability of biological materials in children, especially on the left side, is unclear. Mechanical mitral valves could provide a reasonable solution in these patients because of their long-term durability; however, the need for anticoagulation in the young population may increase the risk profile of mechanical valves [13]. Several factors can jeopardize the long-term outcomes of MVR. Worse survival was reported with the supra-annular valve position [2] and with a small valve size [14]; therefore, tailoring surgical techniques to avoid small valves was associated with improved outcomes [14]. Recent studies have shown that MVR in children is associated with low morbidity and mortality, especially with new mitral valve prostheses [13, 15,16,17,18].
Studies evaluating the outcomes of MVR in children younger than 2 years are scarce, and the outcomes could be further affected by the use of a smaller prosthesis, the need for anticoagulation, and the need for mitral valve reoperations [13]. Therefore, the goal of this study was to report the outcomes of mechanical MVR in children under 2 years old and to evaluate factors affecting long-term valve-related thrombosis, mitral valve reoperation, and survival.
Methods
Patients
This retrospective cohort study included children under 2 years of age who underwent MVR at a single center from 2000 to 2023. The study included patients who underwent mechanical MVR. Patients who were older than 2 years, had univentricular hearts, had biological valves, or had concomitant aortic or pulmonary valve replacement were excluded. The study was approved by the hospital’s institutional review board.
Study data and outcomes
The data collected included age in months at the time of the indexed operation, sex, weight in kg, cardiac diagnosis, the presence of mitral valve lesions, and history of previous cardiac surgery. Reported operative data included mitral valve sizes, types, and positions (annular vs. supra-annular). Postoperative complications included renal failure, sepsis, complete heart block (CHB) requiring permanent pacemaker (PPM) insertion, left ventricular outflow tract obstruction, extracorporeal membrane oxygenation insertion, intensive care unit (ICU) and hospital stay, and hospital mortality. The follow-up outcomes included bleeding, valve-related thrombosis, mitral valve reoperation, and survival.
Anticoagulation protocol
Heparin infusion was started 24 h postoperatively along with warfarin until the target international normalization ratio (INR) of 2.5–3.5 was achieved. Patients were followed up after discharge at the outpatient clinic. Low-molecular-weight heparin was used for patients who were not tolerant or noncompliant with warfarin or who were at high risk of bleeding. Additional antiplatelet therapy with aspirin was used in selected patients according to the discretion of the treating physicians.
Surgical techniques
All patients underwent mitral valve surgery through median sternotomy and cardiopulmonary bypass. The mitral valve was approached via left or right atriotomies depending on the concomitant pathology and the surgeons’ preference. The mitral valve was inserted into the annular or supra-annular position.
Follow-up protocol
The patients were followed for 1 week after discharge at the anticoagulation clinic and postoperative clinic. The frequency of follow-up in the anticoagulation clinic was determined according to the INR. For patients with a stable INR, follow-up was scheduled every 4 weeks.
Statistical analysis
The normality of the distribution of continuous variables was evaluated, and normally distributed data are presented as the mean and standard deviation, while nonnormally distributed data are expressed as the median (25th and 75th quartiles). Categorical data are presented as numbers and percentages. Time-to-event data are presented as Kaplan–Meier curves, and the curves were compared between annular and supra-anular positions using the log-rank test. Factors affecting time-to-event outcomes were evaluated with univariate Cox regression analysis, and the hazard ratio (HR) and its confidence interval were reported. Analyses were performed using Stata 17 (Stata Corp, College Station, TX, USA).
Results
Baseline and operative data
The study included 23 patients who underwent mechanical MVR; their ages ranged from 2 to 24 months. The mean weight was 7.6 ± 2.9 kg, and 11 (48%) patients were males. The most common diagnosis was congenital MR (n = 6; 26%). Mitral regurgitation was the most common mitral valve lesion (n = 17; 74%), two patients had mitral stenosis (9%), and four had mixed lesions (17%).
