Abstract
Background
T1 mapping is a robust and highly reproducible application to quantify myocardial relaxation of longitudinal magnetisation. Available T1 mapping methods are presently site and vendor specific, with variable accuracy and precision of T1 values between the systems and sequences. We assessed the transferability of a T1 mapping method and determined the reference values of healthy human myocardium in a multicenter setting.
Methods
Healthy subjects (n = 102; mean age 41 years (range 17–83), male, n = 53 (52%)), with no previous medical history, and normotensive low risk subjects (n=113) referred for clinical cardiovascular magnetic resonance (CMR) were examined. Further inclusion criteria for all were absence of regular medication and subsequently normal findings of routine CMR. All subjects underwent T1 mapping using a uniform imaging set-up (modified Look- Locker inversion recovery, MOLLI, using scheme 3(3)3(3)5)) on 1.5 Tesla (T) and 3 T Philips scanners. Native T1-maps were acquired in a single midventricular short axis slice and repeated 20 minutes following gadobutrol. Reference values were obtained for native T1 and gadolinium-based partition coefficients, λ and extracellular volume fraction (ECV) in a core lab using standardized postprocessing.
Results
In healthy controls, mean native T1 values were 950 ± 21 msec at 1.5 T and 1052 ± 23 at 3 T. λ and ECV values were 0.44 ± 0.06 and 0.25 ± 0.04 at 1.5 T, and 0.44 ± 0.07 and 0.26 ± 0.04 at 3 T, respectively. There were no significant differences between healthy controls and low risk subjects in routine CMR parameters and T1 values. The entire cohort showed no correlation between age, gender and native T1. Cross-center comparisons of mean values showed no significant difference for any of the T1 indices at any field strength. There were considerable regional differences in segmental T1 values. λ and ECV were found to be dose dependent. There was excellent inter- and intraobserver reproducibility for measurement of native septal T1.
Conclusion
We show transferability for a unifying T1 mapping methodology in a multicenter setting. We provide reference ranges for T1 values in healthy human myocardium, which can be applied across participating sites.
Similar content being viewed by others
Background
The increasing contribution of cardiovascular magnetic resonance (CMR) in clinical management is due to its accurate measurements and versatility of the imaging readouts, providing a comprehensive assessment of cardiac function and structure. Insights provided by tissue characterization using late gadolinium enhancement (LGE) imaging, inform on the underlying etiology, relate to prognosis and guide treatment [1]-[5]. LGE relies on visual and semi-quantitative determination of relative myocardial differences commonly presenting with characteristic patterns of LGE distribution [6]. As it requires regional relative differences between normal and abnormal tissue, it is limited in revealing diffuse myocardial disease, where abnormal and normal tissue are intertwined [7]. Recent advances in image acquisition and quantitative postprocessing now allow fast tissue characterisation based on the absolute quantifiable differences in recovery rates of longitudinal magnetisation by T1 mapping [8]. T1 mapping is emerging as a robust and highly reproducible application to provide meaningful measures reflecting biologically important myocardial properties [9]. Currently proposed T1-based indices and potential novel measures include native T1, which reflects myocardial disease involving the myocyte and interstitium without the need for gadolinium based contrast agents (GBCA); and extracellular volume fraction (ECV), a direct GBCA-based measurement of the size of the extracellular space, reflecting only interstitial disease. Emerging evidence shows that myocardial T1-values correlate with the content of underlying myocardial fibrosis, they are abnormal in a variety of heart conditions and also reflect pathological substrates such as oedema or infiltration [9].
Despite a recognized potential for clinical application, there is an identified lack of standardization and transferability of T1 mapping methods beyond a single and commonly expert center. Setups of available T1 mapping methods are presently site and vendor specific, with variable accuracy and precision of T1 values between the systems and sequences [10]. Considerable differences in image acquisition, post-processing approaches and proposed clinical applications further complicate the use of T1 values as standard application in clinical decision-making [11],[12]. Finally, because T1 mapping is presently used in a research (or work-in- progress, WIP) domain and not yet a clinical product for all vendors, the experience is concentrated to a few specialist centers. We established a multicenter consortium of CMR centers that adopted a uniform T1 mapping methodology, which is supported by a single vendor platform. We assessed the transferability of a T1 mapping method in terms of the standardized acquisition and postprocessing approach, and determined biological reference values of human myocardium in a multicenter setting.
