Abstract
Coronavirus disease 2019 (COVID-19) is a recent pandemic caused by a novel severe acute respiratory syndrome coronavirus 2 (SARS‑CoV‑2) leading to pulmonary and extra-pulmonary manifestations due to the development of oxidative stress (OS) and hyperinflammation. The underlying cause for OS and hyperinflammation in COVID-19 may be related to the inhibition of nuclear factor erythroid 2-related factor 2 (Nrf2), a master regulator of antioxidative responses and cellular homeostasis. The Nrf2 pathway inhibits the expression of pro-inflammatory cytokines and the development of cytokine storm and OS in COVID-19. Nrf2 activators can attenuate endothelial dysfunction (ED), renin-angiotensin system (RAS) dysregulation, immune thrombosis, and coagulopathy. Hence, this review aimed to reveal the potential role of the Nrf2 pathway and its activators in the management of COVID-19. As well, we tried to revise the mechanistic role of the Nrf2 pathway in COVID-19.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The whole world confronted a disaster situation that first emerged in late December 2019 as purely a few cases of pneumonia in Wuhan, China (Batiha et al. 2021a). A scrupulous investigation employing next-generation sequencing and phylogenetic analysis led to the recognition of the causative agent of this respiratory disease, a novel coronavirus (2019-nCoV) (Al-Kuraishy et al. 2021a). The World Health Organization (WHO) allocated a name,Coronavirus disease 2019, or COVID-19, to the disease and declared it a pandemic on March 11, 2020 (McFee 2020). Later on, the 2019-nCoV was renamed to SARS-CoV-2 by the International Committee on Taxonomy of Viruses based on its genetic match to a previously known coronavirus, severe acute respiratory syndrome coronavirus (SARS-CoV) (McFee 2020). Transmission of SARS-CoV-2 occurs when a healthy individual inhales or comes into contact with respiratory droplets from an infected person (Al-Kuraishy et al. 2022n). The average incubation period before a patient exhibits disease symptoms ranges from 2 to 14 days (El-Saber Batiha et al. 2022). SARS-CoV-2 has shown that it is genetically similar to previously known coronavirus SARS-CoV and hence is placed under the family Coronaviridae (Al-Kuraishy et al. 2021k). Coronavirus contains positive-sense single-stranded RNA as its genetic material which also helps the virus to evade host immune response and assists its entry inside the host cell (Al-Kuraishy et al. 2021l). Interestingly, SARS-CoV-2, similar to SARS-CoV, exploits the angiotensin-converting enzyme 2 (ACE2) receptor to gain access inside human cells (Babalghith et al. 2022). Besides, the trimeric S protein of SARS-CoV-2 is sliced by transmembrane protease serine 2 (TMPRSS2), similar to SARS-CoV (Al-Kuraishy et al. 2021c).
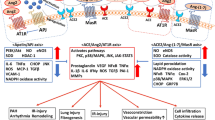
The peptidase ACE2 metabolizes vasoconstrictor angiotensin II (Ang II) to the vasodilator Ang1-7 and Ang1-9 (Al-Kuraishy and Al-Gareeb 2020). ACE2 receptor is highly expressed in various cellular systems, including enterocytes, cardiomyocytes, pulmonary alveolar cells, neurons, and testes (Moubarak et al. 2021). Consequently, the downregulation of ACE2 during SARS-CoV-2 infection provokes vasoconstriction and the development of endothelial dysfunction (ED), oxidative stress (OS), and inflammatory disorders (Al-Kuraishy et al. 2022g). The binding of SARS-CoV-2 with the ACE2 receptor leads to a series of inflammatory cellular events with cytopathic effects causing cell injury and hyperinflammation (Al-Thomali et al. 2022a) (Fig. 1).
The clinical presentation of COVID-19 is mainly asymptomatic or presented with mild symptoms in 85% of cases (Al-Kuraishy et al. 2022y). Nonetheless, 15% presented with moderate-severe form due to the progress of acute lung injury (ALI) (Mostafa-Hedeab et al. 2022a). Also, 5% of COVID-19 patients may be critical and necessitate assessed ventilation due to the development of acute respiratory distress syndrome (ARDS) (Al-Kuraishy et al. 2022p).
SARS-CoV-2-induced OS triggers activation of different signaling pathways such as nuclear factor erythroid 2-related factor 2 (Nrf2), which induces cellular interactions to mitigate SARS-CoV-2-mediated viral toxicity and cellular injury (Zhu et al. 2021). The Nrf2 is a transcription factor that normalizes numerous essential genes that encode body antioxidant and anti-inflammatory signaling systems (Zhu et al. 2021). Downregulation of Nrf2 by SARS-CoV-2 is associated with an unregulated expression of the ACE2 receptor and the development of OS and inflammatory disorders (Qu et al. 2023). Consequently, the objective of the present review was to elucidate the role of Nrf2 in SARS-CoV-2 infection.
Overview of the Nrf2
Nrf2, a master regulator of antioxidative responses, is very crucial in maintaining cellular homeostasis (Vomund et al. 2017). Nrf2 belongs to the NFE2 family of transcription factors and contains seven Neh domains that regulate Nrf2 activity by binding to DNA or proteins (Vomund et al. 2017). Nrf2 is a transcription factor that regulates the expression of antioxidant enzymes and antioxidant response elements during the development of OS and inflammatory reaction (Pall and Levine 2015). Nrf2 is activated in two ways; in canonical Nrf2 activation, specific cysteine residues on Keap1 are oxidized by oxidative stress or electrophiles, resulting in a conformational change in the adaptor protein and the inhibition of E3 ubiquitin ligase activity (Gan et al. 2010). Alternatively, non-conical mechanisms can disturb the interaction of keap1 and Nrf2 through Nrf2 phosphorylation (Gan et al. 2010).
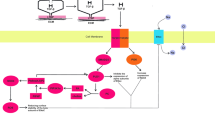
Nrf2 triggers the expression of phase II enzymes and heme oxygenase 1 (HO-1), and inhibiting inflammatory signaling pathways (Gan et al. 2010). As well, Nrf2 has pleiotropic effects in controlling the immune response, and cellular metabolism (Jung and Kwak 2010). Noteworthy, Nrf2 is engaged with Kelch-like ECH-associated protein 1 (Keap1), which regulates the anti-inflammatory and antioxidant effects of Nrf2 (Zhong et al. 2019). Keap1 regulates the expression of adaptor protein and ubiquitin ligase complex via binding Culin-3 and Rbx1 with an anchoring effect on the cytoplasmic Nrf2 (Hikichi et al. 2019). During the development of OS and generation of reactive oxygen species (ROS), Nrf2 is rapidly dissociated from KEAP1 and translocated to the nucleus and activation the expression of antioxidant proteins to maintain cellular homeostasis (Li et al. 2014). Following the ending of OS, Nrf2 is inactivated by cytoplasmic KEAP1 and nuclear beta-transducing repeat-containing protein glycogen synthase kinase 3 (β-TrCP-GSK3) (Iizuka et al. 2021) (Fig. 2).
Molecular mechanism of nuclear factor erythroid 2-related factor 2 (Nrf2): (1): Under balanced conditions, Nrf2 is anchored with Kelch-like ECH-associated protein 1 (KEAP1). Nrf2 binds keap, gets ubiquinat3d, and degraded by β-TrCP in the cytoplasm. (2): Under oxidative stress, Nrf2 is dissociated and enters and binds small maf protein (sMaf) to bind antioxidant response element (ARE), which increases the expression of the antioxidant gene. Nrf2 is degraded by beta-transducin repeat-containing protein glycogen synthase kinase 3 (β-TrCP-GSK3). Dissociation of Nrf2 from Kelch-like E.C.H. associated protein 1 (KEAP1) with activation of antioxidant response element (ARE), which increases the expression of antioxidant genes heme oxygenase 1 (HO-1) and quinone oxidoreductase (NQO1), which blocks the progression of oxidative stress (OS) and maintains redox balance and cytoprotective effect
Furthermore, Nrf2 supports different metabolic processes such as the production of nicotinamide adenine dinucleotide phosphate (NADPH) and the metabolism of amino acids, lipids, nucleotides, and iron/heme (DeBlasi and DeNicola 2020). Nrf2 activates glutaminase, which converts glutamine into glutamate which enter the nucleus and involved in the production of GSH (Hamada et al. 2021). Nrf2 significantly influences the regulation of the enzymes involved in the production pathway of serine which is a component of different macromolecules, such as nucleotides, ceramide, and sphingolipid (Ishii et al. 2022). As well, Nrf2 is a critical factor in cellular components, which helps in cellular repair and OS control (Yu and Xiao 2021).
Of interest, Nrf2 attenuates OS-induced ALI/ARDS by mitigating endothelial dysfunction. In an in vitro study conducted, Canella et al. (Canella et al. 2018) illustrated that Nrf2 pathway activators prevent OS-induced ALI via mitigation of outward rectifier chloride channels (ORCCs) in human lung epithelial cells (A549 line). On the other hand, Wu and colleagues revealed that the Nrf2 pathway attenuates the development of diabetic cardiomyopathy through the mitigation of OS (Wu et al. 2022). Flavonoids have been shown to mitigate OS in cell lines via activation of the Nrf2 pathway (Sindhu et al. 2021). Similarly, chrysin reduces sepsis-induced cardiac injury by ameliorating the Nrf2 pathway (Xingyue et al. 2021). Furthermore, the underlying mechanism of the antioxidant effects of Nrf2 is through the induction expression of antioxidant protein genes like HO-1 and quinone oxidoreductase (NQO1), which block the progression of OS and maintain redox balance (Sugimoto et al. 2021). These findings suggest that Nrf2 reduces the propagation of OS-induced tissue injury and organ dysfunction.
