Abstract
Background
Proper assessment of mammographically detected asymmetries is essential to avoid unnecessary biopsies and missed cancers as they may be of a benign or malignant cause. According to ACR BIRADS atlas 2013, mammographically detected asymmetries are classified into asymmetry, focal asymmetry, global asymmetry, and developing asymmetry. We aimed to assess the diagnostic performance of artificial intelligence in mammographically detected asymmetries compared to breast ultrasound as well as combined mammography and ultrasound.
Results
This study was a prospective study that comprised 51 women with breast asymmetry found on screening as well as diagnostic mammography. All participants conducted full-field digital mammography and ultrasound. Then the obtained mammographic images were processed by the artificial intelligence software system. Mammography had a sensitivity of 100%, specificity of 73%, a positive predictive value of 56.52%, a negative predictive value of 100%, and diagnostic accuracy of 80%. The results of Ultrasound revealed a sensitivity of 100.00%, a specificity of 89.47%, a positive predictive value of 76.47%, a negative predictive value of 100.00%, and an accuracy of 92.16%. Combined mammography and breast ultrasound showed a sensitivity of 100.00%, a specificity of 86.84%, a positive predictive value of 72.22%, a negative predictive value of 100.00%, and an accuracy of 90.20%. Artificial intelligence results demonstrated a sensitivity of 84.62%, a specificity of 94.74%, a positive predictive value of 48.26%, a negative predictive value of 94.47%, and an accuracy of 92.16%.
Conclusions
Adding breast ultrasound in the assessment of mammographically detected asymmetries led to better characterization, so it reduced the false-positive results and improved the specificity. Also, Artificial intelligence showed better specificity compared to mammography, breast ultrasound, and combined Mammography and ultrasound, so AI can be used to decrease unnecessary biopsies as it increases confidence in diagnosis, especially in cases with no definite ultrasound suspicious abnormality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Background
Mammography is the primary imaging modality used to diagnose breast cancer. The breasts usually show a symmetrical distribution of fibro glandular tissue and fat. Asymmetry of this pattern is a challenging mammographic finding that may signify a normal or benign finding [1]. However, it can be a sign of breast cancer if asymmetry is novel, palpable, or associated with distortion, mass, or calcifications so it usually needs further assessment [2, 3].
According to ACR BIRADS Mammography, 2013 Asymmetries are classified into asymmetry, focal asymmetry, global asymmetry, and developing asymmetry. Each type has a proper assessment category in the BIRADS lexicon. When associated suspicious clinical, mammographic, or ultrasound findings are present, this leads to upgrades of the BIRADS [4, 5].
Asymmetry is seen only in one of the standard mammographic projections and this finding is not visible on additional views and it usually represents superimposed fibro glandular tissue and summation artifact in 80% of cases and usually contains interspersed fat. It is usually benign [4, 6].
Global asymmetry occupies more than 25% of the breast or more than one quadrant. It is seen in more than mammographic projection [4, 7]. Global asymmetry is usually a sequel of benign causes such as postoperative, normal variations, or hormonal influence. However, it may be due to malignant causes, but as global asymmetry extends over a large region; a malignancy of this size is almost always associated with a palpable mass or other clinical abnormality [7]. Global asymmetries are usually assigned BIRADS category 2 if there are no corresponding palpable abnormalities, masses, architecture distortion, significant calcifications, or reduced breast size [4, 8].
Focal asymmetry is defined as the asymmetry which is visible on more than mammographic projection), occupying less than a quadrant of the breast and lacking a convex outer border of a mass [4, 9]. Focal asymmetry may be due to benign or malignant causes, a focal asymmetry with no abnormality seen in the US, and with no clinical or suspicious finding in mammography is classified as BIRADS 3 category. While if focal asymmetry is associated with suspicious clinical, US, or other mammographic findings, then it will be classified BIRADS 4 category [5].
Developing asymmetry refers to focal asymmetry that is novel, expanding, or becoming more noticeable when compared with a previous examination [9, 10]. Developing asymmetry is classified as BIRADS 4 according to ACR BIRADS atlas 2013[4].
Breast US has shown the capability of differentiation of solid breast lesions from cystic lesions with several advantages including assessment of lesions in real-time, low cost, and lack of ionizing radiation [11].
