Abstract
Background
Coronavirus disease 2019 (COVID-19) was declared a global pandemic by the World Health Organization on March 11, 2020. COVID-19 infection is considered a multi-system disease with neurological, digestive, and cardiovascular symptoms and complications. It can trigger acute and diffuse endothelial dysfunction, resulting in a cytokine storm, most likely induced by the interleukin-6 (IL-6) amplifier. The peripheral and central neurological complications may explain some clinical manifestations such as vagus nerve palsy. The known main CT chest findings of COVID-19 pneumonia include ground glass patches, pulmonary consolidations, inter-lobar septal thickening, crazy paving appearance, and others. We presented our experience in the incidental discovery of phrenic nerve paralysis as atypical chest finding in patients with a known history of COVID-19-associated pneumonia, proved by RT-PCR and coming for evaluation of the lung changes. Patients with evidence of diaphragmatic paralysis underwent close follow-up with a re-evaluation of the phrenic nerve palsy at their routine follow-up for COVID-19 pneumonia. The association of the phrenic nerve palsy was correlated with the CT chest severity score.
Results
Among 1527 scanned patients with known COVID-19 pneumonia, we had recognized 23 patients (1.5%) with unilateral diaphragmatic paralysis, accidentally discovered during CT chest examination. Twenty-one patients had shown complete recovery of the associated diaphragmatic paralysis during their follow-up CT chest with regression or the near-total resolution of the pulmonary changes of COVID-19- pneumonia. No significant correlation between the incidence of unilateral diaphragmatic paralysis and CT severity score with p value = 0.28.
Conclusion
Phrenic paralysis is considered a serious but rare neurological complication of COVID-19 pneumonia. No significant correlation between the CT severity score and the incidental discovery of unilateral diaphragmatic paralysis. The majority of the cases show spontaneous recovery together with the improvement of the pulmonary changes of COVID-19 pneumonia. The association of phrenic paralysis with anosmia and dysgeusia could suggest a direct viral attack on the nerve cells.
Similar content being viewed by others
Background
COVID-19 is an infectious disease of the respiratory system caused by (SARS-CoV-2), which is a strain of coronavirus. COVID-19 usually presents with clinical manifestations related to pneumonia [1]. The clinical presentation of COVID-19 infection varies from asymptomatic cases up to acute respiratory distress syndrome (ARDS) [2]. The spectrum of infection extends to include gastrointestinal and neurological complaints. It has been considered a pandemic crisis by the WHO in March 2020 [3, 4]. The high rate of false-negative results of RT-PCR, particularly early in the course of the disease process, and the inconsistent availability of testing, necessitate a systematic approach to diagnosis, including the use of radiologic imaging [5,6,7]. Imaging plays an important role in the detection and follow-up of cases with COVID-19 infection. CT chest examination is considered the preferred imaging tool for cases with clinical suspicion of COVID-19 as well as evaluation of the degree of pulmonary affection [8, 9]. Phrenic paralysis should be considered in cases of COVID-19 pneumonia when orthopnoea and paradoxical abdominal respiration are present [10]. The virus may attack the olfactory nerve cells resulting in anosmia and dysgeusia. Moreover, SARS-CoV-19 may invade peripheral nerve terminals and such findings could explain the phrenic paralysis in COVID-19 patients, resulting in pulmonary decompensation [11–13].
The present study aimed to focus upon incidental detection of the diaphragmatic paralysis in known COVID-19 patients and correlate the incidence with the CT chest severity score. Also, our study aims to detect the prognosis and recovery of the phrenic paralysis together with the time interval changes of the pulmonary changes of COVID-19 pneumonia.
Methods
Patients
This cross-sectional study was done in the period from the first of May till 30 November 2020 and included 1527 patients with known COVID-19 pneumonia, proved by RT-PCR and referred for CT chest for assessment of the pulmonary changes.
Non-contrast high-resolution chest CT examinations, using (PHILIPS; Ingenuity 8 multislice CT scanner; USA) were done for all patients. All patients were placed in a supine position with both arms elevated. Scan direction was caudocranial in all patients. Scout was taken starting from 1 cm below the lowest costo-phrenic angle to 1 cm above the lung apices.
