Abstract
Background
Cardiac magnetic resonance (CMR) is a powerful and robust imaging modality with the capability for tissue characterisation by quantifying the intrinsic magnetic properties of tissues. The current international consensus recommendation is to determine the local normal T1 mapping value. Recent advancement suggests pooled data or benchmarking of CMR mapping reference values. We present the adult myocardial native T1 mapping value of a common MR acquisition in healthy Malaysians who are predominantly brown skin Asians.
Methods
This is a prospective, cross-sectional study in a single centre. CMR was performed on a 1.5 T MR using 5(3s)3 MOLLI sequence with motion correction, TE = 1.31 ms, TR = 2.78 ms. The analysis of the mid-septal T1 value was done using third-party software (CVi42) and the OsiriX software by two blinded cardiac radiologists.
Results
A total of 51 eligible adults were recruited of which 1 was excluded due to incidental finding of moderate mitral regurgitation. The mean age was 30 years ± 7.0, the majority (64%) was female, the median heart rate was 72 ± 20 bpm and mean the BSA was 1.6 ± 0.1 m2. The ethnicity distribution is Malay 76%, Indian 12%, Chinese 8% and Sabahan 4%. The normal range for mid-septum myocardial T1 using CVi42 was 1012 ± 33 ms and using OsiriX was 1018 ± 37 ms. There is no correlation observed against gender, ethnicity and heart rate. The moderate correlation observed with age is not gender specified. The interobserver reliability was good (coefficient 0.813).
Conclusions
For T1 mapping to be applied clinically, it is imperative to determine the local normal value as recommended by international consensus. The first-ever database on native T1 mapping for 1.5 T CMR reference value for Malaysians who are predominantly brown skin Asians is provided in this pilot study.
Similar content being viewed by others
Background
Cardiac magnetic resonance (CMR) is a powerful and robust imaging modality with superior capability for tissue characterisation. The T1 mapping, derived from T1 relaxometry (spin–lattice relaxation time) can quantify the intrinsic magnetic properties of human body tissues. It is useful to quantify diffuse myocardial processes such as fibrosis, oedema, storage disorders, or hemochromatosis [1,2,3].
The native (referring to non-contrast) T1 mapping is increased in chronic pathologies in which the myocardium has an expanded interstitial space where free water can accumulate, such as in areas of chronic diffuse or replacement fibrosis, water (oedema) or infiltration by amyloidosis [3, 4]. An increase in T1 mapping value has also been shown to be sensitive in myocarditis [5]. On the other hand, native T1 values are lowered by iron or fat content within the myocardium, which makes it useful to detect myocardial siderosis and Anderson-Fabry disease [6, 7].
In theory, cardiac T1 mapping has great potential as a biomarker [8] in cardiomyopathies against the current standard of endomyocardial biopsy, which carries significant procedural risk. Native T1 mapping is acquired without any contrast administration which is a great advantage. Apart from the normal minimal MRI risk, there is no other added risk to the native T1 mapping scan.
The normal native T1 values of 1.5 Tesla CMR system in adult has been documented at 943 ms [9] to 976 ms [9,10,11,12] and in children between 990–1042 ms [13]. Another study documented a higher adult T1 native value of 1027ms [14]. The previous study has shown that the normal native T1 values are heterogeneous within even healthy subjects [11]. A more recent review paper quoted the value ranging between 960–989 ms for 1.5 T MRI [15]. These data were pooled from various studies, predominantly from Western countries with mainly Caucasian subjects. Previous work has also suggested that partial volume artefact, heart rate compensation [16] and age/gender matching as confounders that affect the native T1 values. While the potential benefit of T1 mapping in cardiac tissue characterisation is both novel and revolutionary, standardisation of the T1 normal value is challenging as it may be affected by gender, technical scan sequences [17] and magnet field strength [18]. This hampers the ability for maximum clinical application across different centres.
