Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the virus responsible for coronavirus disease 2019. It presents one of the most threatening pandemics in the history of humanity. The mortality and morbidity represent an unprecedented challenge to the modern medical era. SARS-CoV-2 results in acute respiratory distress syndrome, high concentrations of proinflammatory mediators, cytokine storm (CS) due to massive release of cytokines, hypercoagulation, and hemoglobin disintegration. Dysregulation of iron homeostasis, iron overload as indicated by high ferritin level, and ferroptosis are major factors in the pathogenesis of the disease. We report a case of SARS-CoV-2 in which the use of epinephrine (Epi) resulted in an unexpected attenuation of CS, decreasing ferritin level and inhibiting ferroptosis.
Case presentation
A 64-year-old male patient with a history of multiple medical comorbidities had been diagnosed with SARS-CoV-2. Further evaluation showed marked increase in inflammatory markers, severe hyperferritinemia, and lymphopenia in laboratory blood tests. The characteristic score of CS was strongly positive, and in addition to regular treatment, the patient received Epi due to development of acute generalized skin rash, severe itching, and edema of lips and tongue. Epi may have successfully terminated not only the acute cutaneous condition, but also have attenuated CS, decreased ferritin level, and other inflammatory markers in addition to complete patient’s recovery.
Conclusion
Epinephrine may attenuate CS and inhibit ferroptosis which is an iron-dependent, non-apoptotic mode of cell death. Epi interacts with ferric and/or ferrous iron and built a stable complex that impedes activation of beta-adrenergic receptors. Epi may cause marked decrease of ferritin and other inflammatory markers. Epi may be used to decrease iron overload which is associated with many medical diseases like type 2 diabetes mellitus and cardiometabolic diseases such as coronary heart disease and cerebrovascular disease. As a new clinical indication extensive studies are required for further assessment and possible therapeutic uses.
Similar content being viewed by others
Background
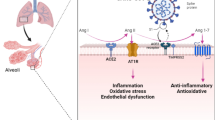
SARS-CoV-2 is the virus responsible for coronavirus disease 2019 (COVID-19) which presents one of the most threatening pandemics in the history of humanity. The mortality and morbidity represent an unprecedented challenge to the modern medical era [1]. Until March 2023, nearly 761.07 million cases and 6.87 million deaths have been reported according to statistics from the World Health Organization [2]. Clinical manifestations are absent or mild in a substantial proportion of patients who test positive for SARS-CoV-2. However, bilateral pneumonia is the main finding in hospitalized patients [3] and at least 5% initially present in serious condition, and require admission to intensive care unit (ICU) [4]. SARS-CoV-2 infects cells by attaching [5] to angiotensin converting enzyme receptor 2. SARS-CoV-2 has many complications which include acute respiratory distress syndrome (ARDS) [6], high concentrations of proinflammatory mediators [7], CS due to massive release of cytokines [8], hypercoagulation [9], and hemoglobin disintegration [10]. Dysregulation of iron homeostasis [11], iron overload [12, 13], and ferroptosis are major factor in the pathogenesis of SARS-CoV-2 [14, 15]. Atypical presentation of ARDS is not only caused by alveolar damage [16, 17] but also due to vascular endothelial injury, destruction of the beta-1 chain of hemoglobin that releases iron into the circulation [10]. Increased iron overload is associated with increased blood viscosity as well as recurrent and diffuse micro and macro vascular thrombosis which leads to elevated levels of D-dimer and death in many cases [10, 16, 17]. In 2012, Dixon proposed the concept of ferroptosis, an iron-dependent, non-apoptotic mode of cell death characterized by ferritin degradation, lipid peroxidation, and accumulation of reactive oxygen species (ROS) [18]. Free unbounded iron (Fe+2) is characterized by a high reactivity and toxicity due to formation of free radical oxygen species (ROS) through Fenton and Haber–Weiss reaction [19]. ROS formation may contribute not only to lung injury, but also to increased endothelial permeability, increased cytokine level in the lungs, and neutrophilic alveolar infiltrates [20]. High serum ferritin is also associated with ARDS progression [20, 21], and as tissue damage progresses and iron level increases, ferritin increases also to isolate iron [21]. We report a case of SARS-CoV-2 in which Epi was used and resulted in an unexpected attenuation of CS, decreasing ferritin level and inhibiting ferroptosis. The mechanism of action and the new indication of Epi will be discussed.