The size of the mitral valve prosthesis ranged from 16 to 25 mm. The most common types were St. Jude (n = 15) (St. Jude Medical Inc.; Minneapolis, MN, USA), and CarboMedics valve (n = 4) (CarboMedics, Inc.; Austin, Texas). In 6 patients (26%), the mitral valve was inserted in a supra-annular position, and in the remaining 17, the mitral valve was inserted in the annular position (Table 1).
Postoperative outcomes
Renal failure occurred in three patients (13%), sepsis in four patients (17%), and complete heart block in seven patients (32%). There was no significant difference in PPM insertion between annular (n = 5, 31%) and supra-annular (n = 2, 33%) valves (p > 0.99). The median duration of ICU stay was 14 days (range 7–25), and the median duration of hospital stay was 27.5 days (range 14–60). Operative mortality occurred in three patients (13%). Warfarin was used in 17 patients (Table 2).
Follow-up events
Bleeding
Bleeding was reported in five (22%) patients; four were managed conservatively, but one had major intracranial hemorrhage (ICH) requiring craniotomy. Three of the patients who had bleeding were on warfarin, and two were not (p = 0.58). The sites of bleeding were dental (n = 2), mouth (n = 1), pulmonary (n = 1), and ICH (n = 1).
Valve thrombosis
Valve thrombosis was defined as a 50% increase in the mean mitral inflow gradient by echocardiography or a stuck mitral valve leaflet on fluoroscopy or echocardiography. Nine patients had thrombosis (39%) at least once (n = 4), twice (n = 2), five times (n = 2), or nine times (n = 1). Alteplase was used in 7 patients on 26 occasions [once (n = 2), twice (n = 2), five times (n = 2), and nine times (n = 1)], and it was successful in treating the thrombus in 24 instances. In two events, the patients presented too late with severe pulmonary edema and died. Two patients were managed by redo surgery: one with removal of a clot and one with redo mitral valve replacement.
The percentages of patients who were free from thrombosis at 1, 2, and 5 years were 67%, 55%, and 55%, respectively. Four out of the 6 patients with supra-annular valves had thrombosis, and 5 out of the 17 patients with annular valves had thrombosis. The rates of freedom from valve-related thrombosis in the annular position were 86%, 71%, and 53% after 1, 2, and 5 years, respectively, while they were 50% in the supra-annular position after 6 months (p < 0.001) (Fig. 1).
Thrombosis was not related to age [HR: 0.95 (95% CI: 0.84–1.07); p = 0.381], sex [HR: 0.99 (95% CI: 0.26–3.71); p = 0.988], weight [HR: 1.06 (95% CI: 0.85–1.33); p = 0.607], or valve size [HR: 0.92 (0.72–1.17); p = 0.487]. The incidence of valve thrombosis was significantly greater in patients who were not on warfarin (p < 0.001) (Fig. 2).
Four patients required postoperative extracorporeal membrane oxygenation (ECMO) support, two of whom underwent additional repair of the atrioventricular canal, and one patient underwent additional tetralogy of Fallot repair. Mortality occurred in two patients with ECMO support [19].
Mitral valve reoperation
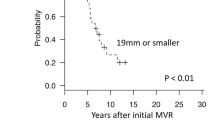
Seven patients underwent redo MVR, two of whom underwent reoperations twice. The median time to reoperation was 19 months (range 4–45). The second redos occurred after 121 and 130 months. One patient had a redo with a tissue valve, while the others had mechanical valves. The rate of freedom from valve reoperation was 76% after 1 year, 69% after 2 years, and 57% after 5 years. Four patients had annular valves, and three patients had supra-annular valves. The rates of freedom from mitral valve reoperation at 1, 2, and 5 years were 92%, 84%, and 70%, respectively, for annular valves, and they were zero% after 7 months in the supra-annular position (p < 0.001) (Fig. 3).
