Abstract
Suicidal ideation in patients with major depressive disorder (MDD) presents a critical challenge in mental health, with conventional antidepressants often having delayed onset. This systematic review and meta-analysis investigates the efficacy of ketamine in rapidly reducing acute suicidal ideation in this patient group. A comprehensive literature search up to June 2023 across PubMed (Medline), Scopus, Web of Science, and Embase yielded 12 studies, including 7 randomized controlled trials (RCTs). The evaluation of the impact of ketamine on Montgomery-Åsberg Depression Rating Scale-Suicidal Ideation (MADRS-SI) scores was conducted using the standardized mean difference (SMD) through the Cohen’s d method for analysis. The assessment of risk of bias was executed utilizing the Cochrane Risk of Bias Tool (RoB2). Subgroup assessments considered study period, geographic location, and follow-up duration. Ketamine administration showed a significant reduction in MADRS SI scores (mean difference, -1.16; 95% CI, -1.89, -0.23). Subgroup analysis revealed varying efficacy based on the study period, geographical location, and follow-up length. Intravenous ketamine demonstrated the most substantial reduction in suicidal thoughts. High heterogeneity among studies was observed. Ketamine offers a rapid and significant reduction in acute suicidal ideation in patients with MDD. It holds promise as an intervention during high-risk periods where conventional treatments are limited by slower onset. However, variability in study results and concerns over long-term safety necessitate further research to optimize treatment protocols and understand the implications of different administration routes. These findings have important implications for developing clinical guidelines in managing acute suicidal ideation in MDD.
Similar content being viewed by others
Introduction
Suicidal ideation, defined as thoughts of self-harm or intent to take one’s own life, represents a critical and escalating challenge in global health. This complex issue profoundly impacts not only individuals but also their families and communities at large [1]. The situation is particularly alarming among those diagnosed with major depressive disorder (MDD), where up to 15% may eventually succumb to suicide [2]. This stark reality underscores the urgent need for effective interventions that can rapidly reduce acute suicidal ideation in patients with depression.
Despite the gravity of this need, the therapeutic landscape has been limited. Conventional antidepressants, typically prescribed for depressive symptoms, often require weeks or even months to mitigate suicidal thoughts. This delay poses a significant risk, particularly during the initial phases of treatment, leaving a critical care gap for high-risk patients [3].
In this context, ketamine, primarily known as an N-methyl-D-aspartate (NMDA) receptor antagonist, has emerged as a potential lifeline. Historically used for anesthesia, ketamine’s unique pharmacological profile enables rapid antidepressant effects, often within hours, a feat not matched by traditional antidepressants [4]. This rapid action positions ketamine as a promising candidate for quick alleviation of depressive symptoms and, by extension, acute suicidal thoughts [5,6,7]. However, the current literature presents a mixed picture of ketamine’s efficacy in this regard. While some studies report a significant decrease in suicidal ideation, others indicate a more modest or minimal effect [5, 8]. Such variation could stem from differences in study designs, dosage regimens, patient demographics, and methods of measuring suicidal ideation.
To address these inconsistencies and provide clearer clinical guidance, this systematic review and meta-analysis consolidates data from randomized controlled trials (RCTs). Our aim is twofold: to systematically review RCTs assessing the impact of ketamine on acute suicidal ideation, and through meta-analysis, to quantitatively evaluate both the extent and duration of ketamine’s anti-suicidal effects. By focusing specifically on emergency department settings, where rapid intervention is critical, this research could significantly influence the clinical management of patients at acute suicide risk [9]. The insights gained from this study are poised to reshape the understanding and application of ketamine in urgent mental health interventions.
Methods
Search strategy and selection criteria
To assemble a comprehensive collection of studies for our meta-analysis, we conducted a detailed literature search up to June 2023. This search spanned across four major electronic databases: PubMed (Medline), Scopus, Web of Science, and Embase. The search strategy involved the use of specific keywords including “ketamine,” “suicide,” and “suicidal ideation.” To enhance the accuracy and breadth of our search, we utilized medical subject headings (MeSH) and EMTREE for each database.
Beyond the electronic database search, we manually examined the reference lists of all selected studies and relevant review articles. This step was crucial to identify additional studies that might have been missed in the initial database search.