Case presentation
A 64-year-old male patient with a medical history of ischemic heart disease, previous coronary artery bypass grafting surgery, dyslipidemia, and cerebral transient ischemic attacks. He was receiving clopidogrel, atorvastatin, and bisoprolol. He presented with fever, dry cough, severe myalgia, and bony aches. Real-time reverse transcription-polymerase chain reaction assay of nasopharyngeal (NP) swab was positive for SARS-CoV-2. Clinical examination revealed fever: 38.8 °C (degree Celsius), blood pressure: 117/71 mmHg, heart rate: 113 beat/min, respiratory rate: 14 times/min, and peripheral oxygen saturation: 95% on room air. Chest examination revealed normal breath sounds in upper lung zones, while coarse crackles and wheezes were more in lower lung zones. Normal first and second heart sounds were detected and accompanied by mild tachycardia. Chest radiography showed bilateral mainly peripheral rounded opacities mostly in lower lung fields, and chest computed tomography revealed bilateral ground glass opacities with peripheral distribution mainly in lower lung fields. Serial laboratory blood tests are shown in Table 1. The patient was isolated and given acetaminophen 500 mg/6-h, azithromycin 500 mg/day for 10 days, rivaroxaban 10 mg/day for 3 weeks, clopidogrel 75 mg/day, prednisone 20 mg/day for 10 days followed by slow tapering every 3 days for 2 weeks, ascorbic acid 1 g/day, and zinc 30 mg/day. On the morning of the ninth day the peak level of serum ferritin and other inflammatory markers such as fibrinogen, C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), and lowest level of lymphocytes were found in the blood sample. On the night of the same day, the patient complained of generalized skin rash accompanied by severe itching. He received intravenous hydrocortisone and after 15 min the itching worsened as he developed swelling of lips and tongue. For fear of compromising his airway Epi 0.6 mcg/kg had been prescribed; thus, 50 mcg was injected subcutaneous (S/C) in the upper thigh. The dose was repeated after 20 min and resulted in successful resolution of itching, lips and tongue edema. Four hours later the same condition was recurred and resolved by injecting 50 mcg Epi S/C; meanwhile, the absence of tachycardia and hypertension was noticed. The cutaneous eruption continued for 5 days, and Epi was injected 4–5 times daily to manage this condition under medical supervision and monitoring. Serial biochemical laboratory tests showed normal renal and hepatic function tests and marked decrease of serum ferritin from 2000 to 855 ng/ml. Meanwhile, other inflammatory markers decreased markedly (Table 1), and the patient’s general condition improved dramatically, so he was discharged on day 20 after 2 negative NP swabs. Phlebotomy was done to avoid deleterious effects of elevated ferritin, and resulted in decreasing ferritin from 860 to 843 ng/ml. However, Epi was administered three times daily under monitoring and medical supervision and we observed a decrease of ferritin level to 258 ng/ ml in 3 weeks.
Discussion
The CS represents the most furious and serious complication of SARS-CoV-2. It is due to an excessive immune response to the virus and abundant release of proinflammatory cytokines into the circulation [22]. CS score (CSs) was proposed to accurately identify patients who are in this hyperinflammatory state. CSs is considered positive if there is lymphopenia and at least two other inflammatory markers of either: serum levels of ferritin, D-dimer, CRP, or LDH, are elevated [23]. Lymphopenia is defined as a lymphocytic count below 1 × 109/L as reported in severe cases [24,25,26]. Our patient showed lymphopenia (0.92 × 109/L). Ferritin is marker of inflammation and is elevated in cases associated with CS. Mean value reported in severe cases [27,28,29] was 800 ng/ml, while in our case, it was 2000 ng/ml. D-dimer is a fibrin degradation product, and median value correlated with severity [30,31,32] is 1 mg/L, while in our case it was 1.48 mg/L. CRP levels are considered an independent risk factor for poor prognosis and at a cutoff level of 100 mg/L is associated with mechanical ventilation and mortality [33]. Our patient’s value was 164.8 mg/L. LDH is a marker of tissue damage that is related to severe cases [24] with a mean value > 300 U/L. Our case had a value of 317 U/L. Fibrinogen is a clotting factor that increased during inflammatory response and the cutoff level to predict ICU admission [34] was 571.0 mg/dl. In our case, it was 767 mg/dl.