Redo-MVR was more common in younger patients [HR: 0.84 (95% CI: 0.72–0.98); p = 0.029], while it was not related to sex [HR: 0.83 (95% CI: 0.18–3.82); p = 0.814], weight [HR: 0.90 (95% CI: 0.69–1.17); p = 0.420], or valve size [HR: 0.73 (95% CI: 0.49–1.09); p = 0.121].
Survival
The median follow-up duration was 26 months (range 4–126). Six deaths (26%) were reported, with thrombosis being the direct cause of death in two patients: three died because of sepsis, and one died because of pulmonary hypertension crisis. Survival was 78% after 1 year and 73% after 5 years. Survival at 5 years was 88% in the annular position, 50% after 3 months, and 25% after 14 months in the supra-annular position (p = 0.009) (Fig. 4).
Survival was not related to age [HR: 0.78 (95% CI: 0.60–1.004); p = 0.054], sex [HR: 0.99 (95% CI: 0.20–4.96); p = 0.994], weight [HR: 0.90 (95% CI: 0.67–1.20); p = 0.464], or valve size [HR: 0.82 (95% CI: 0.57–1.18); p = 0.275].
Discussion
Cardiac valve defects are common congenital cardiac lesions in children, and repair is the procedure of choice for restoring long-term cardiac function [20]. The optimal mitral valve prosthesis in children is still a subject of ongoing research, and the results of the available prostheses remain suboptimal. Mechanical valves offer a durable solution but have a high risk of thromboembolic complications and lifelong anticoagulation therapy [21]. On the other hand, tissue valves are prone to early degeneration when implemented in children [22], and both valve types have fixed diameters that do not grow with the patients. A small valve size predicts worse outcomes after MVR in children, and several techniques have been adapted for inserting larger prostheses that might persist longer [14]. The long-term outcomes after MVR in children younger than 2 years remain a concern. This study evaluated the outcomes after mechanical MVR in children younger than 2 years and assessed possible factors that might affect the outcomes. The study included 23 patients whose mitral valve size ranged between 16 and 25 mm, and 6 patients had a supra-annular valve. The most common postoperative complication was a complete heart block requiring PPM insertion (32%), and operative mortality was reported in three patients (13%). Seven patients had CHB, two of whom had AV canals, one who had VSD repair, and one who had TOF repair. Valve-related thrombosis was a common complication, and 28 attacks were reported in 9 patients, two of whom underwent surgical interventions. Thrombosis was more common in patients not on warfarin and with a supra-annular valve. Additionally, patients with supra-annular valves had more mitral valve reoperations and lower survival.
Supra-annular valve implantation has been described in children with small native annular diameters [23]. The technique was theoretically introduced to expand the life of the valve; however, Barker and associates reported that the use of the supra-annular mitral valve did not extend the time to the second replacement, and on the contrary, it affected hemodynamics [24]. Kanter and colleagues evaluated 15 children with supra-annular MVR and reported permanent pacemaker insertion in 3 patients and early thrombosis in 3 patients. On follow-up, six patients underwent mitral valve reoperations for pannus formation or thrombus [23]. Giordano and coworkers studied seven children with supra-annular MVR and reported reoperation in two patients for thrombus or pannus formation [25]. Tierney and colleagues reported lower survival in patients with supra-annular MVR, similar to our results, but with a lower rate of complete heart block [2]. Kwon and associates compared supra-annular MVR using polytetrafluoroethylene grafts (n = 8) to annular MVR (n = 33) in children < 20 kg. They did not report differences in survival between the groups [26].