Nrf2 and SARS-CoV-2 infection
In SARS-CoV-2 infection, Nrf2 is highly dysregulated, causing abnormal expression of ACE2 with further increasing viral entry (Nguyen et al. 2022). SARS-CoV-2 induces protein kinase receptor (PKR) activation, which acts as endoplasmic reticulum kinase to promote the degradation of Nrf2 (Mostafa-Hedeab et al. 2022b). The Nrf2 pathway is inhibited during SARS-CoV-2 infection leading to augmentation of OS and related inflammatory disorders (Nguyen et al. 2022). Therefore, NF-κB and NADPH oxidases are activated in SARS-CoV-2 infection, causing hyperinflammation and OS, respectively (Al-Kuraishy et al. 2022q). In addition, Nrf2 inhibits the activation of stimulator of interferon genes (STING) which regulate the expression of interferon (IFN) response (Ryan et al. 2022). In turn, IFN blocks the expression of Nrf2, leading to hyperinflammation and OS (Ryan et al. 2022). Concerning the clinical significance of Nrf2 level in COVID-19 patients, a comparative study including 40 children with COVID-19 compared with matched 35 healthy controls showed that Nrf2 level was lower in children with COVID-19 as compared with healthy controls due to tissue damage and OS (Gümüş et al. 2022). Furthermore, various studies suggested that Nrf2 level is highly dysregulated in COVID-19 patients (Cuadrado et al. 2020; Singh et al. 2021).These findings proposed that exaggerated immune response in SARS-CoV-2 infection induces a substantial reduction in the activity of the Nrf2 pathway.
Moreover, Nrf2 improves antiviral response against different viral infections for example Nrf2 activators restrict the replication of herpes simplex virus-1 (HSV-1) in human primary fibroblasts (Wyler et al. 2019). Likewise, Nrf2 protects against infections with the respiratory syncytial virus (RSV) and metapneumovirus (MNV) by modifying the innate immune response and preventing viral replication. Infections with RSV and MNV are associated with OS formation and hyperinflammation, which promote Nrf2 gene expression (Ivanciuc et al. 2018). Additionally, it has been noted that Nrf2 is essential for preventing OS-induced neurocognitive problems in people with human immunodeficiency virus (HIV) (Reddy et al. 2012). Reddy et al. (Reddy et al. 2012) found that HIV glycoprotein 120 induces expression of the Nrf2/HO-1/NQO1 axis in parallel with the activation of pro-inflammatory cytokines, mainly TNF-α (Reddy et al. 2012). Furthermore, Nrf2 is upregulated in response to the chronic effects of viral infections. In addition, Nrf2 prevents the spread of the influenza virus infection (Ramezani et al. 2018). Nrf2 is a major regulator during viral infections; some infections activate Nrf2, though other viral infections may provoke Nrf2 independent of the antioxidant pathway (Cherupanakkal et al. 2017). Remarkably, Nrf2 is involved in the host immune response in patients with dengue infection (Cherupanakkal et al. 2017). According to comparison research involving 88 dengue patients and 31 patients with other febrile illnesses, dengue patients had higher levels of Nrf2 expression in their human peripheral mononuclear cells than those with other febrile illnesses (Cherupanakkal et al. 2017). Therefore, Nrf2 may be implicated in the virulence and pathogenesis of viral infections. These findings proposed that Nrf2 plays a crucial role in viral infections to counteract the associated OS and exaggerated inflammatory reactions. Nevertheless, Nrf2 could be implicated in the propagation of viral infections.
Nrf2 has an integral role in the regulation of immune response and propagation of inflammation (Vomund et al. 2017). It reduces the expression of pro-inflammatory cytokines, including MCP-1, TNF-α, IL-6, and IL-1β, by inhibiting the recruitment of RNA polymerase II and macrophage activation (Battino et al. 2018). Interestingly, Nrf2 blocks the expression of NF-κB, which plays an essential role in primary immune response and induction of inflammation (Lee et al. 2015). Therefore, depletion of Nrf2 signaling enhances lipopolysaccharide (LPS)-induced lung inflammation by exaggerating NF-κB/TNF-α (Rushworth et al. 2011). Yan and coworkers revealed that Nrf2 signaling attenuates ALI and lung inflammation development in mice by inhibiting the expression of TLR4 (Yan et al. 2018). The net effect of Nrf2 on the inflammatory reaction is through the activation of HO-1, which produces an anti-inflammatory effect. As well, Nrf2 has a direct anti-inflammatory by inhibiting the NF-κB-dependent release of pro-inflammatory cytokines (Fig. 3).
Nuclear factor erythroid 2-related factor 2 (Nrf2) and SARS-CoV-2: SARS-CoV-2 induces protein kinase receptor (PKR) activation that promote the degradation of NRF2. Toll-like receptor (TLR) signaling promotes the expression of nuclear factor kappa B (NF-κB), which plays an essential role in primary immune response and induction of inflammation. Nrf2 has direct anti-inflammatory by inhibiting the NF-κB-dependent release of pro-inflammatory cytokines. Nrf2 produces an anti-inflammatory effect through the activation of heme oxygenase 1 (HO-1)
Mechanistic role of Nrf2 in SARS-CoV-2 infection
Nrf2 and inflammatory signaling pathways
NLRP3 inflammasome
Nod-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome are multiprotein complexes formed in the cytosol driving caspase-1 cleavage and the secretion of the pro-inflammatory cytokines IL-1β and IL-18 and other damage-associated molecular patterns (DAMPs) (Batiha et al. 2022a; Alrouji et al. 2023a). NLRP3 inflammasome promotes antigen presentation and the induction of an adaptive immune response (Batiha et al. 2023a).
It has been shown that SARS-CoV-2 infection and abnormal immune response are associated with the activation of different inflammatory signaling pathways leading to hyperinflammation (Al-Kuraishy et al. 2022f). In COVID-19, an excessive immunological response results in a high level of NLRP3 inflammasome activation (Al-Kuraishy et al. 2021j). In the SARS-CoV-2 infection, the endogenous adjuvant activity is caused by the direct activation of NLRP3 by a viral protein, named viroporin protein 3a, suggesting that SARS-CoV-2 can directly activate NLRP3 inflammasome (Al-Kuraishy et al. 2022t). Targeting of NLRP3 inflammasome pathway by selective inhibitors may reduce COVID-19-induced complications (Batiha et al. 2021b). Liu et al. (Liu et al. 2017a) showed that Nrf2 negatively regulates the expression of NLRP3 inflammasome via inhibition of ROS generation (Liu et al. 2017b). An experimental study demonstrated that rotenone-induced NLRP3 inflammasome expression by ROS was inhibited by Nrf2 mediated activation of NQO1 in mice (Liu et al. 2017b). Therefore, by inhibiting the NLRP3 inflammasome in COVID-19, Nrf2 activators may significantly decrease SARS-CoV-2 infection-induced inflammation (Mendonca and Soliman 2020). Besides, activation of NLRP3 inflammasome is associated with more expression of NF-κB, which increases inflammatory disorders via the release of pro-inflammatory cytokines in COVID-19 (Batiha et al. 2022b). Importantly, Nrf2 attenuates the expression of NF-κB, leading to potent anti-inflammatory effects (Mendonca and Soliman 2020). It has been observed that the expression of NLRP3 was upregulated, and the expression of IL-1β and IL-18 was downregulated after Nrf2 silencing (Chen et al. 2019). It has been reported that bardoxolone methyl offers an effective pharmacological approach to increasing Nrf2 activity and mitigating cholestasis in hepatic ischemia–reperfusion injury (Ruiz et al. 2013). Nrf2 activator isoliquiritigenin prevents the development of ALI in mice by suppressing the NF-κB pathway through induction expression of Nrf2 and adenosine monophosphate protein kinase (AMPK) (Liu et al. 2017a).
TLR4 and high mobility box protein 1 (HMBP1)
Interestingly, immune response during acute cell injury promotes the expression of TLR4 and HMBP1 (Alkhayyat et al. 2022). Nrf2 reduces the expression of pro-inflammatory cytokines, including MCP-1, TNF-α, IL-6 and IL-1β by inhibiting the recruitment of RNA polymerase II and macrophage activation (Yu et al. 2019).Therefore, Nrf2 attenuates the development of inflammatory changes straight by its anti-inflammatory effects or indirectly via modulation the expression of HO-1 and reduction of OS (Saha et al. 2020). In COVID-19, the exaggerated expression of TLR4 and HMBP1 induce the release of pro-inflammatory cytokine and immune thrombosis, respectively (Al-kuraishy et al. 2022a). HMBP1 induction in COVID-19 aggravates the recruitment of neutrophils with the formation of neutrophil extracellular traps (NETs), which causes more inflammation and thrombosis termed immune thrombosis (Al-Kuraishy et al. 2022c, 2022m). Interestingly, Nrf2 agonist resveratrol suppresses inflammatory reactions and inhibits the expression of MCP-1, TNF-α, IL-6, and IL-1β, as well as the expression of adhesion molecules through activating the Nrf2/HO-1 pathway (Al-Kuraishy et al. 2022a; Giordo et al. 2021). Resveratrol may have potential therapeutic efficacy in mitigating SARS-CoV-2 infection-associated hemostatic complications and disorders (Giordo et al. 2021; Liao et al. 2021). Giordo et al. (Giordo et al. 2021) proposed that in virtue of its anti-thrombotic, antioxidant, and anti-inflammatory effects, resveratrol can reduce OS, inflammatory disorders, and thrombotic events in COVID-19 through activation of the Nrf2/HO-1 pathway (Giordo et al. 2021).