Methods
Multicenter consortium
Standardized T1-mapping sequence and imaging protocol, developed and validated at King’s College London (KCL) [13]-[16], were distributed to 4 CMR centers using 1.5 and 3 T Philips scanners (University of Leeds (UL), United Kingdom; German Heart Institute Berlin (DHZB), Germany and St Vincent University (StVU), Sydney, Australia). The participating centers were identified via the worldwide Philips Healthcare clinical science network and hold individual partnership research agreements, allowing for adequate clinical science support and provision of compatible sequences and scanner software packages. Imaging parameters were unified across participating sites. Concordance of local T1-values with KCL values was established in preliminary experiments using standardized phantoms (Eurospin II Test System®) at each location for each field strength, as well as healthy subjects scanned at each location. The respective local ethical review panels approved the study protocols and all subjects provided written informed consent.
Study population
Healthy subjects with no significant medical history, no evidence of cardiovascular disease (normal ECG, normal cardiac dimensions and function by cine CMR, normal tissue characterization) or taking any regular medication, were included (n = 102). An independent group of normotensive subjects referred for clinical CMR with a low pretest probability of cardiomyopathy or cardiac disease, taking no medication, and with subsequently normal findings on routine CMR, were used as a comparative cohort (n = 113) [17]. Characteristics were recorded for all subjects, including age, gender, body mass index, heart rate and blood pressure. Exclusion criteria were the generally accepted contraindications to CMR (implantable devices, cerebral aneurysm clips, cochlear implants, severe claustrophobia) or a history of renal disease with a current eGFR < 60 mL/min/1.73 m2.
CMR protocol
CMR studies were performed with the patient supine using clinical 1.5 T or 3 T scanners (Achieva or Ingenia, Philips Healthcare, Best, The Netherlands) equipped with advanced cardiac package, radiofrequency magnetic field shimming (MultiTransmit) technology and a 32-channel coil. Subjects underwent a standardized imaging protocol for assessment of cardiac function and size, as described previously [14]-[16]. Contrast-enhanced study was performed in a total of 179 subjects (healthy volunteers, n = 66, 65%; low risk subjects, n = 113, 100%). Balanced steady state free precession, single breath-hold modified inversion recovery Look-Locker (MOLLI) was used for T1 mapping and performed in a single mid-ventricular short axis slice, prior to contrast administration and to scar imaging, respectively (TE/TR/flip-angle (FA): 1.64 ns/3.3 ns/50°, voxel size 1.8 × 1.8 × 8 mm, phase encoding steps n = 166, 11 images from three inversions (3 + 3 + 5) with three-heartbeat pauses prior to the second and third inversions and an adiabatic prepulse) [13]-[16]. Intravenous gadobutrol (Bayer Healthcare, Leverkusen, Germany) was administered in doses 0.1, 0.15 or 0.2 mmol/kg per body weight, as per the local clinical protocol.
Image analysis
All routine CMR analysis was performed using commercially available software (ViewForum, Extended Workspace, Philips Healthcare, The Netherlands), as previously described [14]-[16].
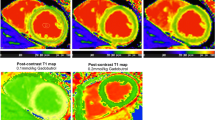
Myocardial T1 relaxation was measured by placing a region of interest (ROI) conservatively within the septal myocardium, as previously described [13]-[16] (Figure 1). T1 values were also measured for the complete mid-ventricular SAX slice as well as for 6 equiangular segments (7–12) [18]. Care was taken to avoid contamination with signal from the blood pool. Following offline image co-registration and motion correction, T1 values were determined by fitting an exponential model to the measured data applying Look-Locker, noise and heart rate correction, as previously described [13]-[16]. In addition to the T1 measurements of native myocardium and blood pool, we calculated the myocardial partition coefficient (λ) and extracellular volume fraction (ECV) according to the formulas [19]:
-
a.
λ = ΔR 1 myocardium /ΔR 1 blood where R 1 = 1/T 1 measured before and twenty minutes after GBCA administration.
-
b.
ECV = λ(1 − hematocrit).
Statistical analysis
Statistical analysis was performed using SPSS software (SPSS Inc., Chicago, IL, USA, version 20.0). Normality of distributions was tested with the Kolmogorov-Smirnov statistic. Categorical data are expressed as percentages, and continuous variables as mean ± SD or median (interquartile range), as appropriate. For comparison of two and more than two normally distributed variables, Student’s t-test and one-way analysis of variance (ANOVA, with Bonferroni’s post-hoc test) as appropriate. Associations were explored by single and multivariate linear regressions. Images considered of sufficient quality and free of artefacts [20] to be deemed diagnostic were used for analysis of septal native T1. Inter- and intraobserver reproducibility and agreement of post-processing approaches was assessed according to the method of Bland and Altman. Because hematocrit has been only available in 34% of all subjects, missing values for calculation of ECV were obtained using multiple imputation method [21]. All values are reported as mean ± SD and a p-value of less than 0.05 was considered statistically significant.