Anticoagulation protocols for mechanical valves in children vary widely between centers [27]. In this study, patients were treated with different anticoagulation regimens after MVR. The main anticoagulation protocol used warfarin to achieve a target INR between 2.5 and 3.5. However, the use of warfarin is challenging in young patients, and some patients require the use of low-molecular-weight or unfractionated heparin. In one study, the durability of mechanical valves in children was affected by the frequent occurrence of valve-related thrombosis, making the reoperation rate comparable to that of biological valves [28]. This study showed that valve-related thrombosis was more common in patients who were not receiving warfarin therapy. The anticoagulation regimen should be tailored to the patient’s characteristics and risk factors. Shin and colleagues reported that the rate of freedom from anticoagulation-related complications (bleeding/thrombosis) was 94% at 5 years after mechanical valve replacement in children [29]. Most of our patients with valve-related thrombosis were managed with alteplase, with successful outcomes. Surgery was required for only two patients with valve-related thrombosis. A previous study reported that alteplase was successful in relieving prosthetic mitral valve thrombosis in 19/20 children [30].
The results of this study demonstrated that mechanical MVR in children younger than 2 years was associated with increased morbidity and mortality. The durability of valves in children is a major concern. A recent study tested the potential use of balloon-expandable heart valves that can adapt to annular growth in children [12]. However, the issue of accelerated bioprosthetic degeneration in children remains challenging. These results indicate that the optimal valve prosthesis in children has not yet been identified, and further research is required to improve the durability and decrease the complication rate in pediatric patients with artificial heart valves.
Limitations
This study has several limitations. First, the retrospective design has inherent biases. Several factors could affect the outcomes and were not thoroughly evaluated, and the choice of technique or prosthesis could be affected by surgeons’ preferences and experience. However, MVR in children younger than 2 years is rare, and prospective randomized studies are not practical. Second, the study evaluated mechanical MVR; no alternative options were compared to this valve type. Third, the study is limited by the sample size, but this is attributed to the low frequency of the procedure. Finally, this was a single-center study, and the protocols used to select patients for surgery and anticoagulation regimens might not be similar to those used in other centers.
Conclusions
Mechanical MVR in children younger than 2 years is associated with high complication rates, especially in patients with supra-annular valves, including thrombosis and reoperation. Anticoagulation with warfarin remains challenging in infants. On the other hand, not using warfarin appears to be associated with an increased risk of valve-related thrombosis. However, further studies evaluating alternative options are needed.
Availability of data and materials
The datasets used or analyzed in the current study are available from the corresponding author upon reasonable request.
Abbreviations
- MVR:
-
Mitral valve replacement
- CHB:
-
Complete heart block
- PPM:
-
Permanent pacemaker
- INR:
-
International Normalised Ratio
- ECMO:
-
Extracorporeal membrane oxygenation
- ICU:
-
Intensive care unit
- ICH:
-
Intracranial hemorrhage
- HR:
-
Hazard ratio
References
Javier Delmo EM, Hetzer R (2020) Mitral valve surgery in infants and children. Translational pediatrics. China. 9:187–90