PI3K/Akt signaling
Certainly, activated PI3K/Akt signaling is necessary for the anti-inflammatory effect of Nrf2 (Al-Kuraishy et al. 2022w; Basile et al. 2022). However, PI3K/Akt pathway may enhance SARS-CoV-2 endocytosis mediated by the clathrin pathway (Khezri et al. 2022). Deregulation of renin-angiotensin system (RAS) in SARS-CoV-2 infection also provokes increasing of AngII, which is a potent activator of this pathway with the development of lung fibrosis (Al-Kuraishy et al. 2022r, 2022v, 2022x; Hussien et al. 2021). PI3K/Akt also activates the NF-κB pathway with further inflammatory and OS disorders in COVID-19 (Basile et al. 2022). Azithromycin suppresses PI3K/Akt pathway by inhibiting abnormal inflammatory reactions in COVID-19 (Al-Kuraishy et al. 2021e, 2020c, 2020d). Based on this evidence, increased activation of PI3K/Akt signaling encourages the expression of anti-inflammatory Nrf2 to neutralize hyperinflammation and OS in COVID-19.
Mechanistic target of rapamycin (mTOR)
Of note, there is a potential interaction between Nrf2 and mTOR, which is concerned in the propagation of inflammatory disorders (Al-Kuraishy et al. 2022e, 2022s). Sestrins which is triggered by environmental stressors promote the expression of Nrf2 and inhibit the mTOR pathway (Rhee and Bae 2015). In addition, Nrf2 blocks the activation of the mTOR pathway during inflammatory reactions (Rhee and Bae 2015). Besides, Nrf2 agonist sulforaphane reduces OS and normalizes autophagy in Parkinson’s disease through inhibition generation of ROS and mTOR pathways, respectively (Zhou et al. 2016). In COVID-19, the mTOR pathway promotes viral replication (Karam et al. 2021). Particularly, mTOR is a serine/threonine kinase that controls cell growth by enhancing mTOR1 and mTOR2 (Battaglioni et al. 2022). It has been identified that mTOR inhibitor metformin effectively treats influenza virus infection (Al-Kuraishy et al. 2021b, 2022i). It has been suggested that the mTOR pathway is essential for the replication of SARS-CoV-2, and mTOR inhibitors and modulators like sapanisertib and metformin, respectively, may reduce COVID-19 severity (Coleman et al. 2021). In this state, Nrf2 signaling may decrease the severity of SARS-CoV-2 infection and associated complications through modulation of the mTOR pathway.
Advanced glycation endproducts
Advanced glycation endproducts (AGEs) provoke the release of pro-inflammatory cytokines and induce the propagation of OS and inflammatory disorders with subsequent activation of the Nrf2 pathway to counterbalance OS and/or inflammatory reactions (Al-Kuraishy et al. 2023d, 2021i, 2020e; Alomair et al. 2022a). Remarkably, Nrf2/HO-1/NQO1 axis is exceedingly activated in the endothelial cells subjected to AGEs activators to give an adaptive response against OS and/or inflammatory reactions in diabetes (He et al. 2011). An experimental study established that some herbal medicine like Eucommia ulmoides attenuates glucotoxicity by inhibiting AGEs through enhancement of the Nrf2 pathway in diabetic mice (Do et al. 2018). These findings suggest that Nrf2 is considered as a potent inhibitor of AGEs in different metabolic and inflammatory disorders. AGEs are generated due to the glycation of DNA, proteins, and lipids, which play a critical role in the pathogenesis of metabolic and inflammatory disorders (Alomair et al. 2022a). The receptor of AGEs (RAGE) expressed in pulmonary epithelial alveolar cells is involved in the pathogenesis of SARS-CoV-2 infection and associated lung inflammation, ALI and ARDS (Al-Kuraishy et al. 2022b, 2021h; Alkazmi et al. 2022). Both diabetes and the aging process accelerate the production of AGEs which, via the interaction with RAGE on the macrophages, trigger lung inflammation in COVID-19 (Alomair et al. 2022b; Al-Kuraishy et al. 2022j). Furthermore, Nrf2 is reduced in different metabolic disorders, including diabetes mellitus (Costa et al. 2019). This may explain the susceptibility of patients with metabolic disorders to the risk of SARS-CoV-2 infection and COVID-19 severity (Batiha et al. 2023b; Al-Kuraishy et al. 2021g, Al-Kuraishy et al. 2023f). Likewise, soluble RAGE (sRAGE) plasma level is associated with COVID-19 severity. A prospective cohort study included 164 COVID-19 patients compared to 23 non-COVID-19 pneumonia demonstrated that high sRAGE plasma level was associated with the need for oxygen therapy and 30-day mortality (Lim et al. 2021). Consequently, sRAGE is a potential biomarker for predicting COVID-19 severity and mortality.
Signal transducer and activator of transcription 3
Signal transducer and activator of transcription factors (STATs) are a family of transcription factors that regulate cell growth, survival, differentiation, and motility (Diallo and Herrera 2022). STAT3 protein exists in a latent or inactive form in the cytoplasm (Diallo and Herrera 2022). STAT3 can be activated by receptor-associated kinases and phosphorylated at various phosphorylation sites, particularly at Tyr-705 and Ser-727 (Dai et al. 2022; Al-Kuraishy et al. 2023h). STAT3 protein is expressed at a basal level in cells but rapidly increases once activated by specific cytokines (Al-Kuraishy et al. 2022d). STAT3 is a critical factor in interleukin-6 (IL-6) induced gene regulation. STAT3 can be phosphorylated by IL-6 signal pathway, whereas IL-6 can also activate STAT3 at the transcriptional level (Al-Thomali et al. 2022b). STAT3 signaling pathway is exaggerated in SARS-CoV-2 infection leading to hyperinflammation, thrombosis, and lung fibrosis (Al-Kuraishy et al. 2022l). STAT3 impairs antiviral immune response and the development of lymphopenia (Al-Kuraishy et al. 2022d). Herein, targeting STAT3 in COVID-19 may mitigate hyperinflammation and related fatal complications (Batiha et al. 2022c). It has been revealed that the Nrf2 pathway negatively regulates STAT3 expression in rats with benign prostatic hypertrophy (Fishel et al. 2015). Downregulation of Nrf2 increases ferroptosis-induced ALI by activating STAT3 expression in mice (Wang et al. 2022). Therefore, the Nrf2 pathway plays a crucial role in attenuating inflammatory disorders in COVID-19 through inhibition of STAT3.
ADAM-metalloproteinase domain 17
ADAM-metalloproteinase domain 17 (ADAM17) is a ubiquitously expressed membrane-bound enzyme that mediates shedding of a wide variety of important regulators in inflammation including cytokines and adhesion molecules (Nadwa et al. 2023; Aleksova et al. 2021; Almishri et al. 2022; Al-Kuraishy and Al-Gareeb 2021a). ADAMs, similarly to MMPs, possess various physiological functions and the ability to regulate many processes such as cell migration, proliferation, angiogenesis, apoptosis, wound healing, and tissue repair and survival. ADAM17 activates TNF-α and sheds ACE2, facilitating SARS-CoV-2 entry (Nadwa et al. 2023; Aleksova et al. 2021; Almishri et al. 2022; Al-Kuraishy and Al-Gareeb 2021a). In spite of a lower ACE2 expression on cells surface, patients with cardiovascular disorders have a higher COVID-19 mortality rate, which is likely driven by the imbalance between ADAM17 protein which is required for cleavage of ACE2 ectodomain resulting in increased ACE2 shedding and TMPRSS2 which is required for spike glycoprotein priming (Aleksova et al. 2021; Almishri et al. 2022; Al-Kuraishy and Al-Gareeb 2021a). Although the membrane-bound form of ACE2 regulates the ACE2/Ang1-7 axis, the role of soluble ACE2 remains largely unclear (Al-Buhadily et al. 2021). It has been recognized that activating the Nrf2 pathway by butyrate releasers reduces COVID-19 severity by inhibiting the ADAM17 pathway (Paparo et al. 2022). Notably, the expression of ADAM17 was significantly increased in Nrf2-deficient macrophages in vivo and in vitro (Reddy et al. 2022). Therefore, promoting expression of Nrf2 reduces cardiovascular injury and inflammatory injury in COVID-19 patients.
These verdicts pointed out that the Nrf2 pathway is intricate with different signaling pathways to reduce the risk of OS and inflammatory disorders in COVID-19.