Exclusion criteria
Our exclusion criteria were carefully designed to focus the meta-analysis on the most relevant and high-quality studies. The criteria for excluding studies from the analysis were as follows:
-
1.
Study design: We excluded studies that were not randomized controlled trials (RCTs) or parallel clinical trials. This included cohort studies, case-control studies, case reports, and cross-sectional studies.
-
2.
Language and accessibility: Articles not published in English and those for which full texts were not accessible were excluded.
-
3.
Relevance and focus: Studies that did not specifically investigate the effect of ketamine on suicidal ideation were excluded. This included studies where ketamine was not the main intervention or where suicidal ideation was not a primary outcome.
-
4.
Quality and structure: Studies that did not adhere to the PICOT (population, intervention, comparison, outcome, time) structure were excluded. This framework was critical for ensuring the relevance and clarity of the research objectives.
-
5.
Duplicate data: Any studies that reported duplicate data or were overlapping significantly with other included studies were excluded to avoid redundancy.
-
6.
Outliers in design or outcome: Studies with intervention and comparison groups that were not aligned with our research objective, or those with outcomes that did not focus on our specified effect size index, were excluded.
These criteria ensured a focused and rigorous selection of studies, thereby enhancing the validity and reliability of our meta-analysis findings (Table 1).
Data management and review process
An Endnote (Version 20) library was set up to manage the articles efficiently, which facilitated the elimination of duplicates and the review of titles and abstracts. Two authors (RRD and FGH) conducted initial reviews independently. In instances of disagreement or ambiguity, the issues were resolved through discussion based on predefined criteria or escalated to a third reviewer (YM) for final arbitration.
Data extraction and quality assessment
Data extraction was performed by two independent reviewers, focusing on study characteristics, participant demographics, details of ketamine administration, and outcomes specific to suicidal ideation. Any discrepancies were resolved through discussion or by consulting a third reviewer. We employed the Cochrane risk-of-bias tool (RoB 2) for assessing the quality of RCTs, categorizing studies based on their risk of bias as low, moderate, or high [10].
Statistical analysis
Effect sizes in this meta-analysis were represented by the standardized mean difference (SMD), calculated using a random model due to the expected similarity in study designs and populations. In the analysis, a random-effects model, specifically the restricted maximum likelihood (REML) method, was employed due to the higher observed heterogeneity rate. The heterogeneity and variance across studies were assessed using the Cochrane Q and I2 tests, with heterogeneity levels classified as per Cochrane guidelines. Meta-regression analyses were conducted to explore the relationships between variables like age and the estimated pooled risk ratio. Egger’s test was employed to assess publication bias. All statistical tests were two-tailed and deemed significant at an alpha level of 0.05. All analyses were carried out using STATA software, version 17, to ensure rigorous statistical evaluation and Subgroup analysis.
Results
Study selection
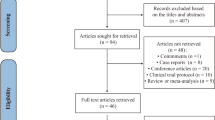
Our systematic search yielded 524 articles. After screening titles and abstracts, 435 were excluded primarily due to irrelevance to the study’s focus on ketamine’s impact on acute suicidal ideation. Of the remaining 89 articles, a detailed full-text review led to the exclusion of 80 more, primarily due to non-alignment with the PICOT structure or differing primary outcomes. This left us with 9 studies for data extraction (Table 2). Figure 1 illustrates this selection process, which culminated in 6 studies being incorporated into the final meta-analysis.
Study characteristics
The 9 trials included in our initial analysis exhibited geographical diversity, with 4 conducted in the US, one jointly in the US and Canada, and 4 in various Asian countries. All studies uniformly administered ketamine at a dosage of 0.5 mg/kg. The participant age range reflecting suicidal ideation varied, with the lowest average age (31.68 years) reported in an Indian study and the highest (47.15 years) in a Taiwanese study. Follow-up periods also differed, ranging from 4 h in Yoav Domany’s U.S.-based study to 30 days in Anna Feeney’s study, also in the US. These variances highlight the diverse contexts in which ketamine’s efficacy was evaluated, potentially influencing the results and their applicability.
Publication bias
For publication bias assessment, we utilized Egger’s test, appropriate for meta-analyses with fewer than 10 studies. The test revealed no significant publication bias (B coefficient, -4.34; standard error, 4.21; P value: .337), suggesting that the likelihood of our results being influenced by unpublished negative studies is low (Fig. 2). This supports the integrity of our findings, although the limited number of studies necessitates cautious interpretation.