Fortunately, there are ongoing, strong concerted initiatives worldwide to find a solution for the standardisation of T1 mapping, such as the use of phantoms [19, 20], three-dimensional heart MR mapping fingerprints [21, 22] and z- score application [23]. Even recently, there has also been a group working on structured clustering [24] in yet another attempt for T1 mapping normal value standardisation. The evolution of open-source standardised tools, such as the total mapping toolbox (TOMATO) [25], may help to investigate sources of T1 mapping variability. There is also a validation study on artificial intelligence and machine learning approach of T1 radiomics analysis [26] to facilitate the application of native T1 mapping in the mainstream clinical setting. Figure 1 illustrates the roadmap of the CMR mapping timeline, from the early work till the current application. All in all, native T1 mapping warrants its rightful place in routine clinical practice as recommended by expert consensus [5]. The challenge in standardisation should not stop its novel potential application. Currently, for the technique to be applied clinically, it is imperative to determine the normal T1 mapping value in local healthy subjects as recommended by international consensus [5].
Though the studies in other continents did not report significant ethnicity variation; these study samples were performed in Europe, presumed predominantly Caucasian. This may have underrepresented the non-Caucasian population. Furthermore, apart from a Singaporean Chinese [27] and a small Japanese [28] cohort study, to our knowledge there is no cardiac T1 map healthy population data on brown skin of the Asians, which is the main population in South East Asia. Table 1 demonstrates a summary of previous studies.
In Malaysia, there are a few emerging centres with similar capabilities to perform T1 mapping. We aim to provide cornerstone data for the reference value of adult myocardial native T1 mapping of a common MR acquisition in Malaysian healthy volunteers. The study also explores if any, the correlation of native T1 value in the healthy population against gender, age, body surface area and heart rate. We hope this pilot study will provide the native T1 normal value for the clinical application of CMR in our centre and the application of our national structured cluster benchmarking.
Methods
This is a prospective, cross-sectional, observational study in a single centre. The study obtained the local ethics (MREC) and national medical research registry (NMRR) approval. Purposive and quota sampling from the hospital’s occupational health and safety department (OSHA) non-communicable disease (NCD) database. Those with normal BMI, normotensive and normal sugar (FBS)/lipid profile (FLP) were contacted via WhatsApp application after agreement with the head of respective departments.
Sample size calculation was done using the population mean formulae [29]. Prior data [10] indicated that the mean of native T1 (ms) was 972 (standard deviation = 43, from the difference of the minimum value of 885 ms and maximum values of 1059 ms of the native T1 range. If the Type I error probability and precision are 0.05 and 0.05.s
From reference study [11]; mean T1 value = 972 ms, \(\frac{\upsigma }{\mathrm{SD}}\) = 43, max 1059, min 885, Δ = 50% of SD = 21.5.
Inclusion criteria |
|---|
1. Adult, 18-year-old and above |
2. No known medical illness and absent of any cardiovascular symptoms (normotensive, normal FLP, normal FBS) |
3. Body mass index (BMI) less than 25; according to the WHO classification |
Exclusion criteria |
|---|
1. Contraindication to MRI (as listed from the departmental MRI checklist) |
2. Pregnancy |
3. Current/active smokers (including vaper) |
4. Images with incidental findings of congenital heart defect and cardiac/pericardial mass |
Data was collected from March 2021 until Dec 2021 and recorded in a data collection sheet. Relevant information was obtained from subject’s history, physical examination, blood profiles and medical records within 1 year of the MRI study. Subjects were physically examined. Weight and height measured. A baseline ECG was performed, and the subjects’ ECG will be performed and interpreted by the cardiologist.
Description of CMR image acquisition
Non-contrast CMR was performed on the 1.5 T Amira MR (Siemens Healthcare, Germany) using a 32‐channel phased‐array cardiac coil and retrospective cardiac gating by ECG. Cine steady‐state free‐precession (SSFP) images were acquired in the long‐axis plane and a stack of 10–13 adjacent short‐axis slices through both ventricles from the cardiac base to the apex, with a slice thickness of 8 mm and gap of 0–2 mm, in end‐expiratory breath‐holding, for assessing biventricular volumes and ejection fractions.