The term "artificial intelligence" was first created by John McCarthy and his colleagues in 1955. Artificial intelligence is a branch of computer science that implement complex algorithms to solve complex situations and problems with accuracy comparable to human intelligence [12]. It is presumed that the AI system will show increased sensitivity and specificity and thus will help in the earlier diagnosis of breast cancer, decreasing recall rate, and unnecessary biopsies [13].
We aimed to assess the diagnostic performance of AI in Mammographic detected asymmetries compared to breast ultrasound as well as combined mammography and ultrasound.
Methods
The study was a prospective study performed from January 2021 to May 2021, this study comprised 51 women who had mammograms for diagnostic or screening purposes. Their mammograms revealed unilateral breast asymmetry in all of them. The ages of the patients ranged from 31 to 61 years. Their mean age was 43.96 ± 7.9 years old (mean ± SD).
All patients performed full-field digital mammography, and breast ultrasound. Then their mammographic images were exported to an AI software system and processed.
The final diagnosis was according to the histopathological analysis of biopsy /postoperative surgical specimen (for 24 patients), fine-needle aspiration cytology (one patient), or close follow-up (for 26 patients every six months for one year). The ethical committee approved this study, and each patient gave informed written consent.
Subjects
Our study included 51 patients, and the sample size was calculated by statistical equation.
Inclusion criteria
-
Patients with mammography detected asymmetries.
Exclusion criteria
-
Patients who had a normal study.
-
Patients with other mammographic findings not associated with breast asymmetry.
-
Pregnant women.
-
Cases who received neoadjuvant chemotherapy.
Equipment
-
Full-field digital mammography was done using FUJI film digital mammography machine.
-
Ultrasound of the breasts was performed using a LOGIQS8-GE ultrasound device by a linear probe with high-frequency (3–12 MHz).
-
AI computer system provided by Lunit INSIGHT MMG ver. 1.10.2(Seoul, South Korea) was used.
Full field digital mammography technique
Four standard mammogram views: craniocaudal, and mediolateral oblique views of each breast were obtained for all patients.
Breast ultrasound technique
-
The patient's arms were relaxed and flexed at the back of the head while radial imaging of the entire breast and axillary tail on both sides was performed. Supine scanning was used for medial lesions, while contralateral oblique scanning was used for lateral lesions, involving the axilla.
Image analysis and interpretation of Mammography and breast ultrasound
-
A radiologist with 10 years of experience in breast imaging interpreted mammogram and ultrasound images.
-
Interpretation of the mammograms was performed using, the standard reporting system in BI-RADS ACR atlas 5th edition 2013 [4]. Each asymmetry was assessed in terms of type and location of asymmetry (asymmetry, developing, focal, global asymmetry) and associated mammographic findings (architectural distortion, calcifications, skin thickening, edema skin retraction, nipple retraction, axillary adenopathy). The mammographic BI-RADS category was given for each breast using the BIRADS Atlas 2013, directed by clinical information and mammographic observations but blind to final pathological diagnosis.
-
Interpretation of breast ultrasound was performed using, the standard reporting system in BI-RADS ACR atlas 5th edition 2013 [4]. Every lesion was assessed regarding mass (margin, shape, echo pattern, orientation, and posterior acoustic features), cyst (simple, complicated), distortion, altered parenchyma calcifications, and associated findings. The ultrasound BIRADS category of each breast was given according to the BIRADS atlas of Ultrasound 2013, guided by the results of clinical data and breast ultrasound findings but blinded to the final pathological diagnosis.
-
And then a combined sono-mammographic BIRADS category was provided for each breast.
Image analysis and interpretation of AI
-
The AI software analyzed and scanned the four standard CC and MLO views of each breast producing location information of detected lesions in the form of heat maps that highlighted the area as well as abnormality scores that indicated the probability of malignancy (PoM) score detected for each lesion, which ranged from 1–100%(100% represents the highest level of suspicion and 1% the lowest level).
-
According to the probability of malignancy score, the AI category was determined for each breast as follows:
-
Normal—Low (< 10%).
-
Benign—10–39%.
-
Probably benign—40–59%.
-
Probably malignant—60–79%.
-
Malignant—80–100%.
Correlation between the sono-mammographic BI-RADS and the AI category for each breast and the histopathological results ± close follow-up were performed.
