Abstract
Background
Isolated congenital ostial stenosis of the left coronary artery (LCA) is extremely rare, and available literature is limited. Long-term treatment success is key in the choice of treatment strategy due to the mostly young age of the patients. Here, we present a clinical case and shed light on the surgical treatment strategies including their pitfalls.
Case presentation
We describe a 20-year-old male who presented to the emergency department with recurrent typical angina pectoris symptoms (CCS class II). Computed tomography and coronary angiography revealed isolated ostial stenosis of the LCA, with prominent collaterals from the right coronary artery. The patient was operated on and intra-operative findings showed a severely narrowed LCA ostium that appeared to be fibrotic, and to originate from a similarly fibrotic left coronary sinus of the aortic root. The LCA was excised from the left coronary sinus, and trimmed until the coronary artery lumen appeared macroscopically normal. The fibrotic left coronary sinus was resected and replaced with a bovine pericardial patch, into which the coronary artery was re-inserted. During weaning from cardiopulmonary bypass, we faced a diminished left ventricular function. We attributed this to insufficient myocardial protection. Isolated antegrade cardioplegia had been used, which, for technical reasons, had to be administered separately to the right and left coronary artery after aortotomy. In the setting of huge collaterals from the right to the left coronary artery, a steal-effect likely occurred. After prolonged reperfusion, the left ventricular function recovered, and the further post-operative course was unremarkable.
Conclusions
For the surgical treatment of congenital ostial stenosis of the LCA, both ostium reconstruction and coronary artery bypass grafting have been described. The advantage of ostium reconstruction as chosen here is to create a physiological flow and supply situation to the affected myocardial areas. Furthermore, in case of other downstream events, such as the development of coronary artery disease, all further therapeutic options are preserved.
Special attention should be paid to the administration of cardioplegia in these patients. Combined ante and retrograde cardioplegia administration probably would have achieved more extensive myocardial protection in our case.
Similar content being viewed by others
Background
Determining the true incidence of congenital coronary anomalies including their respective subtypes in the general population is difficult. Findings from autopsy series as well as from coronary angiograms result in an estimate of 0.46–1.55% [1].
Among coronary anomalies, the congenital ostial stenosis of the left coronary artery (LCA) is absolutely rare, especially when it is a stand-alone congenital anomaly that occurs without other congenital heart defects [2]. However, its consequences are potentially fatal ranging from angina, to myocardial damage, and sudden cardiac death. Therefore, prompt treatment is always indicated after diagnosis. Since the patients are usually young, the long-term treatment success is crucial.
Two surgical concepts exist: coronary artery bypass grafting (CABG) or ostial reconstruction [3,4,5]. Due to the rarity of these coronary anomalies, literature, experience, and standardization of the surgical procedures are extremely limited. Hereby, we aim to present a technique of ostial reconstruction including pitfalls to increase the availability of resources for similar cases.
Case presentation
History and diagnostics
We present a 20-year-old male who suffered from angina CCS class II while running, and presented to the emergency department. The patient did not have any significant past medical history, especially no previous relevant diseases or conditions, no previous surgical procedures, and he was not on any regular medication.
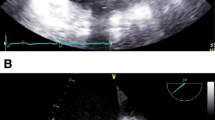
Standard diagnostics were performed, but were inconclusive: the EKG was normal, Troponin I was slightly elevated at 39 pg/ml (reference range 0–26 pg/ml), transthoracic echocardiography was unremarkable. As the patient had been vaccinated against COVID-19 shortly before, he was first suspected of having myocarditis. Magnetic resonance imaging, however, did not show any signs of myocarditis.
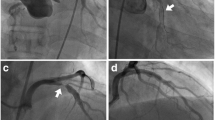
Since the chest pain persisted, a coronary angiography was performed, revealing a filiform 90% stenosis of the left coronary artery ostium, with post-stenotic dilation of the left main, and a huge collateral artery shifting blood from the right coronary artery (RCA) to the left anterior descending (LAD; Fig. 1). The patient was then referred to our center for surgical treatment. A coronary computed tomography angiography was performed confirming the catheterization findings (Fig. 2).