The CT parameter of the CT chest without contrast as follows: The 1.25 mm thickness, 0.625 mm interval using 512 × 512 matrix with tube speed 35 mm/rotation and 0.5 s rotation time. The KV was 120 and mA ranging from 150 to 400 according to the body weight.
A radiologist with 5 years of experience in CT chest examinations has performed the analysis of CT findings. A CT scoring system was used to quantitatively estimate the pulmonary involvement, depending upon the area of pulmonary affection. To calculate the CT-SS, both lungs were first divided into 5 lobes. Then, each lobe was scored on a scale 0–5 by visual inspection. The score 0 indicated no affection. Score 1 indicated less than 5% affection, score 2 indicated 5–25% affection, score 3 indicated 26–49% affection, score 4 indicated 50–75% affection, and score 5 indicated more than 75% affection. The sum of individual lobar scores was done to calculate the total CT severity score which was ranging from 0 (no affection) to 25 (maximum affection).
All CT images were viewed using a PHILIPS workstation, with multi-planar reformation. Written informed consent was obtained from all patients, and the study protocol was approved by the institutional research ethics committee.
Unilateral diaphragm paralysis is often first suspected during the CT chest examination after the finding of an abnormally elevated hemidiaphragm, which can be defined as a right hemidiaphragm sitting > 2 cm higher than its left counterpart or a left hemidiaphragm sitting equal or higher than the right hemidiaphragm.
The first step to confirm the diagnosis of uni-lateral diaphragmatic paralysis was the exclusion of possible alternative explanations for this finding. Among these, congenital diaphragmatic hernias, atelectasis of various causes, pulmonary and diaphragmatic masses as well as intra-abdominal processes, such as ascites or subphrenic masses were excluded. Another common finding that could suggest uni-lateral diaphragmatic paralysis was atelectasis at the base of the lung on the affected side.
The second step was to evaluate that the diaphragmatic copula was homogenous, regular, and continuous, with no ipsilateral retraction.
The third step was to confirm the diagnosis of uni-lateral diaphragmatic paralysis using Fluoroscopy, using sniff test for 23 cases with suspected uni-lateral diaphragmatic paralysis on CT chest examination.
The sniff test is a quick and easy real-time assessment of diaphragmatic movement. It can be done by asking the patient to practice sniffing before the study with the patient either standing (preferred) or at a supine position, breathing quietly through an open mouth, in front of fluoroscopy. Then, the patient was asked to take few quick short breaths in with a closed mouth (“sniffs”) causing rapid inspiration.
In normal diaphragmatic motion, both hemi-diaphragms move together up and down during expiration and inspiration respectively. In healthy patients, 1–2.5 cm of excursion is normal in quiet breathing.
In-unilateral diaphragmatic paralysis, the affected hemidiaphragm does not move downwards during inspiration and paradoxical movement can occur.
During routine follow-up of the pulmonary changes of COVID-19, complete recovery of the phrenic paralysis was suggested when there was a restoration of the normal position of the affected diaphragmatic copula and the two hemi-diaphragms were almost at the same level.
The Statistical Package for the Social Sciences (version 24; IBM Corp., Armonk, NY, USA) was utilized in the data manipulation and significance testing. The chi-square correlation analysis was conducted, and p values of < 0.1 were used to denote statistical significance.
Results
Our study included 1527 patients [953 males (62.4%) and 574 females (37.6%)] with ages ranging between 22 to 86 years (mean age of 58 ± 5.2 years with positive RT-PCR for COVID-19 pneumonia (Table 1).
The patients with age group between 58 and 86 years (778 patients; 50.9%) were the most affected group. The second affected age group was between 28 and 58 years age group (687 patients; 45%), then the < 28 years age group (62 patients; 4.1%). There was a significant correlation between the disease severity and age group 58–86 years old (p = 0.031).