Acute urticarial lesions have been noticed in several SARS-C0V-2 case series and typically are characterized by erythematous slightly raised papular rash followed by intense pruritic sensations [35]. Pruritus was reported in 92% of patients with urticarial lesions and was associated with a severe infection [36]. Herrero-Moyano et al. [37] proposed that a CS could be the cause of these rashes rather than the virus itself. Our case (Fig. 1) showed also an acute onset of urticarial lesion that coincided with the peak values of inflammatory markers. CS usually occurs in the second week of infection [38] which was the same time of urticarial rash, itching, tongue, and lips swellings as well as peak level of fever, ferritin, and other inflammatory markers in our patient. The time between mechanical ventilation due to respiratory failure and recognition of a positive CSs was found to be 12–96 h [23]. The timely control of CS through immunomodulators and cytokine antagonists is the key to reduce the mortality rate [39]. In our case, regular treatment and Epi may have successfully attenuated the CS as shown by decrease in ferritin and other inflammatory markers during the 5 days following the use of Epi (Fig. 2). This case report shows a clear-cut temporal association between Epi administration and rapid clinical and biochemical improvement in our patient with positive CSs. This may be explained by the interaction between Epi and labile plasma iron which may inhibit ferroptosis. The interaction between ferritin and Epi was first reported in 1956, and it was concluded that circulating ferritin can inhibit the vasoconstrictor response to Epi in an experimental study [40] (Table 2). More recently, it was found that EPI interacts with ferric (Fe+3) or ferrous (fe+2) iron from plasma labile iron pool and results in impeding activation of adrenergic receptors experimentally [41]. This explains lack of tachycardia and hypertension, which might be harmful to our patient.
Epinephrine is cutting the vicious circuit of iron, ferritin, and ferroptosis which are the ambiguous, devious, and vicious culprits in SARS-CoV-2. Epinephrine interacts with F2 and F3 to decrease ferritin that may attenuate cytokine storm and inhibit ferroptosis. ARDS adult respiratory distress syndrome, Hb hemoglobin, F2 ferrous iron, F3 ferric iron, CRP C-reactive protein, LDH lactate dehydrogenase
Surgical shock [47] and cardiac arrest [48] are the first clinical indications of Epi that were discovered by Crile in 1903 (Table 2). Epi may present a therapeutic option to lower increased iron stores, reflected by high serum ferritin levels, associated with type 2 diabetes mellitus (T2DM) [52] and other cardiometabolic diseases such as coronary heart disease (CHD) and cerebrovascular disease (CEVD) [53]. It is concluded that inhibiting ferroptosis significantly reduces ischemia/reperfusion-related cardiac injury [54] and may also suppress inflammation and improve wound healing in diabetic ulcer [55].
Conclusions
Epi interacts with ferric and/or ferrous iron to build a stable complex that impedes activation of beta-adrenergic receptors. Epi may attenuate cytokine storm and decrease ferritin levels associated with viral and medical diseases such as T2DM and cardiometabolic diseases. Epi may inhibit ferroptosis thus significantly reduces ischemia/reperfusion-related cardiac injury and may improve wound healing in diabetic ulcer. As a new clinical indication of Epi, extensive studies are required for further assessment and possible therapeutic uses.
Availability of data and materials
All data related to this case report are contained within the manuscript.