Pre-operative coronary computed tomography angiogram. A Short segment (3 mm) high-grade filiform ostial stenosis of the left coronary artery, with post-stenotic dilation of the left main (yellow arrow). B Dominant right coronary artery with outgoing collateral vessel supplying the left anterior descending (yellow star)
Surgical procedure and intra-operative findings
After median sternotomy and opening the pericardium, the large collateral artery immediately stood out (Fig. 3). Cardiopulmonary bypass was instituted via the ascending aorta and the right atrium. The aorta was cross-clamped and cardioplegic arrest was achieved using Calafiore’s blood cardioplegia, administered initially via the aortic root, and later on selectively via the coronary ostia. The aorta was opened by transverse aortotomy and then completely detached above the sinutubular junction.
Subsequent inspection of the aortic root showed the following: The aortic valve was tricuspid, and all three leaflets were normal. The left coronary ostium was located in the typical position, but with a maximum diameter of 1 mm. Macroscopically, it appeared to be constricted by fibro-membranous tissue (Fig. 4), including fibrotic transformation of the left coronary aortic sinus. The right coronary artery ostium was unremarkable.
The left coronary artery was cut out from the aortic sinus as a button. The excised left main was then gradually trimmed until a normally sized lumen was reached. Remarkably, the wall of the left main was of normal thickness only on the pulmonary side, the opposite side was rather membranous and thin. Because of the very thin tissue, cardioplegia had to be administered separately to the left and right coronary artery for technical reasons at this stage of the procedure. Next, the fibrotic left coronary aortic sinus was resected and reconstructed using a bovine pericardial patch (Supple Peri-Guard® Patch 6.0 × 8.0 cm; Baxter International Inc., Deerfield, IL, USA), and a neo-ostium was created using a commercially available punch. The left main was then inserted into the created neo-ostium using a running 6–0 polypropylene suture, and the aortotomy was closed.
After de-airing, the cross-clamp was removed. Initially, the left ventricular function was severely impaired, but gradually improved with reperfusion and moderate inotropic support (milrinone 0.17 µg/kg/min, dobutamine 3.2 µg/kg/min). After weaning from cardiopulmonary bypass and decannulation, the chest was closed in a standard fashion, and the patient was transferred to the intensive care unit.
Outcomes
The post-operative course was unremarkable. Immediately post-operatively, Troponin I was 1917 pg/ml, it peaked 12 h post-operatively at 9235 pg/ml, with a sharp decline afterwards. The initially moderately reduced left ventricular ejection fraction improved continuously to about 50% at discharge. On post-operative day 7, a coronary computed tomography angiography was performed and showed the neo-ostium of the left coronary artery widely patent without any residual stenosis (Fig. 5).
The intra-operatively resected tissue had been sent for histological assessment which found a bulgingly constricted ostium, but no atherosclerotic changes or atheromatosis. Altogether, although differential etiologies such as inflammatory processes cannot be fully excluded, congenital ostium stenosis was the most likely diagnosis.
On the ninth post-operative day, the patient was discharged to rehabilitation in good general condition. Lifelong intake of acetylsalicylic a cid (ASA) 100 mg daily was recommended.
Conclusions
Surgically, two concepts exist for the management of congenital LCA ostial stenosis. The first option is coronary artery bypass surgery. With regard to long-term treatment success, the preferred graft is the left internal thoracic artery. In addition to the well-known superior long-term patency rates [3, 5], it also offers excellent growth characteristics in patients who are still very young and small at the time of surgery. As an example, an in-situ internal thoracic artery graft was found to have grown by 125% in length with 112% of body growth (112% body surface area), and to have enlarged by 149% in diameter [3].
The second surgical concept is ostium or coronary artery reconstruction, as demonstrated here. In our opinion, this technique offers several crucial advantages. First, this approach restores antegrade blood flow in all areas of the coronary system, resulting in a physiological flow and supply situation to the affected myocardial areas. Second, all options are preserved for future operations or interventions that may become necessary [4]. We elected to replace the entire fibrosed left coronary aortic sinus and to reimplant the left coronary artery after resection of the ostial stenosis and trimming until a normal lumen was reached. Patch angioplasty of the ostium and left main by incision of the ostium and coronary artery followed by patch implantation is another option, but was not feasible here because of the severely fibrosed tissue and extremely narrow ostium opening. Whereas for aortic sinus replacement with neo-ostium creation and coronary artery reimplantation autologous or bovine pericardium will be most suitable, for patch angioplasty autologous or bovine pericardium, venous, or arterial patch grafts have been described [6]. After ostium or coronary artery reconstruction, different strategies for anticoagulation or platelet inhibition are used. Besides lifelong mono antiplatelet therapy, several authors have described temporary anticoagulation with coumadin or warfarin derivates with a target international normalized ratio of 2.0–3.0 for 3 or 6 months, often combined with a platelet inhibitor. The latter is usually given as a lifelong medication. More recent reports have replaced this strategy with dual antiplatelet therapy using Clopidogrel and ASA for the first 3 or 6 months, followed by lifelong monotherapy using ASA [4, 6]. We advocate for a case-by-case decision, taking into account the extent of the repair, as well as the individual risk for bleeding complications.