Nrf2 and renin angiotensin system
SARS-CoV-2 infection induces downregulation of ACE2, which involves the metabolism of AngII to angiotensin 1–7 (Ang1-7) (Al-Kuraishy et al. 2022o, 2023j, 2023k). This interaction leads to the overexpression of pro-inflammatory AngII and the reduction of anti-inflammatory Ang1-7 with subsequent development of ALI/ARDS (Al-Kuraishy et al. 2023l, 2022k, 2022u, 2023g). Deregulation of the RAS is associated with the development of OS and inflammation by inducing the expression of NADPH and pro-inflammatory cytokines, respectively (Al-Kuraishy et al. 2021m). A higher circulating AngII level inhibits endogenous antioxidant capacity and may inhibit the expression of the Nrf2 pathway (Al-Kuraishy et al. 2021n; Alkazmi et al. 2023a). Exaggerated AngII in rats with experimental kidney injury leads to OS by inhibiting the Nrf2 pathway (Uddin et al. 2021). Pepe et al. (Pepe et al. 2019) observed that induction of the Nrf2 pathway attenuates AngII-induced intestinal epithelial injury in mice. In addition, activators of the Nrf2 pathway can reduce exaggerated intra-renal AngII in diabetic patients and the development of diabetic nephropathy (Abdo et al. 2015). The SARS-CoV-2 viral spike protein binds to ACE2, which aids viral entrance into the host cell (Al-Kuraishy et al. 2023c, 2023e, 2022b; Alsaidan et al. 2023). In light of the probable elevation the expression of ACE2 by these drugs, there has been growing suspicion that ACE inhibitors and Ang II receptor blockers may raise the risk of the onset and severity of COVID-19 (Al-Kuraishy et al. 2022h). SARS-CoV-2, on the other hand, stimulates ACE2 shedding from the cell surface, downregulates ACE2 expression, and enhances ACE2 endocytosis, which raises Ang II concentration and lowering Ang-(1–7) (Al-Maiahy et al. 2021). Due to Ang II’s pro-inflammatory effects and the lack of Ang-(1–7)-mediated counter-regulation is probably significant in the pathophysiology of COVID-19 (Al-Kuraishy and Al-Gareeb 2021c). COVID-19 hypercoagulability may be caused by the prothrombotic effects of increased Ang II (Al-Kuraishy et al. 2023b). The COVID-19-associated vasculopathy may be facilitated by the upregulation of ACE/Ang II and downregulation of ACE2/Ang-(1–7) in the vascular endothelium (Al-kuraishy et al. 2023b). An updated study revealed that local RAS contribute to the pathogenesis and progression of diabetic nephropathy by exacerbating oxidative stress and inflammation (Razliqi et al. 2023). Activation of Nrf2 pathway by gentisic acid attenuates diabetic nephropathy in animal model study (Razliqi et al. 2023). Therefore, dysregulation of RAS in COVID-19 could be the possible reason behind the reduction of Nrf2 activity. Thus, AT1R blockers may be beneficial in preventing OS and inflammatory reactions via upregulation of the Nrf2 pathway (Karan et al. 2020).
Nrf2 and endothelial dysfunction
SARS-CoV-2 infection primarily affects the vascular endothelium leading to endothelial dysfunction and the development of coagulopathy (Alrouji et al. 2023b; Alkazmi et al. 2023b; Alomair et al. 2023). SARS-CoV-2 infects the endothelial cells directly due to the abundance expression of ACE2, causing cellular injury and apoptosis with subsequent reduction of endothelial cells’ capacity to release anti-thrombotic factors (Alomair et al. 2023). In addition, injury of pulmonary vascular endothelial cells by direct cytopathic effects of SARS-CoV-2 or due to OS and hyperinflammation lead to pulmonary micro-thrombosis, a prominent feature of COVID-19 (Moubarak et al. 2021; Batiha et al. 2023b; Al-Kuraishy and Al-Gareeb 2021b). Particularly, circulating endothelial cells and soluble intercellular adhesion molecule-1 levels are augmented in severely affected COVID-19 patients (Bonaventura et al. 2021). ED is a risk factor for developing micro-vascular dysfunction and immune thrombosis due to NETs formation and platelet activation (Bonaventura et al. 2021). It has been reported that activation of the Nrf2 pathway by ellagic acid prevents OS-induced ED in mice (Ding et al. 2014). Chen et al. (Chen et al. 2015) illustrated that Nrf2 activators decrease the propagation of ED in animal model studies. It has been demonstrated that resveratrol has an essential role in preventing the development of ED through the activation of the Nrf2 pathway (Parsamanesh et al. 2021). In addition, Nrf2 improves endothelial function by activating nitric oxide (NO) synthase and the release of NO (Luo et al. 2015). Furthermore, Nrf2 tempers the development of immune thrombosis and coagulopathy through the mitigation of hyperinflammation and OS, which are intricate in the propagation of ED and linked coagulopathy (Takahashi et al. 2020). In vitro study conducted by Takahashi et al. (Takahashi et al. 2020) demonstrated that Nrf2 plays an essential role in the prevention of coagulopathy by negative regulation of tissue plasminogen activator and fibrinolytic activity. As well, activation of Nrf2 reduces the risk of venous thrombosis by alleviating inflammatory changes and OS (Li et al. 2021). These verdicts suggested that the Nrf2 pathway may mitigate ED and associated coagulopathy in COVID-19.
Nrf2 and cytokine storm in COVID-19
SARS-CoV-2 can rapidly activate pathogenic Th1 cells to secrete pro-inflammatory cytokines, such as granulocyte–macrophage colony-stimulating factor (GM-CSF) and IL-6 (Al-Kuraishy et al. 2021d, 2021f, 2020b; Onohuean et al. 2021). GM-CSF further activates inflammatory monocytes to produce large quantities of IL-6, TNF-α, and other cytokines (Al-Kuraishy et al. 2020a). Membrane-bound immune receptors such as TLR4 may contribute to an imbalanced inflammatory response, and weak IFN-γ induction may be an important amplifier of cytokine production (Hussien et al. 2018). Together, the impaired acquired immune responses and unrestrained inflammatory innate responses to SARS-CoV-2 may cause cytokine storms (Rasheed et al. 2019; Song et al. 2020). It has been shown that the Nrf2 pathway inhibits the expression of pro-inflammatory cytokines and the progression of cytokine storm in COVID-19 (Zinovkin and Grebenchikov 2020). Particularly, Nrf2 has a role in regulating immune response and inflammation. It decreases the expression of pro-inflammatory cytokines, including MCP-1, TNF-α, IL-6, and IL-1β, by inhibiting the recruitment of RNA polymerase II and macrophage activation (Zhang et al. 2022). In critically affected COVID-19, Nrf2 activators decrease systemic inflammation and OS (McCord et al. 2020). Different experimental studies confirmed that Nrf2 activators inhibit the expression and release of pro-inflammatory cytokines (Motterlini et al. 2019; Thimmulappa et al. 2006). Similarly, Nrf2 activators attenuate the airway inflammatory process and ED development (Al-Kuraishy et al. 2019a, 2019b, 2022k). A clinical trial also revealed the protective effect of Nrf2 activators in preventing lung inflammation (Kobayashi et al. 2016). Furthermore, Nrf2 inhibits the activation of different inflammatory signaling pathways, including NLRP3 inflammasome, TLR4, HMBP1, NF-κB, and STAT3 that are involved in the development of cytokine storm (Ren et al. 2019). As well, Nrf2 blocks the OS pathway, which activates inflammatory signaling pathways like NF-κB and NLRP3 inflammasome (Ren et al. 2019). Similarly, Nrf2 inhibits abnormal and exaggerated immune response through inhibition of INF activation, thereby preventing the excessive release of pro-inflammatory cytokines (Bhaskar et al. 2020). Therefore, the Nrf2 activator could be effective in the attenuation of the SARS-CoV-2 infection-induced cytokine storm.
Nrf2 activators and KEAP1 inhibitors
Sources and mechanism of actions of Nrf2 activators are listed (Table 1). It has been reported that Nrf2 activators reduces airway inflammation as documented by many clinical trials (Al-Kuraishy et al. 2022o; Carlson et al. 2020; Müller et al. 2016). For example, a flavonoid sulforaphane inhibits SARS-CoV-2 infection-induced expression of IL-6 and IL-8 in bronchial epithelial cells (Gasparello et al. 2021). Sulforaphane hinders the interaction between SARS-CoV-2 spike protein and ACE2 with inhibition of the release of pro-inflammatory cytokines and development of cytokine storm (Gasparello et al. 2021). Kiser et al. (Kiser et al. 2021) revealed that sulforaphane inhibits the expression of NLRP3 inflammasome and NF-κB with a suppression effect on the development of cytokine storms. Therefore, sulforaphane could be a candidate for treating COVID-19 through modulation of OS and hyperinflammation.