Images using a MOLLI sequence T1 mapping with motion correction were acquired in basal, mid‐ventricular, and apical short-axis planes, in end‐expiratory breath‐holding. The MOLLI images were acquired with a 5(3s)3 sequence, acquiring 8 images in 11 heartbeats (five in subsequent heartbeats, followed by a pause of 3 s, again three in subsequent heartbeats). These 8 images were acquired using end‐diastolic triggering, echo time (TE) 1.31 ms, repetition time (TR) 2.78 ms, inversion time (TI) 190 ms, trigger delay (TD) 194–434 ms, flip angle 35°, bandwidth 108.5 kHz, matrix 160 × 148, number of excitations 1, FOV 320–340 mm, FOV in phase direction 100%, slice thickness 8 mm. This yielded a spatial resolution of 1.9 to 2.1 mm × 2 to 2.3 mm.
Image analysis
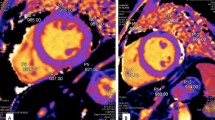
The analysis was performed by two cardiac radiologists using firstly using the CVi42software/ CVI (Circle Cardiovascular Imaging Inc. Calgary, Canada and then the OsiriX software. The reason for using these two software applications is simply due to the fact that all the centres in our country with CMR mapping capability have OsiriX and a couple of centres have Cvi42. The septal T1 value for each slice (basal, mid, and apical) was generated using the T1 maps (using the MOLLI sequences described above) by drawing a free-hand crescent shape ROI [30]. See Fig. 2. Special care was taken to exclude areas of artefacts or where multiple phases did not contain the respective myocardial segment within the prescribed ROI due to cardiac motion between readouts. Examples of artefacts include motion artefacts or inappropriate inclusion of pericardium, lung, or blood in areas of thin myocardium.
Heart rates, FOV, TI and TR were recorded from T1 mapping images. Interobserver variability was assessed from a subset of measurements by the two independent investigators blinded to each other’s results.
Data analysis
The data analyses will be performed using IBM SPSS Statistics for Windows Version 22.0. First, the data will be manually entered into the software. The data cleaning was conducted to detect any errors that could affect the accuracy of the results. After that, the actual analysis was carried out. In this study, descriptive statistics is employed for selected variables. The findings are presented based on the types and distribution of the data. Categorical data (age, gender, ethnicity) will be presented as frequencies and percentages, while numerical data (body surface index) will be presented as means and standard deviations (if normally distributed), or as medians and interquartile ranges (if not normally distributed). To study the correlation between two sets of numerical data, Pearson’s product-moment correlation will be used if homoscedasticity is assumed, while Spearman’s rank-order correlation will be used if heteroscedasticity is assumed. To study the association between categorical data (gender and ethnicity) with numerical data (T1 mapping value), the independent T test for independence will be used, while the Mann–Whitney test will be used if the assumptions for the independent T test for independence are violated. The intraclass correlation coefficient is used to study how closely two readers are agreeing with each other on the T1 mapping value. A value of at least 0.75 generally means good agreement. All probability values are two-sided, and a level of significance of less than 0.5 (p value < 0.05) will be considered statistically significant.
Results
A total of 85 volunteers were screened initially; only 51 eligible, healthy adults were included in the study. Out of these, 1 was excluded due to an incidental finding of asymptomatic moderate mitral regurgitation. About two third of the volunteers are female and the mean age was 30 ± 7.0 years old. The majority of the population is brown-skinned. The overall population demographics, heart rate, scan parameters and cardiac volume measurements are summarised in Table 2.
The normal range for mid-septum myocardial native T1 of the entire cohort for using CVI was 1012 ± 33 ms and using the OsiriX 1018 ± 37 ms. The T1 map values of the rest of the basal and apical septum are shown in Table 3. It is interesting but surprising to observe that the mid-septum native T1 map value has the narrowest standard deviation as described in other studies.