Statistical analysis
The statistical package SPSS (Statistical Package for the Social Sciences) version 26 (IBM Corp., Armonk, NY, USA) was used to code and entered the data. Quantitative data were summarized using mean, standard deviation, minimum and maximum. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), Positive Likelihood Ratio 3, Negative Likelihood Ratio, and diagnostic accuracy were calculated.
Results
This study was conducted on 51 women with detected breast asymmetry by screening or diagnostic mammography. The ages of the patient ranged from 31 to 61 years, their mean age was 43.96 ± 7.9 years (mean ± SD).
The distribution of symptoms among the studied population is shown in Table 1
In the current study, 1/51(2%) case was assigned an ACR score of A, 22/51(43.1%) cases were assigned an ACR score of B, 26/51(51%) cases were assigned an ACR score of C, and 2/51 (3.9%) cases were assigned an ACR score of D according to the ACR breast density classification.
A correlation was done with the final diagnosis either by histological results of the biopsy and surgical specimen (24 lesions) fine-needle aspiration cytology (one patient), or close follow-up (for 26 patients); 38/51 (74.50%) were benign and 13/51 (25.50%) were malignant. The distribution of various pathological entities is shown in Table 2
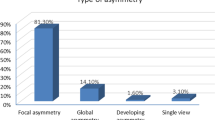
Regarding Mammographic findings in our study, the type of asymmetries was assessed; 43/51 (84.3%) of the asymmetries were focal asymmetry, 1/51 (2.0%) were asymmetry, and 7/51 (13.7%) were global asymmetry. One or more associated mammographic findings were detected as shown in Table 3
Mammographic BIRADS was assigned to each lesion according to mammography morphology descriptors; 28 out of 51 lesions (54.9%) were deemed benign (BIRADS 2 and 3), and 23 out of 51 lesions (45.1%) were deemed malignant (BIRADS 4 and 5). Upon correlating the mammography findings to the final diagnoses, 13 lesions were true positives, 10 lesions were false positives, no lesions were detected as false negatives, and 28 lesions were true negatives. Mammography showed a sensitivity of 100% (95% CI 75.29% to 100.00%), a specificity of 73% (95% CI 56.90% to 86.60%), a positive predictive value of 56.52% (95% CI 43.30% to 68.88%), a negative predictive value of 100%, Positive Likelihood Ratio 3.8 (95% CI 2.23 to 6.47), Negative Likelihood Ratio 0.00, and diagnostic accuracy 80.39% (95% CI 66.88% to 90.18%).
The ultrasound findings in each case were assessed, where one or more findings were detected as shown in Table 4. Ultrasound BIRADS was assigned to each lesion according to ultrasound morphology descriptors; 34/51 (66.7%) lesions were considered benign (BIRADS 1, 2, and 3), while 17/51 (33.3%) lesions were considered malignant (BIRADS 4 and 5). After revising the pathology results, 13 lesions were true positives, 4 lesions were false positives, we had no false-negative results, and 34 lesions were true negatives. So, ultrasound showed a sensitivity of 100.00% (95% CI 75.29% to 100.00%), a specificity of 89.47% (95% CI 75.20% to 97.06%), a positive predictive value of 76.47% (95% CI 56.26% to 89.15%) a negative predictive value of 100.00%, Positive Likelihood Ratio 9.50 (95% CI 3.76 to 24.01), negative Likelihood Ratio 0.00, and accuracy 92.16% (95% CI 81.12% to 97.82%).
Then each lesion was given a Sonomamography BIRADS category based on the combined BIRADS mammography and US morphological characteristics; 33/51 (65%) lesions were considered benign (BIRADS 1, 2, and 3), while 18/51 (35%) lesions were considered malignant (BIRADS 4 and 5). So, the combined sono-mammography showed a sensitivity of 100.00% (95% CI 75.29% to 100.00%,) a specificity of 86.84% (95% CI 71.91% to 95.59%), a positive predictive value of 72.22% (95% CI 53.46 to % 85.46%) to, and a negative predictive value of 100.00%, positive Likelihood ratio7.6 (95% CI 3.36 to 17.20), negative likelihood ratio 0.00, and accuracy 90.20% (95% CI 78.59% to 96.74%).