Results
Contribution of cases per recruiting centers was as follows: KCL (n = 146, 68%; 1.5 T: n = 101, 3 T, n = 45), DHZB (n = 46, 22%, all 3 T), UL (n = 14, 7%, all 3 T), StVU (n = 9, 4%, all 1.5 T). Subject characteristics for healthy volunteers and low risk patients are presented in Table 1. There were no differences in mean age or gender representation and routine CMR measures between field strengths (p > 0.05), as well as between the two groups (Figure 2).
Comparison of T1 values per field strength
In healthy controls, mean native T1 values were 950 ± 21 msec at 1.5 T and 1052 ± 23 at 3 T, whereas λ and ECV values were 0.44 ± 0.06 and 0.25 ± 0.04 at 1.5 T, and 0.44 ± 0.07 and 0.26 ± 0.04 at 3 T, respectively (Table 1). There were no significant differences between healthy controls and low risk subjects in routine CMR parameters and T1 values (Figure 2). Mean native T1 was significantly higher at 3 T compared to 1.5 T for both groups. There was a close association between the two partition coefficient variables: 1.5 T: r = 0.92, 3 T: r = 0.94, (p < 0.001 for both).
Comparison of T1 values for post-processing approaches
Age-gender matched samples of subjects from each site (n = 7 per site, a total of 28) were examined for segmental values and average T1 value per short-axis (SAX) slice (Figure 3). As expected, there were considerable regional differences in T1 values in controls with the highest values in inferior septal segments (segment 9, native T1 (msec) – 1.5 T: 995 ± 23; 3 T: 1107 ± 21) and the lowest values in anterolateral segments (segment 12, native T1 (msec) – 1.5 T 902 ± 59; 3 T: 1029 ± 67) at both field strengths (native T1, one-way ANOVA for 6 segments comparisons: 1.5 T: F = 8.0, p < 0.01; 3 T F = 3.1, p < 0.05). Compared to septal native T1 (msec, 1.5 T: 3 T:), native T1 values averaged per SAX slice were significantly lower (native T1 (msec) septal vs. SAX: 1.5 T: 957 ± 22 vs. 941 ± 58; 3 T 1043 ± 23 vs. 1072 ± 63, p < 0.05) [16].
Comparisons of T1 values between the core lab and investigator sites
There was close agreement between T1 values obtained in core-lab and locally for all sites for native septal and SAX T1 measurements (a mixed sample of 1.5 T and 3 T cases from all sites, n = 9, p = 0.57 and p = 0.42; between sites comparisons, repeated measures ANOVA) (Figure 4). Post-contrast values showed lower concordance for both septal and SAX T1 measurements (p = 0.104 and p = 0.08).
Concordance between measurements obtained at core lab and at participating sites for native and postcontrast septal and short axis slice (SAX) measurements in a random sample of 9 cases from all sites (KCL – King’s College London, Leeds – University of Leeds, DHZB – Deutsches Herzzentrum Berlin (German Heart Institute Berlin), Sydney – Vincent University, Sydney Australia.
Comparisons of T1 indices for age, gender, contrast dose and investigator sites
Age groups were determined by separating the entire study population into quartiles (group 1: ≤30 years (1.5 T, n = 27, 3 T = 26), group 2: 31 and 42 years (1.5 T, n = 28, 3 T = 27); group 3: 42 and 53 years (1.5 T, n = 27, 3 T = 24), group 4: ≥ 53 years (1.5 T, n = 28; 3 T = 28) (Figure 5). T1-values were similar across the age groups for any field strength (Table 2). Comparisons of mean values between the participating sites revealed no significant difference for native T1 (F = 1.9, p = 0.86), λ (F = 0.4, p = 0.89) and ECV (F = 0.3, p = 0.91). Mean time from administration to post-contrast T1 mapping acquisition was 18 ± 4.9 min, with no significant departures between the sites (F = 2.9, p = 0.61). A sub-analysis for GBCA dose (Gadovist® 0.1 mmol/kg vs. 0.15 mmol/kg vs. 0.2 mmol/kg) revealed a significant difference for λ and ECV (Table 3) between the protocols.
Distribution of native T1 values for age and gender. Age groups were determined by separating the entire study population into quartiles (group 1: ≤30 years (1.5 T, n = 27, 3 T = 26), group 2: 31 and 42 years (1.5 T, n = 28, 3 T = 27); group 3: 42 and 53 years (1.5 T, n = 27, 3 T = 24), group 4: ≥ 53 years (1.5 T, n = 28; 3 T = 28).