The result shows no statistically significant correlation between the native T1 map value with gender, ethnicity and, heart rate (Table 4). There is, however, a correlation with age and negative but weak correlation with BSA From the whole population scatterplot diagram (Fig. 3), there is a moderate positive correlation between the T1 mapping and age, r = 0.361, N = 50, p = 0.005. Further, in the gender-specific scatterplot diagram (Fig. 3), in males there was a significant strong positive correlation between the T1 mapping and age, r = 0.539, N = 18, p = 0.021, whereas in females there was a weak positive correlation between the T1 mapping and age, r = 0.297, N = 32; however, the relationship was not significant (p = 0.099).
Though there is no statistically significant correlation between the T1 map value with gender, our cohort documented that the mean T1 value in female is higher T1 than in the male group. Additionally, the T1 map value is higher in older age group with exemption for the 41–50 years old which the T1 map value is lower than the prior age group. See Table 5.
Our result shows good agreement with the measurement done using CVI software. There is a moderate correlation using the OsiriX software. See Table 6.
Discussion
We provide the first-ever reference values for a native T1 map of a common mapping sequence in 1.5 T magnetic field strength in healthy human myocardium of Southeast Asian population with predominantly brown skin. Despite the apparent small sample size of this study, from our literature review, only 3 out of the 12 published data on normal T1 map values have a sample size more than our study. The rest has either a similar or less sample size. Our value is higher than the values given in a previous review analysis [18] but similar to a more recent study [31].
The important scanning parameters (TR = 2.78 ms, TE = 1.31 ms and FA = 35°) are specified in this study which will facilitate clustered benchmarking by other centres in our country as suggested by Popescu et al. [24].
Native T1 mapping has been shown to be robust with a narrow and stable normal value range [9]. Its application in daily clinical practice has been supported by a study previously which showed significantly elevated native T1 value in myocarditis, acute myocardial infarction, dilated and hypertrophic cardiomyopathy and amyloidosis [32].
Amongst the different MR sequences, the MOLLI scheme acquisition is very flexible and has high precision [17]. The development of the MOLLI 5(3)3 scheme has improved heart rate sensitivity [33]. As most of our country’s cardiac centres are equipped with mapping capability using the MOLLI scheme, it is only reasonable to obtain a national benchmark normal value of this specific mapping scheme. An example of another sequence, ShMOLLI (shortened MOLLI) is also used elsewhere and has been shown to be comparable to MOLLI [34]. We believe that using the native T1 mapping in a clinical context along with clustered benchmarking will reduce the dilemma of mapping standardisation. Soon, radiomics will be relevant, readily available and can be used for clinical application of the native T1 map values. A validation study [26] has already taken place which will inevitably pave its application in real-life clinical settings.
We found that the mid-cavity MOLLI native T1 map value using the readily available OsiriX software is 1018 ms (SD ± 37 ms) which is similar to another study [35]. As most centres with T1 mapping capability in this country use OsiriX, we believe that currently this is the best measurement tool for clinical practice in centres embarking into this technology. In addition to that, our data show moderate agreement between the two software applications. This shows that for clinical applications, either software is acceptable to be used for the mapping analysis. The simple crescent shape ROI advocated by advanced centres is both practical and reproducible [6, 30]. The smaller SD for the mid cavity value in both CVI and OsiriX software supports that the best representative native T1 measurement is at the mid-septum as recommended by international society [36]. This was also advocated by the same group from their study of more than 700 subjects with non-ischaemic cardiomyopathy which conclude that the mid-native T1 map can inform the presence of abnormal myocardium [30]. We advocate that this be routinely measured and reported in all CMR performed.
Nonetheless, the interobserver result from this study shows that dedicated cardiac software will improve readers’ variability. Thus, it would be efficient to equip cardiac imaging centres with dedicated cardiac software.