The CT-SS value was ranging from 1 up to 25, with a mean value of 10.8 and a median value of 12.5. The cut-off value of the CT severity score between the mild and severe cases was 15 with 81.3% sensitivity and 91% specificity. 42.5% of the patients had CT-SS of 11–15. The mild group (CT-SS of 1–15) included 1374 patients, whereas the severe group (CT-SS of 16–25) was composed of 152 patients (Table 2).
Regarding the predominant disease distribution, we found that COVID-19 has typical peripheral and predominant sub-pleural distribution, with lower lobar predilection and bilateral involvement (92.5%). Also, there was a higher CT severity score for the lower lobe affection which was observed in 59.6% of the patients (Table 3).
We had found 23 patients (about 1.5%) presented with incidental unilateral diagrammatic paralysis (14males and 9 females). No cases were found with bilateral diaphragmatic paralysis. 12 patients (52.2%) with unilateral diaphragmatic paralysis had a mild form of the disease process with CT-SS from 1 to 15, while 11 patients (47.8%) had a severe form of the disease process with CT-SS from 16 to 25. No significant correlation between unilateral diaphragmatic paralysis and CT-SS with p value = 0.28.
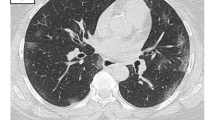
Among the 23 patients with unilateral diaphragmatic paralysis, one patient died from respiratory failure, and representing 0.07% of all cases died with COVID-19 pneumonia. The remaining 22 patients underwent close follow up and the phrenic paralysis was assessed during routine follow-up for pulmonary changes. Twenty-one patients have shown complete recovery of the phrenic paralysis and one patient still has unilateral phrenic paralysis after 2 months from the initial diagnosis (Fig. 1).
Discussion
The clinical presentation and the pulmonary involvement of COVID-19 are heterogeneous, with a wide spectrum from asymptomatic cases up to respiratory failure [14–17].
Ran et al. found that the CT-SS was higher in severe cases when compared with mild cases. Moreover, the CT-SS threshold of 19.5 could identify severe cases with COVID-19 pneumonia, with a sensitivity of 83.3% and a specificity of 94%, resulting in a negative predictive value of 96.3%. They reported that this easy and available method could provide objective means to identify the patients with severe disease, especially in situations of limited availability of health care resources [18, 19].
In addition, Francone et al. have stated that CT-SS of ≥ 18 is highly predictive of poor prognosis with increased risk of mortality in short-term follow-up. Therefore, they reported that the CT severity score of 18 was considered the cutoff value to differentiate between the mild and severe cases [20]. In our study, we found that the cut–off value of CT severity score between the mild and severe cases was 15 with 81.3% sensitivity and 91% specificity.
We have found that COVID-19 has typical peripheral and sub-pleural distributions, with lower lobar predilection and bilateral involvement. Also, there were higher CT severity scores for the lower lobes with an insignificant p value. These findings were similar to the study by Salehi et al. who have reported that CT features of COVID-19 infection had predominant bilateral involvement and peripheral distribution in 87.5% and 76.0% of their patients, respectively [21]. Also, Zhou et al. have reported that 77.4% of the patients had a predominantly peripheral distribution of lesions; and the mean CT-SS for middle and lower zones was significantly higher than that for the upper lung zone, and no significant difference in the mean CT-SS was observed between the middle and lower zones [22].
We have incidentally discovered 23 patients with unilateral phrenic paralysis during the initial evaluation of patients with positive RT-PCR for COVID-19 pneumonia. Twelve patients with phrenic paralysis had a mild form of the disease process with CT-SS from 1 to 15, while 11 patients had a severe form of the disease process with CT-SS from 16 to 25. We had 21 patients showing almost complete recovery of the phrenic paralysis, and one patient still had residual phrenic paralysis despite improvement of the pulmonary changes on CT examination. One patient with unilateral phrenic paralysis died from respiratory failure. We suggest this was caused by direct peripheral neurological involvement of phrenic nerves. Virologists have described neurological lesions causing anosmia and dysgeusia, which were not recognized in patients in Wuhan [23]. Moreover, mono-neuritis multiplex, meningoencephalitis, and myelitis were thought to result from a direct viral attack on the nerve cells and were described as a result of SARS-CoV-19 neurotropism [24]. Increasing evidence also shows that SARS-CoV-19 may invade peripheral nerve terminals. This observation suggests the virus may cause phrenic paralysis in COVID-19 patients, resulting in pulmonary decompensation [25].