Analysis of relationships
There was a trend of a positive association between native T1 and age at 1.5 T (r = 0.21, p < 0.1 for all), which was stronger in the male cohort (r = 0.23, p = 0.04) (Figure 6). There were no such associations noted on 3 T. Comparisons of native T1, λ and ECV by gender revealed no significant differences at any field strength (p > 0.05 for all). Native T1 was mildly associated with LV-EDV, LV-ESV and LV mass at both field strengths (native T1: 1.5 T: r = 0.21, p = 0.04; r = −0.24, p = 0.02; r = −0.23, p = 0.03; 3 T: r = 0.13, p = 0.14; r = −0.21, p = 0.05, r = −0.19, p = 0.07). Results of inter and intraobserver reproducibility and agreement are provided in Additional file 1 (Figure 7).
Discussion
We demonstrate that myocardial T1 values obtained in an investigator-led, single vendor, multicenter study based on similar imaging infrastructure and unified imaging parameters, can be reliably reproduced at the core lab as well as the participating sites by standardized image acquisition and post-processing approach. We provide reference values for native T1 and hybrid measures for 1.5 and 3 T magnetic field strengths in healthy human myocardium. The present study provides a proof of transferability of a T1 mapping methodology beyond a single (and expert) center and suggests a pathway towards a wider use of T1 mapping.
A substantial body of evidence suggests that T1 mapping is sufficiently accurate, reproducible and robust to enter into clinical translational pathway; T1-values were found abnormal in a variety of cardiomyopathies and permit discrimination between normal and abnormal myocardium. Thus far, a couple of studies reported on the associations with outcomes, albeit short-term, in a selected patient population and single centre set-ups [22],[23]. Difficulties for immediate clinical translation also relate to the ongoing technical evolution of T1 mapping sequences, scarcity of multicenter and prospective controlled studies as well as outcome evidence in subgroups and in large populations [11],[24]. This is the first study to provide reference values for myocardium healthy humans, including native T1 and ECV based on a unified T1 mapping methodology at both field strengths, in a multicenter setting. The average T1 values obtained at 1.5 T field strength are closest to the segmental values obtained by Messroghli et al. [13],[20],[25] using MOLLI in its original form, 3(3)3(3)5, which was also used for the current data acquisition. Advances in MRI scanning equipment with improved coils and shimming, combined with a rigorous postprocessing approach with motion correction, as well as considerably greater sample size, may explain the lower spread of native T1 values observed in the present study. Subsequent generations of MOLLI sequence, which introduced several parameter modifications, most noticeably a lower flip angle of 35° (compared to 50° in the original sequence), showed similar average myocardial native T1 values in healthy volunteers (Messroghli: 939 ± 24 msec; Piechnik, 976 ± 46 msec) [26],[27], and were also implemented across other vendor platforms. A series of publications from a single centre (National Institutes of Health, NIH) revealed either similar values at 1.5 T (Gai et al. 986 ± 168 msec, FA = 50° [28]; Liu et al.: native T1: 977 ± 42 msec, FA 35° [12]) or higher native T1 values (Nacif et al. native T1: 1034 ± 56 msec, FA 35°) [29], with considerably greater scatter. T1 relaxation times increase with higher field strength, which is also reflected in our findings: myocardial native T1 values at 3 T are approximately 100 msec longer in comparison to 1.5 T. This observation is concordant with previous studies, including by Piechnick et al. (average SAX 1169 ± 45 msec) [27], and several publications from the NIH (Kawel et al. 1286 ± 59 msec [30]; Lee et al. (1315 ± 39 msec) [31], and more recently, Von Knobelsdorff-Brenkenhoff et al. (1159 ± ~73 msec) [32]. The magnitude of native T1 values at 3 T observed in the present multicenter study concord with the values in patient cohort comparisons studies we have reported on previously [14]-[16], supporting the unchanging source of results based on a uniform imaging platform. We also provide further insights into the reference ranges for λ and ECV based on a MOLLI sequence at both field strengths based on the bolus technique [19],[22],[30]. Our findings concord with previous reports concerning the average and spread of ECV values in healthy subjects (at 1.5 T: Liu et al. 0.27 ± 0.03 (calculated as an average of values for men: 0.26 ± 0.03 and women: 0.28 ± 0.03) [12], Wong et al. ~0.24 ± 0.02 [22]; at 3 T: Kawel et al. ~0.29 ± 0.03 (estimated from the Figure 2 at ~20 min time-point) [30], Lee et al. 0.27 ± 0.10 [31]), and the independency of the calculated parameters from the field strengths.