The correlation between native T1 value and age is still a debate. Some studies showed no correlation [35], some increasing with age [37] while others decreasing with age only in females [9]. Our result shows a positive moderate correlation between the native T1 map with age similar to other studies [27]. There is little definitive and conclusive evidence, and some studies put forward contradicting hypothesis. Such as an increase in myocyte size and volume fraction which will result in a decrease in the relative amount of interstitium. Though there is a moderate correlation between the mapping value with age for our population sampling, there is no significance when gender is specified. This, of course, is affected by the sample size and gender/age distribution. Though in this study, T1 map data of the older (more than 50) population was not sampled; it is important to note that cardiomyopathies (such as Fabry’s or iron overload that employs the T1map values are prevalent in the younger age group. Further, the T1 map value is significantly high in diseased patients. Thus, if the imaging were done in the elderly group, the T1 map value will be significantly high, such that it superseded the expected reference value for that age group.
Our data showed no significant statistical correlation with gender, contradicting most literature such as a neighbouring country study [27] and larger cohort elsewhere [31, 37]. The relationship between T1 mapping and gender has been studied and it is known that the T1 mapping is higher in female as compared to male which is consistent in our study. These studies showed the native T1 values to be higher in women. There is one study that showed no gender correlation with the native T1 value [10]. It is possible that gender correlation with native T1 values may not be a profound feature in healthy ageing as hypothesised by Dabir et al. However, the numbers of our female subjects were more than male which may have affected the analysis. Nonetheless, interesting to note that the mean T1map value in our study is documented higher in females in our study.
Limitations of the study
This was a single-centre study, the sample size is small, and the unequal gender distribution inevitably affects the correlation test. Furthermore, this sample’s age group is skewed to the younger age group in view of the strict selection criteria of healthy individuals, hence excluding most of the older population in Malaysia which mostly has some form of non-communicable diseases, i.e. diabetes, hypertension, dyslipidaemia or high BMI. In future work, values from different groups with non-communicable diseases will be important.
Conclusions
A Malaysian reference for the native T1 value of the common CMR sequence has been established in this pilot study. This will be a reference value for clustered benchmarking by other centres in the country which will facilitate a practical, readily available solution for the current dilemma of T1 map normal value standardisation. In turn, the usage of the novel native T1 map could be deployed to pave its way as a biomarker for diffuse cardiomyopathies.
Availability of data and materials
All the data and material for the study are kept with confidentiality. The data sets used and/or analysed during the current study are available from the corresponding author upon reasonable timing request.
Abbreviations
- CMR:
-
Cardiac magnetic resonance
- MRI:
-
Magnetic resonance imaging
- NMRR:
-
National Medical Research Register
- MREC:
-
Medical Research and Ethics Committee
- SSFP:
-
Steady‐state free‐precession
- MOLLI:
-
Modified Look-Locker inversion recovery
- NCD:
-
Non-communicable disease
- FLP:
-
Fasting lipid profile
- FBS:
-
Fasting blood sugar
References