Moreover, dimethyl fumarate, an approved drug for treating multiple sclerosis and psoriasis, inhibits inflammatory disorders in both Nrf2-dependent and independent pathways (Al-Kuraishy et al. 2023i). A case study of COVID-19 patients treated with dimethyl fumarate demonstrated that this drug had immunomodulatory effects that can prevent the development of cytokine storm (Mantero et al. 2021). Furthermore, dimethyl fumarate had antioxidant and anti-inflammatory effects with modulatory effects on the immune cells so that it can attenuate the development of cytokine storms (Timpani and Rybalka 2020). Dimethyl fumarate inhibits neutrophil migration, neutrophil-mediated ROS production, and pro-inflammatory cytokine expression (Müller et al. 2016). Remarkably, dimethyl fumarate competes with SARS-CoV-2 to bind nicotinic acetylcholine receptor, which is involved in the pathogenesis of SARS-CoV-2 infection and the development of dysautonomia (Simões et al. 2021). Therefore, dimethyl fumarate could effectively reduce the development of COVID-19-induced dysautonomia. Likewise, a hydrogen sulfide donor sodium thiosulfate induces activation of Nrf2 and used in the management of cyanide intoxications, has antiviral and anti-inflammatory properties, and could be effective against SARS-CoV-2 infection (Dai et al. 2021). Importantly, sodium thiosulfate inhibits OS by reducing the production of ROS. Sodium thiosulfate has a cytoprotective effect by inhibiting pro-inflammatory cytokines (Marutani et al. 2015). Sodium thiosulfate prevents pneumonia-induced ALI in children (Farese et al. 2011). Therefore, inhalation of sodium thiosulfate could efficiently decrease SARS-CoV-2 infection-induced ALI (Evgen’ev and Frenkel 2020).
Indeed, resveratrol, a plant polyphenol, activates Nrf2 and inhibits the KEAP1 pathway, improving the anti-inflammatory and antioxidant properties of the Nrf2 signaling pathway (Liao et al. 2021). Similarly, resveratrol augments endogenous antioxidant capacity independent of the Nrf2 signaling pathway (Liao et al. 2021). Consequently, resveratrol can be used as adjuvant therapy in managing COVID-19 patients through the alleviation of OS and inflammatory disorders (Russo et al. 2023). A study demonstrated that a diterpenoid lactone and rographolide inhibits the interaction between Nrf2 and KEAP1, leading to the upregulation of Nrf2 expression (Schulte et al. 2022). Therefore, rographolide could be effective against SARS-CoV-2 infection-induced OS.
These findings proposed that activation of Nrf2 by direct activators or inhibition of the KEAP1 pathway augment the anti-inflammatory and antioxidant effect of the Nrf2 pathway. In this state, activation of the Nrf2 pathway can attenuate the development of OS, hyperinflammation, and cytokine storm.
On the other hand, KEAP1 inhibitors increase the activity of anti-inflammatory and antioxidant effects mediated by Nrf2. KEAP1 inhibitors attenuate inflammatory and OS-mediated ALI (Duran et al. 2016). KEAP1 inhibitors like pentoxifylline and pirfenidone improve the antioxidant capacity and reduce COVID-19 severity in patients with ALI/ARDS (Chavarría et al. 2021; Hamidi et al. 2021). In addition, KEAP1 inhibitors prevent the activation of NF-κB and the release of pro-inflammatory cytokines (Bhandari et al. 2021).
Quercetin, a well-known antioxidant was studied in 152 outpatients suffering from COVID-19 (Singh et al. 2021). The randomized controlled and open-labeled study was carried out for 30 days to show that quercetin is helpful as an adjuvant to the standard treatment in COVID-19 patients (Singh et al. 2021). It was reported that during the initial stage of COVID-19 infection, quertcetin reduced the duration of hospitalization, the need for oxygen supplementation and deaths (Pierro et al. 2021). Quercetin activates Keap1-Nrf2 system and has been reported to mediate anti-inflammatory response (Qin and Hou 2016).
Therefore, Nrf2 activators and KEAP1 inhibitors may alleviate OS and inflammatory changes and prevent cytokine storm development in COVID-19. Indeed, there are a lot of Nrf2 activators, but most of them are not approved by FDA. Nevertheless, because of their anti-inflammatory and antioxidant properties, Nrf2 activators could be a possible adjuvant therapeutic strategy in managing severely affected COVID-19 patients.
Limitation
The present critical review had several limitations, including a shortage of clinical studies which intricate Nrf2 activators in the management of COVID-19 patients. Likewise, most clinical trials still do not endorse Nrf2 activators in the management of COVID-19. Targeting Nrf2 by modulators might be helpful in COVID-19. Though, before implementing this novel strategy in this current pandemic, we must address a number of important issues, including a clear concept of SARS-CoV-2–2 interactions, other impacts of downregulation of ACE2 in human lung, clear concepts on the metabolic reprogramming, and adaptation of immune cells such as macrophages and T cells, pharmacological activation of Nrf2and its impact on the viral entry into the host cell. Taken together, more research is necessary with adequate preclinical and clinical trials to establish this strategy.
Conclusions
SARS-CoV-2-induced OS triggers the activation of different signaling pathways, which counterbalances this complication. One of these pathways is Nrf2 which induces a series of cellular interactions to mitigate SARS-CoV-2-mediated viral toxicity and OS-induced cellular injury. The nrf2 pathway inhibits the expression of pro-inflammatory cytokines and the development of cytokine storm in COVID-19. Nrf2 activators could play an essential role in reducing SARS-CoV-2 infection-induced inflammation by suppressing NLRP3 inflammasome in COVID-19. Furthermore, Nrf2 activators can attenuate ED, RAS dysregulation, immune thrombosis, and coagulopathy. Therefore, this review suggests experimental, in vitro, preclinical, and clinical studies to confirm the possible therapeutic effects of Nrf2 activators alone or as adjuvant therapy in the management of COVID-19.
Data Availability
Not applicable.
Data availability
Not applicable.
References
Abdo S, Zhang S-L, Chan JS (2015) Reactive oxygen species and nuclear factor erythroid 2-related factor 2 activation in diabetic nephropathy: a hidden target. J Diabetes Metab 10:6(6):10.4172/2155-6156.1000547. https://doi.org/10.4172/2155-6156.1000547
Alam M, Ali S, Ashraf GM, Bilgrami AL, Yadav DK, Hassan MI (2022) Epigallocatechin 3-gallate: from green tea to cancer therapeutics. Food Chem 379:132135. https://doi.org/10.1016/j.foodchem
Al-Buhadily AK, Hussien NR, Al-Niemi MS, Al-Kuraishy HM, Al-Gareeb AI (2021) Misfortune and spy story in the neurological manifestations of Covid-19. JPMA J Pak Med Assoc 71(12):S157–S160
Aleksova A, Gagno G, Sinagra G, Beltrami AP, Janjusevic M, Ippolito G et al (2021) Effects of SARS-CoV-2 on cardiovascular system: the dual role of angiotensin-converting enzyme 2 (ACE2) as the virus receptor and homeostasis regulator-review. Int J Mol Sci 22(9):4526
Alkazmi L, Al-Kuraishy HM, Batiha GE-S, Mostafa-Hedeab G, De Waard M, Sabatier J-M et al (2022) Roxadustat for SARS-CoV-2 infection: old signaling raised new hopes. Drugs R&D 22(3):183–186
Alkazmi L, Al-kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Ahmed EA, Batiha GES (2023a) Dantrolene and ryanodine receptors in COVID-19: the daunting task and neglected warden. Clin Exp Pharmacol Physiol 50(5):335–352. https://doi.org/10.1111/1440-1681
Alkazmi L, Al-kuraishy HM, Al-Gareeb AI, Alexiou A, Papadakis M, Saad HM et al (2023b) The potential role of scavenger receptor B type I (SR-BI) in SARS-CoV-2 infection. Immun Inflamm Dis 11(4):e786
Al-Kuraishy HM, Al-Gareeb AI (2020) From SARS-CoV to nCoV-2019: Ruction and argument. Arch Clin Infect Dis 15(COVID-19)