Sequence parameters, vendors’ specific set-up and standardized acquisition in part explain the observed diversity in T1 values in the above studies. A standardized approach to postprocessing is an additional important consideration for reproducibility and spread of T1 measurements, allowing robust discrimination between health and disease. We and others have previously shown that there are significant regional variations of native T1-values in SAX slices of normal subjects [16],[27],[33]-[35], with differences between the septal and lateral segments ranging between 60 to 150 msec. We have previously shown that native T1 measurement in the septum provide the most robust post-processing approach with an excellent intra-observer and inter-observer reproducibility irrespective of the field strength [14]-[16]. We now show that this post-processing approach is robust also in a multicenter setting, providing meaningful values on the expected reference ranges in healthy myocardium. Regional variation in segmental values may also account for a higher spread of values in some of the above studies, because T1 measurements in average SAX approach integrate native T1 values observed in all myocardial segments. It is unlikely that these regional differences represent a true difference in tissue composition.
We found no relationship between T1 values and age. Several previous publications showed decreasing native T1 values [36] or increasing ECV values with age [12],[22]. Whereas decreasing trend with age is difficult to explain, the latter studies used a cross-sectional study design and investigated subclinical or overt disease, respectively. We have shown previously that T1 values increase with age in patients with cardiomyopathies [15]. The discrepancy with previous results in healthy cohorts indicates that characterisation of healthy aging of the myocardium is challenging and complicating simple comparisons of age-related changes in T1 values. Previous studies also showed associations with gender, whereby age-dependency was stronger in men, and older females displaying higher native T1 or hybrid values [12],[36]. We found no gender differences in T1 values and a weak association for hybrid indices with males at 1.5 T; however, we could not reproduce such trend at 3 T. Of note, in a selected subgroup of subjects with very low cardiovascular risk, Liu et al. showed no ECV differences between women and men [12], analogous to our findings. It is possible that age and gender differences in T1 values may relate to subclinical and clinical disease in cross-sectional studies, but may not be a profound feature in healthy aging. Lastly, whereas all sites used a single type of GBCA, gadobutrol, we reveal that non-uniformity of contrast agent doses leads to appreciable differences in hybrid indices at both field strengths. These findings were not expected, as it is generally considered that λ and ECV account for most of these sources of variation, which otherwise complicate cross-sites comparisons [9],[28].
Limitations
A few limitations apply to this study. It is recognized that the MOLLI variant used in our study affords a greater precision in terms of tissue characterisation (i.e. discrimination between health and disease) compared to the measurements derived from other MOLLI scheme variants (which are more accurate in terms of T1 value estimation) [10],[11], therefore, our ECV calculation is likely to be more precise. Ongoing advances and optimization of MOLLI schemes and pulse sequence parameters have shown that estimation of true T1 values can be obtained with greater accuracy [10],[11]. However whilst improved accuracy is attainable, high precision of T1 estimates supporting ability to discriminate between health and disease is fundamental, supporting diagnostic and prognostic role of a biomarker in clinical use. Both, precision and high discriminatory ability have been demonstrated for the approach chosen in the current study [14]-[16]. It may be argued that optimized FA (e.g. 35°) may be superior to our choice of 50° due to a higher SNR and less susceptibility to off-resonance effects [25],[37]. However, a higher FA also leads to stronger magnetisation transfer effects, which differ between normal and abnormal myocardium, adding to the discriminatory ability of the chosen method [38]. Asymptomatic healthy subjects represent an ideal target population for derivation of normal ranges. In selecting healthy volunteers in our study, we strived to also account for exclusion of subclinical disease by a further inclusion criterion of normal findings on routine CMR [17],[39]. These same rigorous inclusion criteria were applied to the subgroup of normotensive low risk patients, primarily intended to compensate for the lower number of contrast studies in healthy volunteers. Because groups were similar for clinical characteristics and routine CMR findings (and subsequently T1 values), we believe that bias introduced by their inclusion in age-gender related comparisons is negligible. Hematocrit, which is used in ECV calculation has only been available in a minority of subjects and has not been sampled at the time of CMR studies [9]. Thus, normal ranges for hybrid measures may bear inaccuracies. In the present study we controlled for, reported on or assessed many of the influences, which complicate T1 values comparisons, including CMR platform, technique, pulse sequence parameter selections, field strength, type of GBCA and post-processing of T1 measurement. While a single vendor platform might potentially be perceived as a limitation to a wider transferability of our results, the multicenter nature of our data overcomes several limitations by delivering evidence, which is fundamental and immediately useful to the centers using same hardware set-up. This adds to the future potential of providing T1 mapping as a commercial clinical application as the evidence continues to emerge. Evidence for robustness of clinical applications also enhances the chances for multivendor agreements, providing the clarity on a common minimum standard for clinical application of a T1 mapping sequence.
Conclusions
In this study we provide a proof of transferability for a T1 mapping methodology beyond a single (expert) center, suggesting a pathway for an extended utility of T1 mapping. Based on a unifying and standardized imaging and postprocessing approach we provide reference T1 values in healthy subjects at both field strengths.