Ferreira VM, Piechnik SK (2020) CMR parametric mapping as a tool for myocardial tissue characterization. Korean Circ J 50(8):658–676. https://doi.org/10.4070/KCJ.2020.0157
Pankaj G, Sven P (2017) Native T1 mapping. Circ Cardiovasc Imaging 10(8):e006767. https://doi.org/10.1161/CIRCIMAGING.117.006767
Kim PK, Hong YJ, Im DJ et al (2017) Myocardial T1 and T2 mapping: techniques and clinical applications. Korean J Radiol 18(1):113–131. https://doi.org/10.3348/kjr.2017.18.1.113
Aherne E, Chow K, Carr J (2020) Cardiac T1 mapping: techniques and applications. J Magn Reson Imaging 51(5):1336–1356. https://doi.org/10.1002/jmri.26866
Messroghli DR, Moon JC, Ferreira VM et al (2017) Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2 and extracellular volume: a consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging. J Cardiovasc Magn Reson 19(1):1–24. https://doi.org/10.1186/s12968-017-0389-8
Rommel K-P, Lücke C, Lurz P (2017) Diagnostic and prognostic value of CMR T 1-mapping in patients with heart failure and preserved ejection fraction. Rev Española Cardiol (Engl Ed) 70(10):848–855. https://doi.org/10.1016/j.rec.2017.02.018
Moustafa A, Khan MS, Alsamman MA, Jamal F, Atalay MK (2020) Prognostic significance of T1 mapping parameters in heart failure with preserved ejection fraction: a systematic review. Heart Fail Rev. https://doi.org/10.1007/s10741-020-09958-4
Radenkovic D, Weingärtner S, Ricketts L et al (2017) T1 mapping in cardiac MRI. Heart Fail Rev 22(4):415–430. https://doi.org/10.1007/s10741-017-9627-2
Piechnik SK, Ferreira VM, Lewandowski AJ et al (2013) Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson 15(1):1–11. https://doi.org/10.1186/1532-429X-15-13
Dabir D, Child N, Kalra A et al (2014) Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson. https://doi.org/10.1186/s12968-014-0069-x
Gottbrecht M, Kramer CM, Salerno M (2019) Native T1 and extracellular volume measurements by cardiac MRI in healthy adults: a meta-analysis. Radiology 290(3):317–326. https://doi.org/10.1148/radiol.2018180226
Granitz M, Motloch LJ, Granitz C et al (2019) Comparison of native myocardial T1 and T2 mapping at 1.5T and 3T in healthy volunteers: Reference values and clinical implications. Wien Klin Wochenschr 131(7–8):143–155. https://doi.org/10.1007/s00508-018-1411-3
Burkhardt BEU, Menghini C, Rücker B et al (2020) Normal myocardial native T1 values in children using single-point saturation recovery and modified look–locker inversion recovery (MOLLI). J Magn Reson Imaging 51(3):897–903. https://doi.org/10.1002/jmri.26910
Nickander J, Lundin M, Abdula G et al (2017) Blood correction reduces variability and gender differences in native myocardial T1 values at 1.5 T cardiovascular magnetic resonance—a derivation/validation approach. J Cardiovasc Magn Reson 19(1):1–11. https://doi.org/10.1186/s12968-017-0353-7
Boehm K, Magn JC, Boehm NK et al (2020) Reference ranges (“normal values”) for cardiovascular magnetic resonance (CMR) in adults and children: 2020 update. J Cardiovasc Magn Reson 3:1–63. https://doi.org/10.1186/s12968-020-00683-3
Amano Y, Omori Y, Yanagisawa F et al (2020) Relationship between measurement errors in myocardial t1 mapping and heart rate. Magn Reson Med Sci 19(4):345–350. https://doi.org/10.2463/mrms.mp.2019-0166
Roujol S, Weingärtner S, Foppa M et al (2014) Accuracy, precision, and reproducibility of four T1 mapping sequences: a headto-head comparison of MOLLI, ShMOLLI, SASHA, and SAPPHIRE. Radiology 272(3):683–689. https://doi.org/10.1148/radiol.14140296
Vo HQ, Marwick TH, Negishi K (2020) Pooled summary of native T1 value and extracellular volume with MOLLI variant sequences in normal subjects and patients with cardiovascular disease. Int J Cardiovasc Imaging 36(2):325–336. https://doi.org/10.1007/s10554-019-01717-3