The artificial intelligence software gave scoring and category to each breast, as shown in Table 5; 38/51 (75%) lesions were considered benign (AI categories: Normal, benign, and probably benign), while 13/51 (25%) lesions were considered malignant (AI categories: malignant and probably malignant). Upon correlation of the AI results to final diagnosis, 11 lesions were true positives, two were false positives, two were false negatives, and 36 were true negatives. So, AI showed a sensitivity of 84.62% (95% CI 54.55% to 98.08%), a specificity of 94.74% (95CI: 82.25% to 99.36%), a positive predictive value of 84.62% (95% CI 58.32% to 95.58%) and a negative predictive value of 94.74% (95% CI 83.39% to 98.47%), Positive Likelihood Ratio16.08 (95% CI 4.09 to 63.19), negative Likelihood Ratio 0.16 (95% CI 0.05 to 0.58) and accuracy 92.16% (95% CI 81.12% to 97.82%).
Discussion
Breast cancer has a wide range of appearances, from spiculated margins to faint asymmetry or mild microcalcification, making an accurate diagnosis and reliable interpretation of mammography challenging [13].
Asymmetries contribute to 6% of non-palpable, screen-detected malignancies, and 27% of missed tumors, and are the second most prevalent source of false-negative screening recall evaluations [14].
Conventional CAD system results depend on feature extraction that should be recognized in the image while AI-based CAD system has the advantage of deep learning which can create known and novel features through its learning process [15].
The application of AI-based CAD to digital mammography has shown improvement in the diagnostic performance of radiologists without increasing the reading time of mammography [16].
Our study included 51 patients, whose ages ranged from 31 to 61 years, with a mean age of 43.96 ± 7.9 (mean ± SD). All had mammography detected asymmetry. They all performed sono-mammography, and their mammographic images were processed and analyzed by an AI system. Upon correlating with the final diagnosis, 38/51 (74.5%) lesions were benign while 13/51 (25.5%) were malignant.
Our results were consistent with those of the prospective study of breast asymmetries conducted by Mokhtar et al. and included 57 patients. They found that most breast asymmetries were secondary to benign lesions (68.4%) [1].
Among the group of malignant cases, our study discovered that invasive ductal carcinoma (IDC) was the most prevalent histopathological type, accounting for 69.2% of the malignant lesions, followed by ductal carcinoma in situ (DCIS), which accounted for 30.8% of the malignant cases.
Final Mammographic BIRADS was assigned according to mammography morphology descriptors and after revising the pathology results, 13 lesions were true positives (TP), 10 lesions were false positive (FP), and no lesions were detected as false negatives (FN), and 28 lesions were true negatives (TN).
The 10 false-positive lesions of mammograms were one fibrocystic change with periductal mastitis (Fig. 1), 8 inflammatory lesions (Fig. 2), and, one apocrine metaplasia. Upon correlation with clinical presentation, out of these 10 false positives lesions, 6 were palpable. Also, false-positive findings in this research were caused by overlapping fibro-glandular tissue and/or associated suspicious findings.
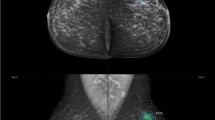
A thirty-eight-year-old lady presented with a right breast lump. Mammography revealed right UOQ focal asymmetry and parenchymal distortion as well as internal amorphous calcifications (a). US revealed right breast UOQ prominent glandular tissue with complicated cyst (b). AI Software revealed no evidence of highlighted areas with PoM scoring less than 10 (low) indicating benignity (a). The final diagnosis was fibrocystic disease with periductal mastitis
A forty-two year-old lady came complaining of a lump sensation in the right breast, Mammography showed right breast UOQ focal asymmetry with mild distortion BIRADS4. a Complementary US revealed right UOQ area of altered parenchyma.AI software revealed right breast upper outer quadrant showed an overlaid heat map, highlighting the area of suspicion with PoM scoring 91% indicating a malignant lesion (b). The final diagnosis was Granulomatous mastitis
Researchers found that false-positive recalls were more prevalent for women who had experienced breast symptoms (pain/tenderness, breast lump, or other breast complaints [17, 18].
So, Mammography in our study showed a sensitivity of 100%, a specificity of 73%, a positive predictive value of 56.52%, a negative predictive value of 100%, and diagnostic accuracy of 80%.
Our results regarding the sensitivity of mammography disagreed with those reported by Mokhtar et al. [1], who reported that the sensitivity of mammography in evaluating asymmetries was about 72.22%. This may be attributed to different population groups and out of these 13 malignant cases, all of them presented by lump, and 11 cases were associated with other suspicious findings.