Additional file
Abbreviations
- BSA:
-
Body surface area
- CMR:
-
Cardiovascular magnetic resonance
- HR:
-
Heart rate
- LGE:
-
Late gadolinium enhancement
- LV:
-
Left ventricle/left ventricular
- LVEDV:
-
Left ventricular end diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- MOLLI:
-
MOdified look locker inversion recovery
- RVEF:
-
Right ventricular ejection fraction
- ROI:
-
Region of interest
References
Kwon DH, Halley CM, Carrigan TP, Zysek V, Popovic ZB, Setser R, Schoenhagen P, Starling RC, Flamm SD, Desai MY: Extent of left ventricular scar predicts outcomes in ischemic cardiomyopathy patients with significantly reduced systolic function: a delayed hyperenhancement cardiac magnetic resonance study. JACC Cardiovasc Imaging. 2009, 2 (1): 34-44. 10.1016/j.jcmg.2008.09.010.
Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ, Gudnason V, Harris TB, Arai AE: Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012, 308 (9): 890-896. 10.1001/2012.jama.11089.
Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG, Jerosch-Herold M, Ghoshhajra BB, Kwong RY: CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013, 6 (9): 944-954. 10.1016/j.jcmg.2013.05.013.
Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR, Di Pietro E, Roughton M, Wage R, Daryani Y, O’Hanlon R, Sheppard MN, Alpendurada F, Lyon AR, Cook SA, Cowie MR, Assomull RG, Pennell DJ, Prasad SK: Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013, 309 (9): 896-908. 10.1001/jama.2013.1363.
Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ: Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011, 15 (57(7)): 821-828. 10.1016/j.jacc.2010.06.062.
Schulz-Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff-Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E: Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013, 15: 35-10.1186/1532-429X-15-35.
Mewton N, Liu CY, Croisille P, Bluemke D, Lima JA: Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 2011, 57 (8): 891-903. 10.1016/j.jacc.2010.11.013.
Nagel E, Narula J: Evolution and revolution in CMR imaging. JACC Cardiovasc Imaging. 2013, 6 (7): 837-838. 10.1016/j.jcmg.2013.05.007.
Moon JC, Messroghli DR, Kellman P, Piechnik SK, Robson MD, Ugander M, Gatehouse PD, Arai AE, Friedrich MG, Neubauer S, Schulz-Menger J, Schelbert EB: Myocardial T1 mapping and extracellular volume quantification: a Society for Cardiovascular Magnetic Resonance (SCMR) and CMR Working Group of the European Society of Cardiology consensus statement. J Cardiovasc Magn Reson. 2013, 15: 92-10.1186/1532-429X-15-92.
Kellman P, Hansen MS: T1-mapping in the heart: accuracy and precision. J Cardiovasc Magn Reson. 2014, 16 (1): 2-10.1186/1532-429X-16-2.
Higgins DM, Moon JC: Review of T1 mapping methods: comparative effectiveness including reproducibility issues. Curr Cardiovasc Imaging Rep. 2014, 7: 9252-10.1007/s12410-013-9252-y.
Liu CY, Liu YC, Wu C, Armstrong A, Volpe GJ, van der Geest RJ, Liu Y, Hundley WG, Gomes AS, Liu S, Nacif M, Bluemke DA, Lima JA: Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol. 2013, 62 (14): 1280-1287. 10.1016/j.jacc.2013.05.078.
Messroghli DR, Radjenovic A, Kozerke S, Higgins DM, Sivananthan MU, Ridgway JP: Modified Look-Locker inversion recovery (MOLLI) for high-resolution T1 mapping of the heart. Magn Reson Med. 2004, 52: 141-146. 10.1002/mrm.20110.
Puntmann VO, D’Cruz D, Smith Z, Pastor A, Choong P, Voigt T, Carr-White G, Sangle S, Schaeffter T, Nagel E: Native myocardial T1 mapping by cardiovascular magnetic resonance imaging in subclinical cardiomyopathy in patients with systemic lupus erythematosus. Circ Cardiovasc Imaging. 2013, 6: 295-301. 10.1161/CIRCIMAGING.112.000151.
Puntmann VO, Voigt T, Chen Z, Mayr M, Karim R, Rhode K, Pastor A, Carr-White G, Razavi R, Schaeffter T, Nagel E: Native T1 mapping in differentiation of normal myocardium from diffuse disease in hypertrophic and dilated cardiomyopathy. JACC Cardiovasc Imaging. 2013, 6: 475-484. 10.1016/j.jcmg.2012.08.019.