Risk of bias
Meta-analysis
Overall effect
The primary aim was to assess the mean difference in MADRS-SI (Montgomery-Åsberg Depression Rating Scale-Suicidal Ideation) scores, an essential tool for evaluating suicidal ideation, between the ketamine intervention (n = 232) and control (n = 175) groups. Our analysis indicated a significant reduction in suicidal thoughts with ketamine use, as reflected by a decrease of 1.06 points in MADRS SI scores (SMD, -1.16; 95% CI, -1.89, -0.23; I^2, 92.49; P for heterogeneity, 0.01) (Table 3). This finding suggests that ketamine administration may have a tangible effect in diminishing suicidal ideation among patients.
Subgroup assessments
In our subgroup analysis, we considered variables such as the timing of the studies (pre and post-2019), geographic locations, and patient follow-up durations. This approach aimed to uncover potential variations in ketamine’s efficacy across different contexts. For studies conducted after 2019, ketamine led to a more pronounced average reduction in MADRS-SI scores of 1.86 points (SMD, -1.86; 95% CI, -3.42, -0.29; I^2, 95.45; P value for heterogeneity: < 0.001) (Table 4). The high heterogeneity in this group may be attributed to evolving methodologies or patient demographics in more recent studies.
Geographically, American and Asian studies showed reductions of 0.83 and 1.29 points, respectively, in MADRS-SI scores. In the American studies, the SMD was -0.83 (95% CI, -1.18 to -0.49; I^2, 29.08; P for heterogeneity, 0.18), while in Asian studies, it was -1.29 (95% CI, -3.25 to -0.97; I^2, 96.41; P for heterogeneity: < 0.001) (Table 5). The variability in these results could reflect differences in patient populations or healthcare practices across regions.
Regarding follow-up duration, we found that shorter follow-up (less than 24 h) resulted in a more substantial decrease in MADRS-SI scores (-2.99 points; 95% CI, -5.38 to -0.60; I^2, 93.11; P for heterogeneity, < 0.001), compared to longer follow-up (more than 24 h), which showed a reduction of 0.55 points (95% CI, -0.88 to -0.22; I^2, 44.54; P for heterogeneity: 0.11) (Table 6). This observation highlights the importance of follow-up duration in assessing the immediate impact of ketamine on suicidal ideation.
Discussion
This study represents a significant advancement in the field, being the first of its kind to comprehensively review and analyze data from 9 studies, including 7 RCTs, with such precision. Our findings demonstrate that ketamine, administered via intravenous, intranasal, and intramuscular routes, is effective in rapidly reducing acute suicidal ideation in patients with major depressive disorder. The substantial standardized mean difference observed across different delivery methods signifies a clinically meaningful effect.
Intravenous ketamine showed the most potent anti-suicidal effect, aligning with pharmacokinetic data that suggest higher bioavailability and quicker onset of antidepressant action with this administration route [20]. Although intranasal ketamine’s effect was smaller, it remains clinically significant, especially considering its non-invasive nature and suitability for a broader range of clinical settings [21]. Notably, patients with higher baseline severity of suicidal ideation exhibited a stronger response to ketamine treatment, indicating its potential as a critical intervention for acutely suicidal patients [22].
These results are particularly relevant considering the gap between the onset of suicidal ideation and the delayed effect of conventional antidepressants. Rapidly reducing suicidal thoughts, ketamine could significantly reduce mortality and morbidity associated with suicide attempts during this high-risk period, potentially revolutionizing the management of acute suicidality in clinical practice.
Nevertheless, the heterogeneity observed among studies underscores the need for further research to refine ketamine treatment protocols. Variations in dosing, adjunct therapies, and outcome measures contribute to this heterogeneity. Future studies should aim for head-to-head comparisons of different administration routes and standardize measures for suicidal ideation to further elucidate optimal treatment strategies. Beyond the factors like age, follow-up duration, and geographical region, other variables such as body mass index, drug dosage, and co-existing medical conditions may play a significant role in the varied responses observed in clinical trials, warranting deeper investigation.