However, our results showed agreement with that of Mokhtar et al. [1] regarding the specificity of mammography as they concluded a specificity of about 71.8% compared to about 73% in our study.
A final Ultrasound BIRADS category was assigned according to the BIRADS mammography morphology descriptors and after comparing them with the pathology outcomes, we found that 13 lesions were true positives, 4 were false positives, there were no false-negative results, and 34 lesions were true negatives.
The false-positive results were 4 cases with inflammatory lesions. They presented by non-mass lesions (showed absence of typical US findings of inflammation and some of them gave a history of incomplete resolution/nonresolution after proper treatment for 2 weeks); 3 cases presented by altered parenchyma, and one presented by parenchyma distortion.
A non-mass finding was seen in benign entities, but it can also occur in malignant tumors and according to Wang et al., identification of non-mass-like breast lesions in the US showed high sensitivity but low specificity, and a definitive diagnosis required US-guided biopsies [19, 20].
So, ultrasound in our study showed a sensitivity of 100.00%, a specificity of 89.47%, a positive predictive value of 76.47%%, a negative predictive value of 100.00%, and an accuracy of 92.16%.
Our results regarding ultrasound sensitivity and specificity were comparable with those reported by Mokhtar et.al, who found that ultrasound had a sensitivity of 100.00% and a specificity of 92.31% in the evaluation of breast asymmetries [1].
A combined digital mammography and ultrasound BIRADS category was assigned for each lesion according to the BIRADS lexicon, and after revising the pathology results, 13 (100.00%) lesions were true positives, 5 (13.2%) lesions were false positive, there were no false-negative results, and 33 (86.8%) lesions were true negative.
The 5 false-positive lesions were; 4 inflammatory lesions and one case with fibrocystic Changes with periductal mastitis.
Prior prospective clinical studies have demonstrated that adding the US to mammography improves the sensitivity and specificity of breast cancer diagnosis, particularly in women with dense breasts and in younger women [21].
Applying an AI computer system to Mammography increases the sensitivity in the detection of breast cancer. So the AI system can aid in the evaluation of doubtful cases [13].
In the current work, an AI abnormality scoring, and the category was given to each breast; 13/50 (25%) breast lesions were deemed malignant (Fig. 3), and 38/50 (74.5%) breast lesions were deemed benign.
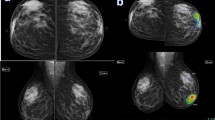
A fifty-five-year-old lady presented with a mass in the right breast. Mammography showed right upper outer quadrant focal asymmetry with a cluster of microcalcifications seen within as shown in a. The US revealed right breast atv0 o'clock altered parenchyma mounting to mass formation. AI software revealed right breast upper outer quadrant showed an overlaid heat map, high lighting the area of suspicion with PoM scoring 99% indicating a malignant lesion as shown in b. The final diagnosis was right breast DCIS
After revising the pathology results, we found 11 lesions were true positives, 2 lesions were false positives, 2 lesions were false-negative results, and 36 lesions were true negatives. The 2 false positives lesions by AI were 2 inflammatory lesions.
The relative decrease in false-positive lesions by AI CAD compared to other modalities is related to the ability of AI-CAD systems to process the images by using segmentations in addition to feature extraction and selection [22, 23].
The 2 false negative lesions by AI were one Invasive Duct Carcinoma and the other one Duct Carcinoma in Situ. The false-negative results of AI-CAD may be because AI focuses on extracting quantitative data related to tumor size, shape, intensity, and texture to display information on pixel distribution within an image and provide a complete results categorization.
Also, a recent study found that an AI system identified less DCIS (83.5%) than a radiologist (89.4) %. [24]. Another recent study reported, that researchers are still unable to determine why the AI system skipped malignancies. However, the AI system appears to have the potential for development [25].
AI in our study showed a sensitivity of 84.62%, a specificity of 94.74%, a positive predictive value of 48.26%, a negative predictive value of 94.47%, and an accuracy of 92.16% (95% CI 81.12% to 97.82%).
In our study, the overall accuracy of AI (92.16%) was greater than that of Mammography alone (80%), and combined mammography and ultrasound (90.20%). The relative higher accuracy of AI in our study compared to Mammography and combined mammography and ultrasound is attributed to a reduction in false positive results by AI.