Rogers T, Dabir D, Mahmoud I, Voigt T, Schaeffter T, Nagel E, Puntmann VO: Standardization of T1 measurements with MOLLI in differentiation between health and disease – the ConSept study. J Cardiovasc Magn Reson. 2013, 15: 78-10.1186/1532-429X-15-78.
Natori S, Lai S, Finn JP, Gomes AS, Hundley WG, Jerosch-Herold M, Pearson G, Sinha S, Arai A, Lima JA, Bluemke DA: Cardiovascular function in multi-ethnic study of atherosclerosis: normal values by age, sex, and ethnicity. AJR Am J Roentgenol. 2006, 186: S357-S365. 10.2214/AJR.04.1868.
Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, Douglas PS, Faxon DP, Gillam LD, Kimball TR, Kussmaul WG, Pearlman AS, Philbrick JT, Rakowski H, Thys DM, Antman EM, Smith SC, Alpert JS, Gregoratos G, Anderson JL, Hiratzka LF, Faxon DP, Hunt SA, Fuster V, Jacobs AK, Gibbons RJ, Russell RO: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee Update the 1997 Guidelines on the Clinical Application of Echocardiography). Circulation. 2003, 108: 1146-1162. 10.1161/01.CIR.0000073597.57414.A9.
Schelbert EB, Testa SM, Meier CG, Ceyrolles WJ, Levenson JE, Blair AJ, Kellman P, Jones BL, Ludwig DR, Schwartzman D, Shroff SG, Wong TC: Myocardial extravascular extracellular volume fraction measurement by gadolinium cardiovascular magnetic resonance in humans: slow infusion versus bolus. J Cardiovasc Magn Reson. 2011, 13: 16-10.1186/1532-429X-13-16.
Messroghli DR, Walters K, Plein S, Sparrow P, Friedrich MG, Ridgway JP, Sivananthan MU: Myocardial T1 mapping: application to patients with acute and chronic myocardial infarction. Magn Reson Med. 2007, 58 (1): 34-40. 10.1002/mrm.21272.
Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR: Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009, 338: b2393-10.1136/bmj.b2393.
Wong TC, Piehler KM, Kang IA, Kadakkal A, Kellman P, Schwartzman DS, Mulukutla SR, Simon MA, Shroff SG, Kuller LH, Schelbert EB: Myocardial extracellular volume fraction quantified by cardiovascular magnetic resonance is increased in diabetes and associated with mortality and incident heart failure admission. Eur Heart J. 2014, 35 (10): 657-664. 10.1093/eurheartj/eht193.
Mascherbauer J1, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, Panzenböck A, Jakowitsch J, Bangert C, Laimer D, Schreiber C, Karakus G, Hülsmann M, Pacher R, Lang IM, Maurer G, Bonderman D: Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circ Cardiovasc Imaging. 2013, 6 (6): 1056-1065. 10.1161/CIRCIMAGING.113.000633.
Mahmoud IZ, Cummins C, Hussain Z, Rogers T, Dabir D, Voigt T, Higgins DM, Nagel E, Schaeffter T, Puntmann VO: T1 values in discrimination between health and disease using different T1 sequences: comparison between 3L3L5-MOLLI, 3 L5-MOLLI, shMOLLI and SASHA. J Cardiovasc Magn Reson. 2014, 15 (S1): 18-
Messroghli DR, Plein S, Higgins DM, Walters K, Jones TR, Ridgway JP, Sivananthan MU: Human myocardium: single-breath-hold MR T1 mapping with high spatial resolution-reproducibility study. Radiology. 2006, 238 (3): 1004-1012. 10.1148/radiol.2382041903.
Messroghli DR, Greiser A, Fröhlich M, Dietz R, Schulz-Menger J: Optimization and validation of a fully-integrated pulse sequence for modified look-locker inversion-recovery (MOLLI) T1 mapping of the heart. J Magn Reson Imaging. 2007, 26 (4): 1081-1086. 10.1002/jmri.21119.
Piechnik SK, Ferreira VM, Dall’Armellina E, Cochlin LE, Greiser A, Neubauer S, Robson MD: Shortened Modified Look-Locker Inversion recovery (ShMOLLI) for clinical myocardial T1-mapping at 1.5 and 3 T within a 9 heartbeat breathhold. J Cardiovasc Magn Reson. 2010, 12: 69-10.1186/1532-429X-12-69.
Gai N, Turkbey EB, Nazarian S, Van der Geest RJ, Liu CY, Lima JA, Bluemke DA: T1 mapping of the gadolinium-enhanced myocardium: adjustment for factors affecting interpatient comparison. Magn Reson Med. 2011, 65 (5): 1407-1415. 10.1002/mrm.22716.