Scheggi V, Olivotto I, Del Pace S et al (2020) Feasibility and outcome of mitral valve repair in patients with infective endocarditis. Cardiothorac Surg 28:27. https://doi.org/10.1186/s43057-020-00037-w
Hetzer R, Delmo Walter EBM, Hübler M, Alexi-Meskishvili V, Weng Y, Nagdyman N et al (2008) Modified surgical techniques and long-term outcome of mitral valve reconstruction in 111 children. Ann Thorac Surg 86(2):604–613
Elmahrouk AF, Ismail MF, Arafat AA, Dohain AM, Helal AM, Hamouda TE, Galal M, Edrees AM, Al-Radi OO, Jamjoom AA (2021) Outcomes of biventricular repair for shone's complex. J Card Surg 36(1):12–20. https://doi.org/10.1111/jocs.15090
Alkhushi N (2023) The management of newborns with critical congenital heart diseases prior to transport to a cardiac center. Cardiothorac Surg 31:1. https://doi.org/10.1186/s43057-022-00090-7
Isaacson E, Lucjak C, Johnson WK, Yin Z, Wang T, Rein L et al (2020) Mitral valve surgery in neonates, infants, and children: surgical approach, outcomes, and predictors. Semin Thorac Cardiovasc Surg. 32(3):541–50 (https://www.sciencedirect.com/science/article/pii/S1043067920300058)
Metras A, Seguela P-E, Roubertie F (2019) Mechanical mitral valve replacement in children: an update. Transl pediatri 8:455–7
Saleem Y, Darbari A, Sharma R, Vashisth A, Gupta A (2022) Recent advancements in pediatric cardiopulmonary bypass technology for better outcomes of pediatric cardiac surgery. Cardiothorac Surg. 30(1):23. https://doi.org/10.1186/s43057-022-00084-5
Ghandour H, Vervoort D, Ravishankar R et al (2022) Cardiac surgery and the sustainable development goals: a review. Cardiothorac Surg 30:14. https://doi.org/10.1186/s43057-022-00072-9
Mashali MH, Yousef AA, Elmahrouk AF et al (2023) Reintervention after repair of tetralogy of Fallot: a one-decade single-center experience. Cardiothorac Surg 31:5. https://doi.org/10.1186/s43057-023-00096-9
Alsoufi B, Manlhiot C, McCrindle BW, Al-Halees Z, Sallehuddin A, Al-Oufi S et al (2010) Results after mitral valve replacement with mechanical prostheses in young children. J Thorac Cardiovasc Surg. 139(5):1189-1196.e2. https://doi.org/10.1016/j.jtcvs.2009.10.038
Hofferberth SC, Saeed MY, Tomholt L, Fernandes MC, Payne CJ, Price K, Marx GR, Esch JJ, Brown DW, Brown J, Hammer PE, Bianco RW, Weaver JC, Edelman ER, Del Nido PJ (2020) A geometrically adaptable heart valve replacement. Sci Transl Med 12(531):eaay4006. https://doi.org/10.1126/scitranslmed.aay4006
Elmahrouk AF, Mashali MH, Ismail MF, Arafat AA, Alamri RM, Baho HA et al (2021) Mitral valve replacement in infants and younger children. Sci Rep. 11(1):15239. https://doi.org/10.1038/s41598-021-94779-0
Yuan H, Wu Z, Lu T, Tang Y, Chen J, Yang Y et al (2021) Long-term outcomes of mitral valve replacement in patients weighing less than 10 kg. J Cardiothorac Surg 16(1):63. https://doi.org/10.1186/s13019-021-01443-9
McPherson I, Bayliss C, Generali T et al (2021) Inverted aortic bioprosthetic for mitral valve replacement with Ross procedure: case report. Cardiothorac Surg 29:3. https://doi.org/10.1186/s43057-021-00041-8
Eltayeb OM, Readdy WJ, Mongé MC, Forbess JM, Sarwark AE, Patel A et al (2019) Mitral valve replacement in infants using a 15-mm mechanical valve. Ann Thorac Surg 108(2):552–557
Samal S, Sharma R, Minhas HS et al (2022) Role of great artery annulus ratio to predict transannular patch enlargement in repair of tetralogy of Fallot. Cardiothorac Surg 30:25. https://doi.org/10.1186/s43057-022-00086-3
Arslanoğlu E, Kara KA, Yiğit F et al (2021) Neurological complications after pediatric cardiac surgery. Cardiothorac Surg 29:19. https://doi.org/10.1186/s43057-021-00056-1
ElMahrouk A, Ismail M, Hamouda T, Shaikh R et al (2019) Extracorporeal membrane oxygenation in postcardiotomy pediatric patients-15 years of experience outside Europe and North America. Thorac Cardiovasc Surg 67(1):28–36