A multileader study that involved 2652 exams and interpreted by 101 radiologists and AI software showed that the AI diagnostic accuracy was 84% (95% CI 82.0% to 86.0%) and the average of the radiologists was 81.4% (95% CI 78.75% to 84.1%) [26]. Upon correlation with our results, the AI and radiologist accuracy in their study show relative lower accuracy indices compared to our study results and this may be attributed to a different population of the study, however, in both studies, the diagnostic accuracy of the AI was statistically non-inferior to that of the mammography.
Regarding the relationship between AI results and final diagnosis, we found that AI had slightly lower sensitivity but much better specificity than sono-mammography. These findings were like those of Roela et al., who did a similar study. AI, AI plus radiologist, and radiologist alone were found to have a sensitivity of 76.08%. (95% CI 66.44–85.71%); 84.02% (95% CI 82.29–85.74%); 80.91% (95% CI 72.51–89.3%), respectively. Specificity for AI, AI plus radiologist, and radiologist alone, respectively, was 96.62% (95% CI 96.42–96.81%), 85.67% (80.02–91.33%), and 84.89% (89.23–91.17%) [27].
Also, our results were comparable to those of Mansour et al., 2022, who studied the role of AI in 6600 dense mammograms, they found that AI had higher diagnostic accuracy and specificity compared to sonomammography (94.5%versus 91.5%, 96.34% versus 88.04%, respectively), yet their AI results showed lower sensitivity compared to Sonomammography(88.2% versus 98.7%) [28].
The main limitation of our study was that being a single institutional–based study, so multiple institutional-based studies are recommended.
Conclusions
Adding breast ultrasound in the assessment of mammographically detected asymmetries led to better characterization, so it reduced the false-positive results and improved the specificity. Also, Artificial intelligence showed better specificity compared to mammography, breast ultrasound, and combined Mammography and ultrasound, so AI can be used to decrease unnecessary biopsies as it increases confidence in diagnosis, especially in cases with no ultrasound suspicious abnormality.
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AI:
-
Artificial intelligence
- BIRADS:
-
Breast imaging reporting and data system
- CC:
-
Craniocaudal
- DCIS:
-
Ductal carcinoma in situ
- FCCS:
-
Fibrocystic changes.
- FAs:
-
Fibroadenomas
- IDC:
-
Invasive duct carcinoma.
- MLO:
-
Mediolateral oblique
- PoM:
-
Probability of malignancy
- SD:
-
Standard deviation
- US:
-
Ultrasound
References
Mokhtar O, Gamal G, Naguib E et al (2020) 3D digital breast tomosynthesis versus the US in evaluating breast asymmetries. Egypt J Radiol Nucl Med 51(1):1–10. https://doi.org/10.1186/s43055-020-00196-0
AbdulWahid HM, Khalel EA, Alwan NA (2019) Mammographic, ultrasonographic and pathologic correlations of focal asymmetric breast densities among a sample of Iraqi women. J Contemp Med Sci. 5(3):131–135
Kamal RM, Moustafa AFI, Fakhry S et al (2019) Adding the merits of contrast to the ease of mammography; can we highlight what’s behind breast asymmetries? Egypt J Radiol Nucl Med. 50(1):1–9
Sickles EA, D’Orsi CJ, Bassett LW et al (2013) ACR BI-RADS® atlas, breast imaging reporting and data system. American College of Radiology, Reston, VA, pp 39–48
Thomassin-Naggara I, Tardivon A, Chopier J (2014) Standardized diagnosis and reporting of breast cancer. Diagn Interv Imaging 95(7–8):759–766
Barazi H, Gunduru M, Mammography BI-RADS Grading (2021). StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2021, StatPearls Publishing LLC.
Ikeda D, Miyake KK (2016) Breast imaging: the requisites E-book. Elsevier Health Sciences; 2016.