Phrenic nerve paralysis could be suggested in patients with orthopnea and paradoxical abdominal respiration. Phrenic paralysis is considered a rare neurological complication of COVID-19 which may aggravate pulmonary decompensation.
The main limitation of this study was the small number of cases incidentally discovered with unilateral phrenic paralysis, although the large sample size of our study. Contribution from multi-center and sharing experience with other countries will be of additive value to better assess such rare but serious complications of COVID-19 pneumonia (Figs. 2, 3, and 4).
Fifty-one-year-old male patient, with a history of COVID-19 pneumonia, referred for initial CT evaluation with CT severity score 8. A, B Axial and coronal CT chest imaging respectively shows bilateral sub-pleural ground-glass patches and pulmonary consolidation with interlobar septal thickening. Incidental discovery of right-sided diaphragmatic paralysis. A notice of large axial hiatus hernia. C, D Images after 4 weeks reveals remarkable regression of bilateral patchy ground glassing and consolidation with resolution of the previous right phrenic paralysis
Seventy-eight-year-old male patient, with a history of COVID-19 pneumonia, referred for initial CT evaluation with CT severity score 7. A, B Axial and coronal CT chest imaging respectively showed right sub-pleural ground-glass patches and pulmonary consolidation with interlobar septal thickening. Incidental discovery of left-sided diaphragmatic paralysis. C, D Images after 4 weeks reveals remarkable regression of the right-sided patchy ground glassing and consolidation with resolution of the previous left phrenic paralysis
Seventy-eight-year-old male patient, with a history of COVID-19 pneumonia, referred for initial CT evaluation with CT severity score 21. A, B Axial and coronal CT chest imaging respectively shows bilateral sub-pleural ground-glass patches and pulmonary consolidation with interlobar septal thickening. Incidental discovery of left-sided diaphragmatic paralysis. The patient died after 2 weeks from respiratory failure
Conclusion
Phrenic paralysis is considered a serious but rare neurological complication of COVID-19 pneumonia. No correlation between the CT severity score and the incidental discovery of unilateral diaphragmatic paralysis. The possibility of neurological disorder caused by SARS-CoV-2 on peripheral nerves, especially the vagus nerve, should be determined by the investigation. Its association with anosmia, dysgeusia, other neurological symptoms, should be considered.
Availability of data and materials
All the datasets used and analyzed in this study are available with the corresponding author on reasonable request.
Abbreviations
- COVID:
-
Coronavirus disease
- MERS-CoV:
-
The Middle East respiratory syndrome coronavirus
- CT:
-
Computed tomography
- CT-SS:
-
Computed tomography severity score
- SARS:
-
Severe acute respiratory syndrome
- RT-PCR:
-
Reverse transcription-polymerase chain reaction
References
Hoffmann M, Kleine-Weber H, Schroeder S et al (2020) SARSCoV-2 Cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271–280.e8
Xu H, Zhong L, Deng J et al (2020) High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci 12(1):8
Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. (JHU). The Johns Hopkins University School of Medicine. https://coronavirus.jhu.edu/map.html. Published 2020. Accessed March 2020.