Captur G, Bhandari A, Brühl R et al (2020) T 1 mapping performance and measurement repeatability: results from the multi-national T1 mapping standardization phantom program (T1MES). J Cardiovasc Magn Reson 22(1):1–17. https://doi.org/10.1186/s12968-020-00613-3
Zhang Q, Werys K, Popescu IA et al (2021) Quality assurance of quantitative cardiac T1-mapping in multicenter clinical trials—a T1 phantom program from the hypertrophic cardiomyopathy registry (HCMR) study. Int J Cardiol 330:251–258. https://doi.org/10.1016/j.ijcard.2021.01.026
Jaubert O, Cruz G, Bustin A et al (2020) Free-running cardiac magnetic resonance fingerprinting: joint T1/T2 map and Cine imaging. Magn Reson Imaging 68(January):173–182. https://doi.org/10.1016/j.mri.2020.02.005
Velasco C, Cruz G, Lavin B et al (2022) Simultaneous T1, T2, and T1ρ cardiac magnetic resonance fingerprinting for contrast agent–free myocardial tissue characterization. Magn Reson Med 87(4):1992–2002. https://doi.org/10.1002/mrm.29091
Kranzusch R, Wiesemann S, Zange L et al (2020) Z-score mapping for standardized analysis and reporting of cardiovascular magnetic resonance modified Look-Locker inversion recovery (MOLLI) T1 data: normal behavior and validation in patients with amyloidosis. J Cardiovasc Magn Reson 7:1–10
Popescu IA, Werys K, Zhang Q et al (2021) Standardization of T1-mapping in cardiovascular magnetic resonance using clustered structuring for benchmarking normal ranges. Int J Cardiol 326:220–225. https://doi.org/10.1016/j.ijcard.2020.10.041
Werys K, Dragonu I, Zhang Q et al (2020) Total Mapping Toolbox (TOMATO): an open source library for cardiac magnetic resonance parametric mapping. SoftwareX 11:100369. https://doi.org/10.1016/j.softx.2019.100369
Antonopoulos AS, Boutsikou M, Simantiris S et al (2021) Machine learning of native T1 mapping radiomics for classification of hypertrophic cardiomyopathy phenotypes. Sci Rep 11(1):1–11. https://doi.org/10.1038/s41598-021-02971-z
Bulluck H, Bryant JA, Tan JZ et al (2017) Gender differences in native myocardial T1 in a healthy Chinese volunteer cohort. Cardiovasc Imaging Asia 1(2):110. https://doi.org/10.22468/cvia.2016.00129
Matsumoto S, Okuda S, Yamada Y et al (2019) Myocardial T1 values in healthy volunteers measured with saturation method using adaptive recovery times for T1 mapping (SMART1Map) at 1.5 T and 3 T. Heart Vessels 34(11):1889–1894. https://doi.org/10.1007/s00380-019-01401-5
Ogston SA, Lemeshow S, Hosmer DW et al (1991) Adequacy of sample size in health studies. Biometrics 47(1):347. https://doi.org/10.2307/2532527
Puntmann VO, Carr-White G, Jabbour A et al (2016) T1-Mapping and outcome in nonischemic cardiomyopathy all-cause mortality and heart failure. JACC Cardiovasc Imaging 9(1):40–50. https://doi.org/10.1016/j.jcmg.2015.12.001
Meloni A, Martini N, Positano V et al (2021) Myocardial T1 values at 1.5 T: normal values for general electric scanners and sex-related differences. J Magn Reson Imaging 54(5):1486–1500. https://doi.org/10.1002/jmri.27639
Nadjiri J, Will A (2016) T1-mapping in daily cardiac magnetic resonance imaging practice: combined use of native T1 and extracellular volume quantification. Int J Cardiovasc Res 5(3):1–8. https://doi.org/10.4172/2324-8602.1000264
Reiter G, Reiter C, Kräuter C et al (2018) Cardiac magnetic resonance T1 mapping. Part 1: aspects of acquisition and evaluation. Eur J Radiol 109(July):223–234. https://doi.org/10.1016/j.ejrad.2018.10.011
Pica S, Sado DM, Maestrini V et al (2014) Reproducibility of native myocardial T1 mapping in the assessment of Fabry disease and its role in early detection of cardiac involvement by cardiovascular magnetic resonance. J Cardiovasc Magn Reson 16(iii):99. https://doi.org/10.1186/s12968-014-0099-4
Rosmini S, Bulluck H, Captur G et al (2018) Myocardial native T1 and extracellular volume with healthy ageing and gender. Eur Heart J Cardiovasc Imaging 19(6):615–621. https://doi.org/10.1093/ehjci/jey034