Nacif MS, Turkbey EB, Gai N, Nazarian S, Van der Geest RJ, Noureldin RA, Sibley CT, Ugander M, Liu S, Arai AE, Lima JA, Bluemke DA: Myocardial T1 mapping with MRI: comparison of look-locker and MOLLI sequences. J Magn Reson Imaging. 2011, 34 (6): 1367-1373. 10.1002/jmri.22753.
Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA: T1 mapping of the myocardium: intra-individual assessment of post-contrast T1 time evolution and extracellular volume fraction at 3 T for Gd-DTPA and Gd-BOPTA. J Cardiovasc Magn Reson. 2012, 14: 26-10.1186/1532-429X-14-26.
Lee JJ, Liu S, Nacif MS, Ugander M, Han J, Kawel N, Sibley CT, Kellman P, Arai AE, Bluemke DA: Myocardial T1 and extracellular volume fraction mapping at 3 tesla. J Cardiovasc Magn Reson. 2011, 13: 75-10.1186/1532-429X-13-75.
Von Knobelsdorff-Brenkenhoff F, Prothmann M, Dieringer MA, Wassmuth R, Greiser A, Schwenke C, Niendorf T, Schulz-Menger J: Myocardial T1 and T2 mapping at 3 T: reference values, influencing factors and implications. J Cardiovasc Magn Reson. 2013, 15 (1): 53-10.1186/1532-429X-15-53.
Kawel N, Nacif M, Zavodni A, Jones J, Liu S, Sibley CT, Bluemke DA: T1 mapping of the myocardium: intra-individual assessment of the effect of field strength, cardiac cycle and variation by myocardial region. J Cardiovasc Magn Reson. 2012, 14: 27-10.1186/1532-429X-14-27.
Rogers T, Puntmann VO. T1 mapping - beware regional variations.Eur Heart J Cardiovasc Imaging. 2014.,
Puntmann VO, Ucar EA, Baydes RH, Ngah NB, Kuo YS, Dabir D, Macmillan A, Cummins C, Higgins DM, Gaddum N, Chowienczyk P, Plein S, Carr-White G, Nagel E. Aortic stiffness and interstitial myocardial fibrosis by native T1 are independently associated with left ventricular remodeling in patients with dilated cardiomyopathy.Hypertension. 2014 [Epub ahead of print].,
Piechnik SK, Ferreira VM, Lewandowski AJ, Ntusi NA, Banerjee R, Holloway C, Hofman MB, Sado DM, Maestrini V, White SK, Lazdam M, Karamitsos T, Moon JC, Neubauer S, Leeson P, Robson MD: Normal variation of magnetic resonance T1 relaxation times in the human population at 1.5 T using ShMOLLI. J Cardiovasc Magn Reson. 2013, 15: 13-10.1186/1532-429X-15-13.
Kellman P, Herzka DA, Arai AE, Hansen MS: Influence of Off-resonance in myocardial T1-mapping using SSFP based MOLLI method. J Cardiovasc Magn Reson. 2013, 15: 63-10.1186/1532-429X-15-63.
Robson MD, Piechnik SK, Tunnicliffe EM, Neubauer S: T1 measurements in the human myocardium: the effects of magnetization transfer on the SASHA and MOLLI sequences. Magn Reson Med. 2013, 670: 664-70. 10.1002/mrm.24867.
Hinojar R, Botnar R, Kaski JC, Prasad S, Nagel E, Puntmann VO: Individualized cardiovascular risk assessment by cardiovascular magnetic resonance. Future Cardiol. 2014, 10 (2): 273-289. 10.2217/fca.13.102.
Acknowledgements
Department of Health via the National Institute for Health Research (NIHR) comprehensive Biomedical Research Centre award to Guy’s & St Thomas’ NHS Foundation Trust in partnership with King's College London and King’s College Hospital National Health Service Foundation Trust. Local radiographer support at participating sites is thoroughly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
1) Multicenter study design and local funding: VP, EN, SP, AJ, RG; 2) Data acquisition and clinical science support: VP, EN, AD, AK, AJ, RG, AD, SK, AD, CH, DD, NC, TR, DMH, BS; 3) Data analysis: VP, DD, NC, RG, AD, SK, AD, CH, LF; 4) Manuscript: drafting, critical revision and final approval: VP, EN, DD, NC, TR, DMH, AJ, CH, JO, AD, SP, RG, AD, SK, BS, LF. All authors read and approved the final manuscript.
Electronic supplementary material
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Dabir, D., Child, N., Kalra, A. et al. Reference values for healthy human myocardium using a T1 mapping methodology: results from the International T1 Multicenter cardiovascular magnetic resonance study. J Cardiovasc Magn Reson 16, 69 (2014). https://doi.org/10.1186/s12968-014-0069-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12968-014-0069-x