Ai T, Yang Z, Hou H et al (2020) Correlation of Chest CT and RT-PCR Testing in Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. https://doi.org/10.1148/radiol.2020200642 s
Qin C, Liu F, Yen TC et al (2020) 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging 47(5):1281–1286
Manna S, Wruble J, Maron SZ et al (2020) COVID-19: a multimodality review of radiologic techniques, clinical utility, and imaging features. Radiology 2(3):e200210. https://doi.org/10.1148/ryct.2020200210
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. https://doi.org/10.1016/s0140-6736(20)30211-7
Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X et al (2020) CT imaging features of 2019 novel coronavirus (2019-nCoV). Radiology. 295:202–207
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barre syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19(5):383–384
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol 92:552–555
Maurier F, Godbert B, Perrin J (2020) Respiratory Distress in SARS-CoV-2 without Lung Damage: Phrenic Paralysis Should Be Considered in COVID-19 Infection. Eur J Case Rep Intern Med 7(6):001728
Gandhi RT, Lynch JB, del Rio C (2020) Mild or moderate Covid-19. N Engl J Med:1–9
Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C (2020) Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology 295:715–721
Wen Z, Chi Y, Zhang L et al (2020) Coronavirus Disease 2019: Initial Detection on Chest CT in a Retrospective Multicenter Study of 103 Chinese Subjects. Radiology 2:2
Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T et al (2020) Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 30:3306–3309
Lee EYP, Ng MY, Khong PL (2020) COVID-19 pneumonia: what has CT taught us. Lancet Infect Dis. https://doi.org/10.1016/S14733099(20)30134-1
Koo HJ, Lim S, Choe J, Choi SH, Sung H, Do KH (2018) Radiographic and CT features of viral pneumonia. Radiographics. 38:719–739
Ran Y, Li X, Liu H, Zhen Y et al Chest CT Severity Score: An Imaging Tool for Assessing Severe COVID-19. Radiology 2(2) Published Online. Mar 30 2020. https://doi.org/10.1148/ryct.2020200047
Yang R, Li X, Liu H et al (2020) Chest CT severity score: an imaging tool for assessing severe COVID-19. Radiol Cardiothorac Imaging 2:2
Francone M, Iafrate F, Masci GM et al (2020) Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol 4:1–10
Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A (2020) Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. AJR Am J Roentgenol 215:87z93
Zhou S, Wang Y, Zhu T, Xia L (2020) CT features of coronavirus disease 2019 (COVID-19) pneumonia in 62 patients in Wuhan, China. AJR 214:1–8
Matsuda K, Park CH, Sunden Y, Kimura T, Ochiai K, Kida H et al (2004) The vagus nerve is one route of transneural invasion for intranasally inoculated influenza A virus in mice. Vet Pathol 41(2):101–107
Desforges M, Le Coupanec A, Dubeau P, Bourgouin A, Lajoie L, Dubé M et al (2019) Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 12(1)
Chen T, Wu D, Chen H, Yan W, Yang D, Chen G et al (2020) Clinical characteristics of 113 deceased patients with coronavirus disease 2019: a retrospective study. BMJ. 368:m1091
Acknowledgements
I would like to thank the chest team for much co-operation and clinical background, as well as the clinical pathology team for their effort and participants throughout the laboratory workup of our patients.
Declarations
.
Funding
Not applicable (no funding received for this study).
Author information
Authors and Affiliations
Contributions
E.H.A is the editor of the manuscript, contributed to image interpretation, and performed the statistical analysis.
M.G.A participated in the design of the study, image interpretation, and data collection.
A.S.A put the idea of research and shared in the design of the study, image interpretation, and data collection.
The authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Written informed consent was signed by all patients before the examination. The study was done at a private hospital and was approved by the Research Ethics Committee (REC) of the hospital. The protocol of the study was carefully revised by the regulating staff responsible for medical research in the hospital. All ethical approval and consents for the study were archived in a file and marked by the title of the study. The ethical number was not applicable.
Consent for publication
All patients included in this research were fully conscious and older than 16-year-old and gave written informed consent to publish the data contained within this study.
Competing interests
The authors declare that they have no competing interests
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdeldayem, E.H., Abdelrahman, A.S. & Mansour, M.G. Recognition of phrenic paralysis as atypical presentation during CT chest examination of COVID-19 infection and its correlation with CT severity scoring: a local experience during pandemic era. Egypt J Radiol Nucl Med 52, 156 (2021). https://doi.org/10.1186/s43055-021-00527-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-021-00527-9