Al-Kuraishy HM, Al-Gareeb AI (2021a) Acute kidney injury and COVID-19. Egypt J Intern Med 33(1):34. https://doi.org/10.1186/s43162-021-00064-x
Al-Kuraishy HM, Al-Gareeb AI (2021b) COVID-19 and acute kidney injury: a new perspective. Age (years) 1(30):42
Al-Kuraishy HM, Al-Gareeb AI (2021c) Covid-19 in Iraq: Events and wisdom. 71(Suppl 8)(12):S2-S3
Al-Kuraishy HM, Al-Gareeb AI, Abdullah SM, Cruz-Martins N, Batiha GE-S (2021a) Case report: hyperbilirubinemia in gilbert syndrome attenuates Covid-19-induced metabolic disturbances. Front Cardiovasc Med 8:642181
Al-Kuraishy HM, Al-Gareeb AI, Alblihed M, Cruz-Martins N, Batiha GE-S (2021b) COVID-19 and risk of acute ischemic stroke and acute lung injury in patients with type ii diabetes mellitus: the anti-inflammatory role of metformin. Front Med 8:110
Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Alexiou A, Batiha GE (2021c) Levamisole therapy in COVID-19. Viral Immunol 34(10):722–5
Al-Kuraishy HM, Al-Gareeb AI, Almulaiky YQ, Cruz-Martins N, Batiha GE-S (2021d) Role of leukotriene pathway and montelukast in pulmonary and extrapulmonary manifestations of Covid-19: the enigmatic entity. Eur J Pharmacol 904:174196
Al-Kuraishy HM, Al-Gareeb AI, Alqarni M, Cruz-Martins N, El-Saber Batiha G (2021e) Pleiotropic effects of tetracyclines in the management of COVID-19: emerging perspectives. Front Pharmacol 12:642822
Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Alexiou A, Batiha GE-S (2021f) Niclosamide for Covid-19: bridging the gap. Mol Biol Rep 48(12):8195–8202. https://doi.org/10.1007/s11033-021-06770-7
Al-Kuraishy HM, Al-Gareeb AI, Alzahrani KJ, Cruz-Martins N, Batiha GE-S (2021g) The potential role of neopterin in Covid-19: a new perspective. Mol Cell Biochem 476:4161–4166
Al-Kuraishy HM, Al-Gareeb AI, Atanu FO, El-Zamkan MA, Diab HM, Ahmed AS et al (2021h) Maternal transmission of SARS-CoV-2: safety of breastfeeding in infants born to infected mothers. Front Pediatr 9:738263
Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Al-Maiahy TJ, Cruz-Martins N, Batiha GE-S (2021i) The looming effects of estrogen in Covid-19: a rocky rollout. Front Nutr 8:649128
Al-Kuraishy HM, Al-Gareeb AI, Faidah H, Alexiou A, Batiha GE-S (2021j) Testosterone in COVID-19: an adversary bane or comrade boon. Front Cell Infect Microbiol 11:666987
Al-Kuraishy HM, Al-Gareeb AI, Mostafa-Hedeab G, Kasozi KI, Zirintunda G, Aslam A et al (2021k) Effects of β-blockers on the sympathetic and cytokines storms in Covid-19. Front Immunol 12:749291. https://doi.org/10.3389/fimmu.2021.749291
Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Atanu FO, Batiha GE-S (2021l) Arginine vasopressin and pathophysiology of COVID-19: an innovative perspective. Biomed Pharmacother 143:112193
Al-Kuraishy HM, Al-Rubiey HF, Al-Buhadily AK, Al-Gareeb AI (2021m) Anti-histamines and Covid-19: hype or hope. JPMA J Pak Med Assoc 71(12):144–148
Al-Kuraishy HM, Hussien NR, Al-Niemi MS, Al-Gareeb AI (2021n) Colchicine in the management of Covid-19: with or lieu of evidence. JPMA J Pak Med Assoc 71(12):S127–S132
Al-Kuraishy HM, Al-Gareeb AI, Shams HA, Al-Mamorri F (2019a) Endothelial dysfunction and inflammatory biomarkers as a response factor of concurrent coenzyme Q10 add-on metformin in patients with type 2 diabetes mellitus. J Lab Phys 11(04):317–322
Al-Kuraishy HM, Al-Kuraishi AH, Al-Windy S, Al-Gareeb AI (2019b) Toxoplasmosis and risk of endothelial dysfunction: role of oxidative stress and pro-inflammatory mediators. Arch Clin Infect Dis 14(6)
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Al-Buhadily AK, Al-Harchan NA, Lugnier C (2020a) COVID-19 and phosphodiesterase enzyme type 5 inhibitors. J Microsc Ultrastruct 8(4):141
Al-kuraishy HM, Al-Maiahy TJ, Al-Gareeb AI, Musa RA, Ali ZH (2020b) COVID-19 pneumonia in an Iraqi pregnant woman with preterm delivery. Asian Pac J Reprod 9(3):156–158
Al-Kuraishy HM, Al-Naimi MS, Lungnier CM, Al-Gareeb AI (2020c) Macrolides and COVID-19: an optimum premise. Biomed Biotechnol Res J 4(3):189
Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C (2020d) Is ivermectin–azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J (BBRJ) 4(Suppl 1):S101–S103
Al-Kuraishy HM, Sami OM, Hussain NR, Al-Gareeb AI (2020e) Metformin and/or vildagliptin mitigate type II diabetes mellitus induced-oxidative stress: the intriguing effect. J Adv Pharm Technol Res 11(3):142
Al-Kuraishy HM, Al-Buhadily AK, Al-Gareeb AI, Alorabi M, Hadi Al-Harcan NA, El-Bouseary MM et al (2022a) Citicoline and COVID-19: vis-à-vis conjectured. Naunyn-Schmiedeberg’s Arch Pharmacol 395(12):1463–1475
Al-Kuraishy HM, Al-Gareeb AI, Al-Hamash SM, Cavalu S, El-Bouseary MM, Sonbol FI et al (2022b) Changes in the blood viscosity in patients with SARS-CoV-2 infection. Front Med 9:876017
Al-Kuraishy HM, Al-Gareeb AI, Al-Hussaniy HA, Al-Harcan NAH, Alexiou A, Batiha GE-S (2022c) Neutrophil Extracellular Traps (NETs) and Covid-19: a new frontiers for therapeutic modality. Int Immunopharmacol 104:108516
Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ, Alexiou A, Mukerjee N, Batiha GE-S (2022d) An insight into the placental growth factor (PlGf)/angii axis in Covid-19: a detrimental intersection. Biotechnol Genet Eng Rev 1–20. https://doi.org/10.1080/02648725.2022.2122291
Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ, Alexiou A, Mukerjee N, Batiha GE-S (2022e) Prostaglandins and non-steroidal anti-inflammatory drugs in Covid-19. Biotechnol Genet Eng Rev 1–21. https://doi.org/10.1080/02648725.2022.2122290
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Alexiou A, Batiha GE-S (2022f) Calprotectin: the link between acute lung injury and gastrointestinal injury in Covid-19: Ban or boon. Curr Protein Pept Sci 23(5):310–320
Al-Kuraishy HM, Al-Gareeb AI, Al-Niemi MS, Aljowaie RM, Almutairi SM, Alexiou A et al (2022g) The Prospective effect of allopurinol on the Oxidative Stress Index and endothelial dysfunction in Covid-19. Inflammation 45(4):1651–1667. https://doi.org/10.1007/s10753-022-01648-7
Al-Kuraishy HM, Al-Gareeb AI, Albezrah NKA, Bahaa HA, El-Bouseary MM, Alexiou A et al (2022h) Pregnancy and COVID-19: high or low risk of vertical transmission. Clin Exp Med 23(4):957–967. https://doi.org/10.1007/s10238-022-00907-z
Al-Kuraishy HM, Al-Gareeb AI, Albogami SM, Jean-Marc S, Nadwa EH, Hafiz AA et al (2022i) Potential therapeutic benefits of metformin alone and in combination with sitagliptin in the management of type 2 diabetes patients with COVID-19. Pharmaceuticals 15(11):1361
Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Mukerjee N, Al-Hamash SMJ, Al-Maiahy TJ et al (2022l) 5-HT/CGRP pathway and Sumatriptan role in Covid-19. Biotechnol Genet Eng Rev 1–26. https://doi.org/10.1080/02648725.2022.2108996
Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE-S (2022j) Central effects of ivermectin in alleviation of Covid-19-induced dysautonomia. Curr Drug Targets 23(13):1277–1287
Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Batiha GE-S (2022k) Targeting and modulation of the natriuretic peptide system in Covid-19: a single or double-edged effect? Curr Protein Pept Sci 23(5):321–334
Al-Kuraishy HM, Al-Gareeb AI, Alkazmi L, Habotta OA, Batiha GE-S (2022m) High-mobility group box 1 (HMGB1) in COVID-19: extrapolation of dangerous liaisons. Inflammopharmacology 30(3):811–820
Al-Kuraishy HM, Al-Gareeb AI, Batiha GE-S (2022n) The possible role of ursolic acid in Covid-19: a real game changer. Clin Nutr ESPEN 47:414–417
Al-Kuraishy HM, Al-Gareeb AI, Bungau SG, Radu A-F, Batiha GE-S (2022o) The potential molecular implications of adiponectin in the evolution of SARS-CoV-2: Inbuilt tendency. J King Saud Univ-Sci 34(8):102347. https://doi.org/10.1016/j.jksus.2022.102347
Al-Kuraishy HM, Al-Gareeb AI, Butnariu M, Batiha GE-S (2022p) The crucial role of prolactin-lactogenic hormone in Covid-19. Mol Cell Biochem 477(5):1381–1392. https://doi.org/10.1007/s11010-022-04381-9
Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, Sonbol FI, Batiha GE-S (2022q) Hyperviscosity syndrome in COVID-19 and related vaccines: exploring of uncertainties. Clin Exp Med 23(3):679–688. https://doi.org/10.1007/s10238-022-00836-x
Al-kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GE-S (2022r) Dipyridamole and adenosinergic pathway in Covid-19: a juice or holy grail. Egypt J Med Hum Genet 23(1):140
Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E, Batiha GE-S (2022s) Nitazoxanide and COVID-19: a review. Mol Biol Rep 49(11):11169–11176
Al-Kuraishy HM, Al-Gareeb AI, Fageyinbo MS, Batiha GE-S (2022t) Vinpocetine is the forthcoming adjuvant agent in the management of COVID-19. Future Sci OA 8(5):FSO797
Al-Kuraishy HM, Al-Gareeb AI, Jalal NA, Kabrah SM, Alexiou A, Batiha GE-S (2022u) SARS-CoV-2 infection and C1-esterase inhibitor: camouflage pattern and new perspective. Curr Protein Pept Sci 23(7):465–474
Al-Kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Batiha GE-S (2022v) Hemolytic anemia in COVID-19. Ann Hematol 101(9):1887–1895
Al-Kuraishy HM, Al-Gareeb AI, Negm WA, Alexiou A, Batiha GE-S (2022w) Ursolic acid and SARS-CoV-2 infection: a new horizon and perspective. Inflammopharmacology 30(5):1493–1501
Al-Kuraishy HM, Al-Gareeb AI, Onohuean H, El-Saber Batiha G (2022x) COVID-19 and erythrocrine function: the roller coaster and danger. Int J Immunopathol Pharmacol 36:03946320221103151
Al-Kuraishy HM, Al-Gareeb AI, Qusty N, Alexiou A, Batiha GE-S (2022y) Impact of sitagliptin on non-diabetic Covid-19 patients. Curr Mol Pharmacol 15(4):683–692
Al-Kuraishy HM, Al-Gareeb AI, Al-Harcan NAH, Alexiou A, Batiha GE-S (2023a) Tranexamic acid and plasminogen/plasmin glaring paradox in COVID-19. Endocr Metab Immune Disord-Drug (Formerly Current Drug Targets-Immune, Endocrine & Metabolic Disorders) 23(1):35–45
Al-Kuraishy HM, Al-Gareeb AI, Alarfaj SJ, Al-Akeel RK, Faidah H, El-Bouseary MM et al (2023b) Long COVID and risk of erectile dysfunction in recovered patients from mild to moderate COVID-19. Sci Rep 13(1):5977
Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Papadakis M, Batiha GE-S (2023c) Heparanase is the possible link between monkeypox and Covid-19: robust candidature in the mystic and present perspective. AMB Express 13(1):1–13
Al-Kuraishy HM, Al-Gareeb AI, Alsayegh AA, Hakami ZH, Khamjan NA, Saad HM et al (2023d) A potential link between visceral obesity and risk of Alzheimer’s disease. Neurochem Res 48(3):745–766
Al-Kuraishy HM, Al-Gareeb AI, Eldahshan OA, Batiha GE-S (2023e) Oxytocin in diabetic Covid-19 patients: a new perspective. Nat Prod Res 37(11):1907–1908
Al-Kuraishy HM, Al-Gareeb AI, Mostafa-Hedeab G, Dubey R, Prabhakar PK, Batiha GE-S (2023f) COVID-19 and diabetes: will novel drugs for diabetes help in COVID-19? Curr Mol Pharmacol 16(4):494–506
Al-Kuraishy HM, Al-Gareeb AI, Rauf A, Alhumaydhi FA, Kujawska M, El-Saber Batiha G (2023g) Mechanistic insight and possible mechanism of seizure in Covid-19: the nuances and focal points. CNS Neurol Disord-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders) 22(6):875–83
Al-Kuraishy HM, Al-Gareeb AI, Saad HM, Batiha GE-S (2023h) Hippo-YAP signaling and SARS-CoV-2 infection: a new mechanistic pathway. Cell Stress Chaperones 28(2):121–123
Al-Kuraishy HM, Al-Gareeb AI, Saad HM, Batiha GE-S (2023i) The potential therapeutic effect of statins in multiple sclerosis: beneficial or detrimental effects. Inflammopharmacology 31(4):1671–1682. https://doi.org/10.1007/s10787-023-01240-x
Al-Kuraishy HM, Batiha GE-S, Al-Gareeb AI, Al-Harcan NAH, Welson NN (2023j) Receptor-dependent effects of sphingosine-1-phosphate (S1P) in COVID-19: the black side of the moon. Mol Cell Biochem 1–9. https://doi.org/10.1007/s11010-023-04658-7
Al-kuraishy HM, Al-Gareeb AI, Kaushik A, Kujawska M, Ahmed EA, Batiha GES (2023k) SARS-COV-2 infection and Parkinson’s disease: possible links and perspectives. J Neurosci Res 101(6):952–975
Al-kuraishy HM, Hussien NR, Al-Niemi MS, Fahad EH, Al-Buhadily AK, Al-Gareeb AI et al (2023l) SARS-CoV-2 induced HDL dysfunction may affect the host’s response to and recovery from COVID-19. Immun Inflamm Dis 11(5):e861
Alkhayyat SS, Al-Kuraishy HM, Al-Gareeb AI, El-Bouseary MM, AboKamer AM, Batiha GE-S et al (2022) Fenofibrate for COVID-19 and related complications as an approach to improve treatment outcomes: the missed key for Holy Grail. Inflamm Res 71(10–11):1159–1167
Al-Maiahy TJ, Al-Kuraishy HM, Al-Gareeb AI (2021) Pregnancy and risk of vertical transmission in Covid-19. J Pak Med Assoc 71(Suppl 8):S137–S143
Almishri W, Swain LA, D’Mello C, Le TS, Urbanski SJ, Nguyen HH (2022) ADAM metalloproteinase domain 17 regulates cholestasis-associated liver injury and sickness behavior development in mice. Front Immunol 12:779119
Alomair BM, Al-Kuraishy HM, Al-Gareeb AI, Al-Hamash SM, De Waard M, Sabatier J-M et al (2022a) Montelukast and acute coronary syndrome: the endowed drug. Pharmaceuticals 15(9):1147
Alomair BM, Al-Kuraishy HM, Al-Buhadily AK, Al-Gareeb AI, De Waard M, Elekhnawy E et al (2022b) Is sitagliptin effective for SARS-CoV-2 infection: false or true prophecy? Inflammopharmacology 30(6):2411–2415
Alomair BM, Al-Kuraishy HM, Al-Gareeb AI, Al-Buhadily AK, Alexiou A, Papadakis M et al (2023) Mixed storm in SARS-CoV-2 infection: a narrative review and new term in the Covid-19 era. Immun Inflamm Dis 11(4):e838
Alrouji M, Al-kuraishy HM, Al-Gareeb AI, Alexiou A, Papadakis M, Jabir MS et al (2023a) NF-κB/NLRP3 inflammasome axis and risk of Parkinson’s disease in Type 2 diabetes mellitus: a narrative review and new perspective. J Cell Mol Med 27(13):1775–1789. https://doi.org/10.1111/jcmm.17784
Alrouji M, Al-Kuraishy HM, Al-Gareeb AI, Elhadad H, Alexiou A, Papadakis M et al (2023b) Immunological interactions in helminths-SARS CoV-2 coinfection: could old enemy be a friend today? Parasite Immunol 45(5):e12982. https://doi.org/10.1111/pim.12982
Alsaidan AA, Al-Kuraishy HM, Al-Gareeb AI, Alexiou A, Papadakis M, Alsayed KA et al (2023) The potential role of SARS-CoV-2 infection in acute coronary syndrome and type 2 myocardial infarction (T2MI): Intertwining spread. Immun Inflamm Dis 11(3):e798
Al-Thomali AW, Al-Kuraishy HM, Al-Gareeb AI, A KA-B, De Waard M, Sabatier JM, et al (2022a) Role of neuropilin 1 in COVID-19 patients with acute ischemic stroke. Biomedicines 10(8):2032. https://doi.org/10.3390/biomedicines10082032
Al-Thomali AW, Al-Kuraishy HM, Al-Gareeb AI, K Al-buhadiliy A, De Waard M, Sabatier J-M et al (2022b) Role of neuropilin 1 in COVID-19 patients with acute ischemic stroke. Biomedicines 10(8):2032
Babalghith AO, Al-kuraishy HM, Al-Gareeb AI, De Waard M, Sabatier J-M, Saad HM et al (2022) The potential role of growth differentiation factor 15 in COVID-19: a corollary subjective effect or not? Diagnostics 12(9):2051
Basile MS, Cavalli E, McCubrey J, Hernández-Bello J, Muñoz-Valle JF, Fagone P et al (2022) The PI3K/Akt/mTOR pathway: a potential pharmacological target in COVID-19. Drug Discov Today 27(3):848–856
Batiha GE-S, Gari A, Elshony N, Shaheen HM, Abubakar MB, Adeyemi SB et al (2021a) Hypertension and its management in COVID-19 patients: the assorted view. Int J Cardiol Cardiovasc Risk Prev 11:200121
Batiha GE-S, Al-Kuraishy HM, Al-Gareeb AI, Welson NN (2022b) Pathophysiology of post-COVID syndromes: a new perspective. Virol J 19(1):158
Batiha GE-S, Moubarak M, Shaheen HM, Zakariya AM, Usman IM, Rauf A et al (2022c) Favipiravir in SARS-CoV-2 infection: is it worth it? Comb Chem High Throughput Screen 25(14):2413–2428
Batiha GE-S, Al-Kuraishy HM, Al-Gareeb AI, Alruwaili M, AlRuwaili R, Albogami SM et al (2023a) Targeting of neuroinflammation by glibenclamide in Covid-19: old weapon from arsenal. Inflammopharmacology 31(1):1–7