While ketamine shows promise, concerns regarding its long-term safety, particularly the potential for abuse and urotoxicity, remain [23]. Future research must also focus on the sustainability of ketamine’s anti-suicidal effects and the adverse outcomes associated with long-term use. A careful assessment is needed to balance its benefits against potential risks to establish ketamine firmly within depression care pathways.
At last, the study’s findings on ketamine’s rapid reduction of suicidal ideation have substantial implications for suicide prevention. They highlight the need to develop and update clinical guidelines, especially for managing patients with MDD who present with acute suicidal ideation. This could lead to significant advancements in treatment strategies, improving outcomes for a highly vulnerable patient population.
Conclusion
In conclusion, this systematic review and meta-analysis of 12 studies provides evidence that ketamine is associated with a significant reduction in acute suicidal ideation for patients with major depressive disorder. Across intravenous, intranasal, and intramuscular administration, ketamine rapidly decreased suicidal thoughts compared to control conditions. The large, consistent treatment effect suggests clinically meaningful anti-suicidal benefits. Intravenous ketamine demonstrated the greatest efficacy, although benefits remained statistically significant with less invasive delivery methods. Patients with more severe baseline suicidal ideation also showed enhanced treatment response. These findings have important implications for managing acute suicide risk. However, limitations like study heterogeneity and design issues highlight the need for additional high-quality research. While results support the cautious use of ketamine for suicidal ideation, optimal treatment protocols, long-term safety with repeated administration, and ethical application remain unclear. Further controlled trials refining methodology and exploring sustainability of effects will be key as we incorporate ketamine into evidence-based suicide prevention strategies. In closing, our findings reveal promise but also underscore careful steps needed to safely realize ketamine’s therapeutic potential for this dire public health threat.
Limitation
Among the limitations of this study, one can mention the lack of reporting results based on important variables such as body mass index, receipt of other treatments, the presence of other underlying diseases or mental disorders in patients, and substance use or non-use, which were not reported in the primary studies, and subgroup analyses based on these variables were not performed in this study. It is recommended that in future studies, especially clinical trials in this area, attention be paid to these variables.
Availability of data and materials
The datasets used and analyzed during the current study are available by the corresponding author upon a reasonable request.
Abbreviations
- MDD:
-
Major depressive disorder
- NMDA:
-
N-methyl-D-aspartate
- RCTs:
-
Randomized controlled trials
- MeSH:
-
Medical subject headings
- PICOT:
-
Population, intervention, comparison, outcome, time
- SMD:
-
Standardized mean difference
- REML:
-
Restricted maximum likelihood
- MADRS-SI:
-
Montgomery-Åsberg Depression Rating Scale-Suicidal Ideation
References
Organization WH (2021) Suicide worldwide in 2019: global health estimates
Blair-West GW, Cantor CH, Mellsop GW, Eyeson-Annan ML (1999) Lifetime suicide risk in major depression: sex and age determinants. J Affect Disord 55(2–3):171–8
Uher R, Mors O, Rietschel M, Rajewska-Rager A, Petrovic A, Zobel A et al (2011) Early and delayed onset of response to antidepressants in individual trajectories of change during treatment of major depression: a secondary analysis of data from the Genome-Based Therapeutic Drugs for Depression (GENDEP) study. J Clin Psychiatry 72(11):5274
Abdallah CG, Sanacora G, Duman RS, Krystal JH (2018) The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacol Ther 190:148–58
Grunebaum MF, Galfalvy HC, Choo T-H, Keilp JG, Moitra VK, Parris MS et al (2018) Ketamine for rapid reduction of suicidal thoughts in major depression: a midazolam-controlled randomized clinical trial. Am J Psychiatry 175(4):327–35
Xu AJ, Niciu MJ, Lundin NB, Luckenbaugh DA, Ionescu DF, Richards EM, et al. Lithium and valproate levels do not correlate with ketamine’s antidepressant efficacy in treatment-resistant bipolar depression. Neural Plasticity. 2015;2015