De Lara Bendahan V, Ramos FH, Garcia JO et al (2015) Breast asymmetries in mammography: management. ECR2015. https://doi.org/10.1594/ecr2015/C-1026
Price ER, Joe BN (2015) Sickles EA (2015) The developing asymmetry: revisiting a perceptual and diagnostic challenge. Radiology 274(3):642–651
Chesebro AL, Winkler NS, Birdwell RL et al (2016) Developing asymmetries at mammography: a multimodality approach to assessment and management. Radiographics 36(2):322–334
Shen Y, Shamout FE, Oliver JR et al (2021) Artificial intelligence system reduces false-positive findings in the interpretation of breast ultrasound exams. Nat Commun 12(1):5645. https://doi.org/10.1038/s41467-021-26023-2
Mendelson EB (2019) Artificial intelligence in breast imaging: potentials and limitations. Am J Roentgenol 212(2):293–299
McKinney SM, Sieniek M, Godbole V et al (2020) International evaluation of an AI system for breast cancer screening. Nature 577(7788):89–94
Lehman CD, Wellman RD, Buist DS et al (2015) Diagnostic accuracy of digital screening mammography with and without computer-aided detection. JAMA Intern Med 175(11):1828–1837
Chan HP, Samala RK, Hadjiiski LM (2019) CAD and AI for breast cancer—recent development and challenges. Br J Radiol. 93(1108):20190580
Odríguez-Ruiz A, Krupinski E, Mordang JJ et al (2019) Detection of breast cancer with mammography: effect of an artificial intelligence support system. Radiology. 290(2):305–314. https://doi.org/10.1148/radiol.2018181371
Nelson HD, O’Meara ES, Kerlikowske K et al (2016) Factors associated with rates of false-positive and false-negative results from digital mammography screening: an analysis of registry data. Ann Intern Med 164(4):226–235
Ray S, Chen L, Keller BM et al (2016) Association between breast parenchymal complexity and false-positive recall from digital mammography versus breast tomosynthesis: preliminary investigation in the ACRIN PA 4006 trial. Acad Radiol 23(8):977–986
Choe J, Chikarmane SA, Giess CS (2020) Nonmass findings at breast US: definition, classifications, and differential diagnosis. Radiographics 40(2):326–335
Wang ZL, Li N, Li M et al (2015) Non-mass-like lesions on breast ultrasound: classification and correlation with histology. Radiol Med (Torino) 120(10):905–910
Thigpen D, Kappler A, Brem R (2018) The role of ultrasound in screening dense breasts-a review of the literature and practical solutions for implementation. Diagnost (Basel, Switzerl) 8(1):20. https://doi.org/10.3390/diagnostics8010020
Yassin NI, Omran S, El Houby EM et al (2018) Machine learning techniques for breast cancer computer-aided diagnosis using different image modalities: a systematic review. Comput Methods Programs Biomed 156:25–45
Turgut S, Dağtekin M, Ensari T (2018) Microarray breast cancer data classification using machine learning methods. Paper presented at electric electronics, computer science, biomedical engineerings' meeting (EBBT)2018.
Salim M, Wåhlin E, Dembrower K et al (2020) External evaluation of 3 commercial artificial intelligence algorithms for independent assessment of screening mammograms. JAMA Oncol 6(10):1581–1588
Lång K, Dustler M, Dahlblom V, et, al. (2021) Identifying normal mammograms in a large screening population using artificial intelligence. Eur Radiol 31(3):1687–1692
Rodriguez-Ruiz A, Lång K, Gubern-Merida A et al (2019) Stand-alone artificial intelligence for breast cancer detection in mammography: comparison with 101 radiologists. JNCI 111(9):916–922
Roela RA, Valente GV, Shimizu C et al (2021) Deep learning algorithm performance in mammography screening: a systematic review and meta-analysis. Wolters Kluwer Health 39
Mansour S, Soliman S, Kansakar A, Marey A, Hunold C, Hanafy MM (2022) Strengths and challenges of the artificial intelligence in the assessment of dense breasts. BJR| Open 4:20220018
Acknowledgements
Not applicable.
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
MH and EA wrote the manuscript. AA collected patient data, MH is responsible for correspondence to journal. MH and EA participated in the design of the study and performed the statistical analysis. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was approved by the ethical committee of faculty of medicine, Cairo university, the ethical approval number is not available. An informed written consent was taken from all subjects.
Consent for publication
All patients included in this research gave written consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hanafy, M.M., Ahmed, A.A.H. & Ali, E.A. Mammographically detected asymmetries in the era of artificial intelligence. Egypt J Radiol Nucl Med 54, 47 (2023). https://doi.org/10.1186/s43055-023-00979-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-023-00979-1