Abbreviations
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- COVID-19:
-
Coronavirus disease 2019
- ARDS:
-
Acute respiratory distress syndrome
- CS:
-
Cytokine storm
- Epi:
-
Epinephrine
- T2DM:
-
Type 2 diabetes mellitus
- CMDs:
-
Cardiometabolic diseases
- CHD:
-
Coronary heart disease
- CEVD:
-
Cerebrovascular disease
- ICU:
-
Intensive care unit
- ROS:
-
Reactive oxygen species
- Fe+2 :
-
Ferrous iron
- Fe+3 :
-
Ferric iron
- CRP:
-
C-reactive protein
- LDH:
-
Lactate dehydrogenase
References
Lippi G, Sanchis-Gomar F, Henry BM (2020) COVID-19: unravelling the clinical progression of nature’s virtually perfect biological weapon. Ann Transl Med 8(11):693. https://doi.org/10.21037/atm-20-3989
Surendra H, Praptiningsih CY, Ersanti AM et al (2023) Clinical characteristics and factors associated with COVID-19-related mortality and hospital admission during the first two epidemic waves in 5 rural provinces in Indonesia: a retrospective cohort study. PLoS ONE 18(3):e0283805. https://doi.org/10.1371/journal.pone.0283805
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Sharma J, Rajput R, Bhatia M, Arora P, Sood V (2021) Clinical predictors of COVID-19 severity and mortality: a perspective. Front Cell Infect Microbiol 25(11):674277. https://doi.org/10.3389/fcimb.2021.674277. (PMID: 34760713; PMCID: PMC8573222)
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Nai-Huei Wu, Nitsche A, Müller MA, Drosten C, Pöhlmann S (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Colafrancesco S et al (2014) sCD163 in AOSD: a biomarker for macrophage activation related to hyperferritinemia. Immunol Res. https://doi.org/10.1007/s12026-014-8563-7
Xu Z et al (2020) Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. https://doi.org/10.1016/S2213-2600(20)30076-X
Li X et al (2020) Molecular immune pathogenesis and diagnosis of COVID-19. J Pharm Anal. https://doi.org/10.1016/j.jpha.2020.03.001
Merad M, Martin J (2020) Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. https://doi.org/10.1038/S41577-020-0331-4
Rapozzi V, Juarranz A, Habib A, Ihan A, Strgar R (2021) Is haem the real target of COVID-19? Photodiagnosis Photodyn Ther 35:102381. https://doi.org/10.1016/j.pdpdt.2021.102381
Iannaccone G, Scacciavillani R, Del Buono MG et al (2020) Weathering the cytokine storm in COVID-19: therapeutic implications. Cardiorenal Med 10(5):277–287. https://doi.org/10.1159/000509483
Goldberg M et al (2020) Cerebrovascular disease in COVID-19. Am J Neuroradiol. https://doi.org/10.3174/ajnr.a6588
Zhou F et al (2020) Clinical course and risk factors for mortality of adult inpatients with Covid-19 in Wuhan, China: a retrospective cohort study. Lancet. https://doi.org/10.1016/S0140-6736(20)30566-3
Sun Y, Chen P, Zhai B, Zhang M, Xiang Y, Fang J, Xu S, Gao Y, Chen X, Sui X, Li G (2020) The emerging role of ferroptosis in inflammation. Biomed Pharmacother 127:110108
Ursini F, Maiorino M (2020) Lipid peroxidation and ferroptosis: the role of GSH and GPx4. Free Radic Biol Med 152:175–185
Camporota L, Cronin JN, Busana M, Gattinoni L, Formenti F (2022) Pathophysiology of coronavirus-19 disease acute lung injury. Curr Opin Crit Care 28(1):9–16. https://doi.org/10.1097/MCC.0000000000000911
Rawat M et al (2020) COVID-19 in newborns and infants—low risk of severe disease: silver lining or dark cloud? Clin Opin. https://doi.org/10.1055/s-0040-1710512
Dixon SJ, Lemberg KM, Lamprecht MR et al (2012) Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149(5):1060–1072. https://doi.org/10.1016/j.cell.2012.03.042
Zhao Z (2019) Iron and oxidizing species in oxidative stress and Alzheimer’s disease. Aging Med (Milton) 2(2):82–87. https://doi.org/10.1002/agm2.12074