Schulz-Menger J, Bluemke DA, Bremerich J et al (2020) Standardized image interpretation and post-processing in cardiovascular magnetic resonance—2020 update. J Cardiovasc Magn Reson 22(1):1–22. https://doi.org/10.1186/s12968-020-00610-6
Liu C-Y, Liu YC, Wu C et al (2013) Evaluation of age-related interstitial myocardial fibrosis cardiac magnetic resonance contrast-enha. J Am Col Cardiol 23(1):1–7. https://doi.org/10.1016/j.jacc.2013.05.078.Evaluation
White SK, Sado DM, Fontana M et al (2013) T1 mapping for myocardial extracellular volume measurement by CMR: bolus only versus primed infusion technique. JACC Cardiovasc Imaging 6(9):955–962. https://doi.org/10.1016/j.jcmg.2013.01.011
Ferreira VM, Piechnik SK, Dall’Armellina E et al (2014) Native T1-mapping detects the location, extent and patterns of acute myocarditis without the need for gadolinium contrast agents. J Cardiovasc Magn Reson 16(1):1–11. https://doi.org/10.1186/1532-429X-16-36
Sado DM, Maestrini V, Piechnik SK et al (2015) Noncontrast myocardial T1 mapping using cardiovascular magnetic resonance for iron overload. J Magn Reson Imaging 41(6):1505–1511. https://doi.org/10.1002/jmri.24727
Treibel TA, Zemrak F, Sado DM et al (2015) Extracellular volume quantification in isolated hypertension—changes at the detectable limits? J Cardiovasc Magn Reson 17(1):1–11. https://doi.org/10.1186/s12968-015-0176-3
Goebel J, Seifert I, Nensa F et al (2016) Can native T1 mapping differentiate between healthy and diffuse diseased myocardium in clinical routine cardiac MR imaging? PLoS ONE 11(5):1–12. https://doi.org/10.1371/journal.pone.0155591
Luetkens JA, Homsi R, Sprinkart AM et al (2016) Incremental value of quantitative CMR including parametric mapping for the diagnosis of acute myocarditis. Eur Heart J Cardiovasc Imaging 17(2):154–161. https://doi.org/10.1093/ehjci/jev246
Acknowledgements
We acknowledge the Malaysia Ministry of Health Director General, Hospital Sultanah Aminah Director, Dr Hasmein binti Mohamed Razali, Puan Aisyah Ali and Cik Amera Rozali. We also acknowledge the ASCI Cube 2022 Program for the constructive input to the manuscript.
Funding
The study obtained Grant from the National Institute of Health, Malaysia Ministry of Health Grant 2021.
Author information
Authors and Affiliations
Contributions
HA is the guarantor of integrity of the entire study, conceptual, design, funding, supervision, methodology, data collection, handling and analysis, writing of original draft, and review and editing. NO contributed to conceptual, design, supervision, methodology, data collection, review and editing. AAK contributed to funding, data collection, methodology, review and editing. MAS contributed to software, data collection, review and editing. SHT contributed to statistical analysis and software, review and editing. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Obtained from Malaysia Ministry of Health, Medical Research and Ethics Committee (MREC). The approval letter dated 28 May 2021 approval no: NMRR-21-54-57895 (IIR). Informed consent was obtained from all subjects accordingly.
Consent for publication
Consent was also sought for publication from the local institution and the Malaysian National Institute of Health.
Competing interests
All the authors are employees of the Malaysia Ministry of Health. No other relevant conflict of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdullah, H., Abdul Kadir, A., Sha’rani, M.A. et al. Pilot study to establish the myocardium native T1 mapping reference values in 1.5 Tesla cardiac magnetic resonance of healthy Malaysians. Egypt J Radiol Nucl Med 54, 98 (2023). https://doi.org/10.1186/s43055-023-01042-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-01042-9