Special attention should be paid to the administration of cardioplegia in these patients. Collaterals can alter the flow situation, and in the case described here, selective antegrade cardioplegia administered separately to the coronary arteries presumably did not sufficiently reach and protect all parts of the myocardium. Separate administration to the left and right coronary artery was necessary for technical reasons as the LCA tissue was very fragile after trimming. In retrospect, especially when administering cardioplegia not simultaneously via both coronary ostia, efflux was noticed from the RCA ostium when administering cardioplegia via the LCA ostium, likely indicating a steal-effect. Retrograde cardioplegia via the coronary sinus, or a combination of ante and retrograde cardioplegia might have achieved a safer protection especially of the subendocardial myocardium. The post-operative Troponin dynamics support the hypothesis of suboptimal intra-operative myocardial protection.
In summary, the rarity of coronary anomalies results in a risk of delayed diagnosis with potentially fatal consequences, but also in a lack of resources and no standardization of surgical treatment strategies. Although CABG is an option, we favor reconstruction for ostial LCA stenosis, as this creates a physiological flow and supply situation to the affected myocardium, and all options are preserved for future operations or interventions that may become necessary. Special attention needs to be paid to the administration of cardioplegia in these patients to achieve efficient myocardial protection as collaterals may profoundly alter the supply situation.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ASA:
-
Acetylsalicylic acid
- CABG:
-
Coronary artery bypass grafting
- LAD:
-
Left anterior descending
- LCA:
-
Left coronary artery
- RCA:
-
Right coronary artery
References
Musiani A, Cernigliaro C, Sansa M, Maselli D, De Gasperis C (1997) Left main coronary artery atresia: literature review and therapeutical considerations. Eur J Cardiothorac Surg 11:505–514
Laux D, Bessières B, Houyel L, Bonnière M, Magny JF, Bajolle F, Boudjemline Y, Bonnet D (2013) Early neonatal death and congenital left coronary abnormalities: ostial atresia, stenosis and anomalous aortic origin. Arch Cardiovasc Dis 106:202–208
Kitamura S, Seki T, Kawachi K, Morita R, Kawata T, Mizuguchi K, Kobayashi S, Fukutomi M, Nishii T, Kobayashi H (1988) Excellent patency and growth potential of internal mammary artery grafts in pediatric coronary artery bypass surgery. New evidence for a “live” conduit. Circulation 78:129–139
Maureira P, Vanhuyse F, Lekehal M, Tran N, Carteaux JP, Villemot JP (2010) Left main coronary disease treated by direct surgical angioplasty: long-term results. Ann Thorac Surg 89:1151–1157
Mavroudis C (2017) Coronary artery bypass grafting in infants, children, and young adults for acquired and congenital lesions. Congenit Heart Dis 12:644–646
Raanani E, Kogan A, Shapira Y, Sagie A, Kornowsky R, Vidne BA (2004) Surgical reconstruction of the left main coronary artery: fresh autologous pericardium or saphenous vein patch. Ann Thorac Surg 78:1610–1613
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
JMB: conceptualization, patient history and data acquisition, writing and editing of manuscript. RS: conceptualization, patient history and data acquisition, surgical method, editing of manuscript. AR: conceptualization, patient history acquisition, surgical method, editing of manuscript. JFG: conceptualization, surgical method, editing of manuscript. SPWG: conceptualization, patient history and data acquisition, writing and editing of manuscript, supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Buck, J.M., Schramm, R., Renner, A. et al. Management of a rare isolated left main coronary artery ostial stenosis in a young patient: a case report. Cardiothorac Surg 31, 17 (2023). https://doi.org/10.1186/s43057-023-00108-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43057-023-00108-8