Kim J, Wessling-Resnick M (2012) The role of iron metabolism in lung inflammation and injury. J Allergy Ther. https://doi.org/10.4172/2155-6121.S4-004
Khiroya H, Turner A (2015) The role of iron in pulmonary pathology. Multidiscip Respir Med. https://doi.org/10.1186/s40248-015-0031-2
Gasparyan AY, Misra DP, Yessirkepov M, Zimba O (2020) Perspectives of immune therapy in coronavirus disease 2019. J Korean Med Sci 35:e176. https://doi.org/10.3346/jkms.2020.35.e176
Cappanera S, Palumbo M, Kwan SH et al (2021) When does the cytokine storm begin in COVID-19 patients? A quick score to recognize it. J Clin Med 10(2):297. https://doi.org/10.3390/jcm10020297
Zheng Z, Peng F, Xu B, Zhao J, Liu H, Peng J, Li Q, Jinag C, Zhou Y, Liu S et al (2020) Risk factors of critical and mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect 81:e16–e25
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, Wang T, Zhang X, Chen H, Yu H et al (2020) Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Investig 130:2620–2629
Ruan Q, Yang K, Wang W, Jiang L, Song J (2020) Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensiv Care Med 46:846–848
Diamanti AP, Rosado MM, Pioli C, Sesti G, Laganà B (2020) Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci 21:3330. https://doi.org/10.3390/ijms21093330
Ghweil AA, Hassan MH, Mohamed AK, Mohamed AO, Mohammed HM, Abdelazez AA, Osman HA, Bazeed SES (2020) Characteristics, outcomes and indicators of severity for COVID-19 among sample of ESNA Quarantine Hospital’s Patients, Egypt: a retrospective study. Infect Drug Resist 13:2375–2383
Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ (2020) COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 395:1033–1034
Zhang J, Yu M, Tong S, Liu L, Tang L (2020) Predictive factors for disease progression in hospitalized patients with coronavirus disease 2019 in Wuhan, China. J Clin Virol 127:104392
Gao Y, Li T, Han M, Li X, Wu D, Xu Y, Zhu Y, Liu Y, Wang X, Wang L (2020) Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J Med Virol 92:791–796
Arachchillage DRJ, Laffan M (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18:1233–1234
Chalmers JD, Singanayagam A, Hill AT (2008) C-reactive protein is an independent predictor of severity in community-acquired pneumonia. Am J Med 121:219–225
Sui J, Noubouossie DF, Gandotra S, Cao L (2021) Elevated plasma fibrinogen is associated with excessive inflammation and disease severity in COVID-19 patients. Front Cell Infect Microbiol 11:734005. https://doi.org/10.3389/fcimb.2021.734005
Rubio-Muniz CA, Puerta-Peña M, Falkenhain-López D et al (2020) The broad spectrum of dermatological manifestations in COVID-19: clinical and histopathological features learned from a series of 34 cases. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.16734
Galván Casas C, Catalá ACHG et al (2020) Classification of the cutaneous manifestations of COVID-19: a rapid prospective nationwide consensus study in Spain with 375 cases. Br J Dermatol 183:71–77
Herrero-Moyano M, Capusan TM, Andreu-Barasoain M et al (2020) A clinicopathological study of eight patients with COVID-19 pneumonia and a late-onset exanthema. J Eur Acad Dermatol Venereol. https://doi.org/10.1111/jdv.16631
Bertozzi PV, de Oliveira Vicente A, Pereira AS et al (2021) Tocilizumab in HIV patient with severe COVID-19: case report. AIDS Res Ther 18:73. https://doi.org/10.1186/s12981-021-00404-5
Siddiqi HK, Lang J, Nauffal V, Morrow DA, Bohula EA (2020) COVID-19 for the cardiologist: a current review of the virology, clinical epidemiology, cardiac and other clinical manifestations and potential therapeutic strategies. JACC Basic Transl Sci 5:518–536