Kadriu B, Musazzi L, Henter ID, Graves M, Popoli M, Zarate CA Jr (2019) Glutamatergic neurotransmission: pathway to developing novel rapid-acting antidepressant treatments. Int J Neuropsychopharmacol 22(2):119–35
Voort JLV, Ballard ED, Luckenbaugh DA, Bernert RA, Richards EM, Niciu MJ et al (2016) Antisuicidal response following ketamine infusion is associated with decreased nighttime wakefulness in major depressive disorder and bipolar disorder. J Clin Psychiatry 77(8):19661
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg 88:105906
Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343
Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ et al (2014) Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 31(4):335–43
Fan W, Yang H, Sun Y, Zhang J, Li G, Zheng Y et al (2017) Ketamine rapidly relieves acute suicidal ideation in cancer patients: a randomized controlled clinical trial. Oncotarget 8(2):2356
Sinyor M, Williams M, Belo S, Orser B, Vincent M, Mah L et al (2018) Ketamine augmentation for major depressive disorder and suicidal ideation: preliminary experience in an inpatient psychiatry setting. J Affect Disord 241:103–9
Feeney A, Hock RS, Freeman MP, Flynn M, Hoeppner B, Iosifescu DV et al (2021) The effect of single administration of intravenous ketamine augmentation on suicidal ideation in treatment-resistant unipolar depression: results from a randomized double-blind study. Eur Neuropsychopharmacol 49:122–32
Pathak U, Ahuja SK, Dwivedi R, Mishra N, Kumar P, Mishra DK et al (2021) Antisuicidal efficacy of ketamine infusion in suicidal patients of depressive disorder. Indian J Psychiatry 63(5):483
Domany Y, McCullumsmith CB. Single, fixed-dose intranasal ketamine for alleviation of acute suicidal ideation. An emergency department, trans-diagnostic approach: a randomized, double-blind, placebo-controlled, proof-of-concept trial. Arch Suicide Res. 2022;26(3):1250-65
Hochschild A, Keilp JG, Madden SP, Burke AK, Mann JJ, Grunebaum MF (2022) Ketamine vs midazolam: mood improvement reduces suicidal ideation in depression. J Affective Disord 300:10–6
Chen M-H, Lin W-C, Wu H-J, Cheng C-M, Li C-T, Hong C-J et al (2019) Antisuicidal effect, BDNF Val66Met polymorphism, and low-dose ketamine infusion: reanalysis of adjunctive ketamine study of Taiwanese patients with treatment-resistant depression (AKSTP-TRD). J Affect Disord 251:162–9
Chen M-H, Lin W-C, Tu P-C, Li C-T, Bai Y-M, Tsai S-J et al (2019) Antidepressant and antisuicidal effects of ketamine on the functional connectivity of prefrontal cortex-related circuits in treatment-resistant depression: a double-blind, placebo-controlled, randomized, longitudinal resting fMRI study. J Affect Disord 259:15–20
Alnefeesi Y, Chen-Li D, Krane E, Jawad MY, Rodrigues NB, Ceban F et al (2022) Real-world effectiveness of ketamine in treatment-resistant depression: a systematic review & meta-analysis. J Psychiatr Res 151:693–709
Derakhshanian S, Zhou M, Rath A, Barlow R, Bertrand S, DeGraw C, et al. Role of ketamine in the treatment of psychiatric disorders. Health Psychol Res. 2021;9(1)
Lin W-C, Su T-P, Li C-T, Wu H-J, Tsai S-J, Bai Y-M, et al. Baseline cognitive function predicts full remission of suicidal symptoms among patients with treatment-resistant depression and strong suicidal ideation after low-dose ketamine infusion. J Psychopharmacol. 2023:02698811231182107
Aan Het Rot M, Zarate Jr CA, Charney DS, Mathew SJ. Ketamine for depression: where do we go from here? Biol Psychiatry. 2012;72(7):537-47
Acknowledgements
The authors would like to thank the Student Research Committee of the Kurdistan University of Medical Sciences.
Funding
This study has granted no funds.
Author information
Authors and Affiliations
Contributions
Y.M., P.K.: conceptualization, methodology. Y.M., R.R.D., S.A.S.: validation, formal analysis, and supervision. L.A.: project administration, data curation and writing-review and editing. P.K., M.B., E.N.: investigation, resources, data curation, writing-original draft and visualizations. L.A., S.A.S., F.G.: validation. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Seyedoshohadaei, S.A., Darehbagh, R.R., Gholami, F. et al. Ketamine’s efficacy in alleviating acute suicidal thoughts: a comprehensive systematic review and meta-analysis. Middle East Curr Psychiatry 31, 40 (2024). https://doi.org/10.1186/s43045-024-00428-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43045-024-00428-3