Husain SA, Brown JW (2007) When reconstruction fails or is not feasible: valve replacement options in the pediatric population. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 117–24. https://doi.org/10.1053/j.pcsu.2007.01.012
Lubiszewska B, Rozanski J, Szufladowicz M, Szaroszyk W, Hoffman P, Ksiezycka E et al (1999) Mechanical valve replacement in congenital heart disease in children. J Heart Valve Dis 8(1):74–79
Chen PC, Sager MS, Zurakowski D, Pigula FA, Baird CW, Mayer JEJ et al (2012) Younger age and valve oversizing are predictors of structural valve deterioration after pulmonary valve replacement in patients with tetralogy of Fallot. J Thorac Cardiovasc Surg 143(2):352–360
Kanter KR, Kogon BE, Kirshbom PM (2011) Supra-annular mitral valve replacement in children. Ann Thorac Surg. 92(6):2221–9. https://doi.org/10.1016/j.athoracsur.2011.06.023
Barker CL, Daubeney PEF, Shinebourne EA (2005) Complications of supra-annular mitral valve placement in infants. Heart 91(6):e48
Giordano R, Cantinotti M, Pak V, Arcieri L, Poli V, Assanta N et al (2015) Supra-annular mitral valve implantation in very small children. J Card Surg. 30(2):185–9. https://doi.org/10.1111/jocs.12501
Kwon HW, Kim W-H, Lee JR, Kwak JG, Cho S, Bae EJ et al (2020) Outcomes of supra-annular mechanical atrioventricular valve replacement with polytetrafluoroethylene graft in infants and children. Pediatr Cardiol 41(3):607–614
Nguyen N, Sharathkumar A (2015) Current perioperative anticoagulation practices in children with prosthetic mechanical heart valves. Congenit Heart Dis 10(5):E210–E215
Alsoufi B, Manlhiot C, McCrindle BW, Canver CC, Sallehuddin A, Al-Oufi S et al (2009) Aortic and mitral valve replacement in children: is there any role for biologic and bioprosthetic substitutes?☆. Eur J Cardio-Thoracic Surg. 36(1):84–90. https://doi.org/10.1016/j.ejcts.2009.02.048
Shin HJ, Jung JW, Park HK, Park YH (2015) Outcome of mechanical heart valve replacement in children: a 20-year experience. J Cardiothorac Surg. 10(1):A81. https://doi.org/10.1186/1749-8090-10-S1-A81
Mashali MH, Ahmad Z, Omar Galal M, Al-Kouatli A (2023) Effectiveness of alteplase infusion for the management of prosthetic mitral valve thrombosis in paediatric age group and proposed algorithm. Cardiol Young 33(5):747–53 (https://www.cambridge.org/core/article/effectiveness-of-alteplase-infusion-for-the-management-of-prosthetic-mitral-valve-thrombosis-in-paediatric-age-group-and-proposed-algorithm/2D970521DDDEF01450F753322F6749BE)
Acknowledgements
None
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization, MHM, OAA, and AE; methodology, MHM and RAZ; software, AE; validation, AAK, RAZ, AAJ, and ZA; formal analysis, AE; investigation, MHM and OAA; resources, AE and OAA; data curation, MHM and OAA; writing—original draft preparation, AE; writing—review and editing, ZA, AAJ, and RAZ. visualization, AAK, ZA, AAJ, and RAZ; supervision, AAK, ZA, AAJ, and RAZ; project administration, AE; and all authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the institutional review board (IRB) under approval number: IRB# 2023–62. Consent to participate is not applicable for the retrospective nature of the study.
Consent for publication
Not applicable for the retrospective nature of the study.
Competing interests
One of the co-authors of this study is an associate editor of the journal, and he has delegated his editorial roles for this article to the editor in chief. His contribution in the article was conceptualization, software, formal analysis, resources, writing—original draft preparation, and project administration. The other authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mashali, M.H., Elmahrouk, A.F., Ahmad, Z. et al. Mechanical mitral valve endurance in children under 2 years. Cardiothorac Surg 32, 12 (2024). https://doi.org/10.1186/s43057-024-00131-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-024-00131-3