Green S, Mazur A, Shorr E (1956) Mechanism of the catalytic oxidation of adrenaline by ferritin. J Biol Chem 220(1):237–255. https://doi.org/10.1016/S0021-9258(18)65348-7
Jačić JK, Nikolić L, Stanković DM, Opačić M, Dimitrijević M, Savić D, Šipka SG, Spasojević I, Pristov JB (2020) Ferrous iron binding to epinephrine promotes the oxidation of iron and impedes activation of adrenergic receptors. Free Radic Biol Med 20(148):123–127. https://doi.org/10.1016/j.freeradbiomed.2020.01.001
Fine LG (1986) Eustachio’s discovery of the renal tubule. Am J Nephrol 6:47–50
Addison T (1855) On the constitutional and local effects of disease of the suprarenal capsules. Highley, London
Oliver SEA (1895) The physiological effects of extracts of the suprarenal capsules. J Physiol 18:230–276
Pohar M, Hansson N (2021) Between two stools? Pharmacologists nominated for Nobel prizes in “physiology or medicine” and “chemistry” 1901–1950 with a focus on John Jacob Abel (1857–1938). Naunyn Schmiedebergs Arch Pharmacol 394(3):503–513. https://doi.org/10.1007/s00210-020-01993-0
Parascandola J (2010) Abel, Takamine, and the isolation of epinephrine. J Allergy Clin Immunol 125(2):514–517. https://doi.org/10.1016/j.jaci.2009.11.044
Ball CM, Featherstone PJ (2017) Adrenaline-a therapeutic history. Anaesth Intensive Care 45(5):531–533. https://doi.org/10.1177/0310057X1704500501
Hermann RE (1994) George Washington Crile (1864–1943). J Med Biogr 2(2):78–83. https://doi.org/10.1177/096777209400200204
McFadden ER (2004) A century of asthma. Am J Respir Crit Care Med 170:215–221. https://doi.org/10.1164/rccm.200402-185OE
Boughton T (1919) Anaphylactic death in asthmatics. JAMA 73:1912–1915
Bodon C (1923) The intracardiac injection of adrenalin. Lancet 1:586–590
Kunutsor SK, Apekey TA, Walley J, Kain K (2013) Ferritin levels and risk of type2 diabetes mellitus: an updated systematic review and meta-analysis of prospective evidence. Diabetes Metab Res Rev 29:308–318
De Das S, Krishna S, Jethwa A (2015) Iron status and its association with coronary heart disease: systematic review and meta-analysis of prospective studies. Atherosclerosis 238:296–303
Pan Y, Wang X, Liu X, Shen L, Chen Q, Shu Q (2022) Targeting ferroptosis as a promising therapeutic strategy for ischemia-reperfusion injury. Antioxidants 11:2196. https://doi.org/10.3390/antiox11112196
Feng J, Wang J, Wang Y, Huang X, Shao T, Deng X, Cao Y, Zhou M, Zhao C (2022) Oxidative stress and lipid peroxidation: prospective associations between ferroptosis and delayed wound healing in diabetic ulcers. Front Cell Dev Biol 10:898657. https://doi.org/10.3389/fcell.2022.898657
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
AE-M is the primary consultant who managed the case, administered Epi, made the research regarding ferritin and Epi, wrote the manuscript, and critically reviewed the literature. FAF managed the case, made a critical revision of Epi, and contributed to history and supervision of the manuscript. SB discussed, shared, and reviewed the manuscript. YA, YA, MB, YG, and FB prepared and shared the manuscript, reviewed the ferritin and Epi interaction, and critically reviewed the manuscript. AAB and AHA collected and organized the references and reviewed the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report.
Competing interests
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
EL-Molla, A., Fetouh, F.A., Bawazir, S. et al. Role of epinephrine in attenuating cytokine storm, decreasing ferritin, and inhibiting ferroptosis in SARS-CoV-2. Egypt Heart J 76, 22 (2024). https://doi.org/10.1186/s43044-024-00455-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-024-00455-9