Batiha G-S, Shaheen H, Al-Kuraishy H, Teibo J, Akinfe O, Al-Garbee A et al (2021b) Possible mechanistic insights into iron homeostasis role of the action of 4-aminoquinolines (chloroquine/hydroxychloroquine) on COVID-19 (SARS-CoV-2) infection. Eur Rev Med Pharmacol Sci 25(23):7565–7584. https://doi.org/10.26355/eurrev_202112_27456
Batiha GE, Al-Gareeb AI, Rotimi D, Adeyemi OS, Al-Kuraishy HM (2022a) Common NLRP3 inflammasome inhibitors and Covid-19: divide and conquer. Sci Afr 18:e01407. https://doi.org/10.1016/j.sciaf.2022.e01407
Batiha GE-S, Al-Kuraishy HM, Al-Gareeb AI, Ashour NA, Negm WA (2023b) Potential role of tirzepatide towards Covid-19 infection in diabetic patients: a perspective approach. Inflammopharmacology 1–11
Battaglioni S, Benjamin D, Wälchli M, Maier T, Hall MN (2022) mTOR substrate phosphorylation in growth control. Cell
Battino M, Giampieri F, Pistollato F, Sureda A, de Oliveira MR, Pittalà V et al (2018) Nrf2 as regulator of innate immunity: a molecular Swiss army knife! Biotechnol Adv 36(2):358–370
Bhandari R, Khanna G, Kaushik D, Kuhad A (2021) Divulging the intricacies of crosstalk between NF-Kb and Nrf2-Keap1 pathway in neurological complications of COVID-19. Mol Neurobiol 58:3347–3361
Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS et al (2020) Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM consortium position paper. Front Immunol 11:1648
Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon DL, Van Tassell BW et al (2021) Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol 21(5):319–329
Canella R, Benedusi M, Martini M, Cervellati F, Cavicchio C, Valacchi G (2018) Role of Nrf2 in preventing oxidative stress induced chloride current alteration in human lung cells. J Cell Physiol 233(8):6018–6027
Cao L, Zhao J, Ma L, Chen J, Xu J, Rahman SU et al (2021) Lycopene attenuates zearalenone-induced oxidative damage of piglet sertoli cells through the nuclear factor erythroid-2 related factor 2 signaling pathway. Ecotoxicol Environ Saf 225:112737
Carlson J, Price L, Deng H (2020) Nrf2 and the Nrf2-interacting network in respiratory inflammation and diseases. Nrf2 and its Modulation in Inflammation 51–76
Chavarría AP, Vázquez RRV, Cherit JGD, Bello HH, Suastegui HC, Moreno-Castañeda L et al (2021) Antioxidants and pentoxifylline as coadjuvant measures to standard therapy to improve prognosis of patients with pneumonia by COVID-19. Comput Struct Biotechnol J 19:1379–1390
Chen B, Lu Y, Chen Y, Cheng J (2015) The role of Nrf2 in oxidative stress-induced endothelial injuries. J Endocrinol 225(3):R83-99
Chen Z, Zhong H, Wei J, Lin S, Zong Z, Gong F et al (2019) Inhibition of Nrf2/HO-1 signaling leads to increased activation of the NLRP3 inflammasome in osteoarthritis. Arthritis Res Ther 21(1):1–13
Cherupanakkal C, Ramachadrappa V, Kadhiravan T, Parameswaran N, Parija SC, Pillai AB et al (2017) Differential expression of NADPH oxidase-2 (Nox-2) and nuclear factor-erythroid 2-related factor 2 (Nrf2) transcripts in peripheral blood mononuclear cells isolated from dengue patients. VirusDisease 28:54–60
Coleman N, Naing A, Zhang S, Piha-Paul SAA, Tsimberidou AM, Janku F et al (2021) Phase I study of mTORC1/2 inhibitor sapanisertib (TAK-228) in combination with metformin in patients (pts) with mTOR/AKT/PI3K pathway alterations and advanced solid malignancies. Wolters Kluwer Health
Cuadrado A, Pajares M, Benito C, Jiménez-Villegas J, Escoll M, Fernández-Ginés R et al (2020) Can activation of NRF2 be a strategy against COVID-19? Trends Pharmacol Sci 41(9):598–610
da Costa RM, Rodrigues D, Pereira CA, Silva JF, Alves JV, Lobato NS et al (2019) Nrf2 as a potential mediator of cardiovascular risk in metabolic diseases. Front Pharmacol 10:382
Dai X, Shiraishi K, Muto J, Utsunomiya R, Mori H, Murakami M et al (2022) Nuclear IL-33 plays an important role in IL-31-mediated downregulation of FLG, keratin 1, and keratin 10 by regulating signal transducer and activator of transcription 3 activation in human keratinocytes. J Invest Dermatol 142(1):136–44. e3
Dai J, Teng X, Jin S, Wu Y (2021) The antiviral roles of hydrogen sulfide by blocking the interaction between SARS-CoV-2 and its potential cell surface receptors. Oxid Med Cell Longev 2021:7866992. https://doi.org/10.1155/2021/7866992
DeBlasi JM, DeNicola GM (2020) Dissecting the crosstalk between NRF2 signaling and metabolic processes in cancer. Cancers (Basel) 12(10):3023
Diallo M, Herrera F (2022) The role of understudied post-translational modifications for the behavior and function of signal transducer and activator of transcription 3. FEBS J 289(20):6235–6255
Ding Y, Zhang B, Zhou K, Chen M, Wang M, Jia Y et al (2014) Dietary ellagic acid improves oxidant-induced endothelial dysfunction and atherosclerosis: role of Nrf2 activation. Int J Cardiol 175(3):508–514
Do MH, Hur J, Choi J, Kim M, Kim MJ, Kim Y et al (2018) Eucommia ulmoides ameliorates glucotoxicity by suppressing advanced glycation end-products in diabetic mice kidney. Nutrients 10(3):265
Duran CG, Burbank AJ, Mills KH, Duckworth HR, Aleman MM, Kesic MJ et al (2016) A proof-of-concept clinical study examining the NRF2 activator sulforaphane against neutrophilic airway inflammation. Respir Res 17:1–4
El-Saber Batiha G, Al-Gareeb AI, Saad HM, Al-Kuraishy HM (2022) COVID-19 and corticosteroids: a narrative review. Inflammopharmacology 30(4):1189–1205. https://doi.org/10.1007/s10787-022-00987-z
Evgen’ev MB, Frenkel A (2020) Possible application of H2S-producing compounds in therapy of coronavirus (COVID-19) infection and pneumonia. Cell Stress Chaperones 25(5):713–715
Farese S, Stauffer E, Kalicki R, Hildebrandt T, Frey BM, Frey FJ et al (2011) Sodium thiosulfate pharmacokinetics in hemodialysis patients and healthy volunteers. Clin J Am Soc Nephrol: CJASN 6(6):1447
Fishel ML, Wu X, Devlin CM, Logsdon DP, Jiang Y, Luo M et al (2015) Apurinic/apyrimidinic endonuclease/redox factor-1 (APE1/Ref-1) redox function negatively regulates NRF2. J Biol Chem 290(5):3057–3068
Gan L, Johnson DA, Johnson JA (2010) Keap1-Nrf2 activation in the presence and absence of DJ-1. Eur J Neurosci 31(6):967–977
Gasparello J, D’Aversa E, Papi C, Gambari L, Grigolo B, Borgatti M et al (2021) Sulforaphane inhibits the expression of interleukin-6 and interleukin-8 induced in bronchial epithelial IB3-1 cells by exposure to the SARS-CoV-2 Spike protein. Phytomedicine 87:153583
Giordo R, Zinellu A, Eid AH, Pintus G (2021) Therapeutic potential of resveratrol in COVID-19-associated hemostatic disorders. Molecules 26(4):856
Gümüş H, Erat T, Öztürk İ, Demir A, Koyuncu I (2022) Oxidative stress and decreased Nrf2 level in pediatric patients with COVID-19. J Med Virol 94(5):2259–2264
Hamada S, Matsumoto R, Tanaka Y, Taguchi K, Yamamoto M, Masamune A (2021) Nrf2 activation sensitizes K-Ras mutant pancreatic cancer cells to glutaminase inhibition. Int J Mol Sci 22(4):1870
Hamidi SH, Kadamboor Veethil S, Hamidi SH (2021) Role of pirfenidone in TGF-β pathways and other inflammatory pathways in acute respiratory syndrome coronavirus 2 (SARS-Cov-2) infection: a theoretical perspective. Pharmacol Rep 73:712–727
Han R, Xiao J, Zhai H, Hao J (2016) Dimethyl fumarate attenuates experimental autoimmune neuritis through the nuclear factor erythroid-derived 2-related factor 2/hemoxygenase-1 pathway by altering the balance of M1/M2 macrophages. J Neuroinflammation 13(1):1–14
He M, Siow RC, Sugden D, Gao L, Cheng X, Mann GE (2011) Induction of HO-1 and redox signaling in endothelial cells by advanced glycation end products: a role for Nrf2 in vascular protection in diabetes. Nutr Metab Cardiovasc Dis 21(4):277–285
He T, Li X, Wang X, Xu X, Yan X, Li X et al (2020) Chemical composition and anti-oxidant potential on essential oils of Thymus quinquecostatus Celak. from Loess Plateau in China, regulating Nrf2/Keap1 signaling pathway in zebrafish. Sci Rep 10(1):1–18
Hikichi M, Mizumura K, Maruoka S, Gon Y (2019) Pathogenesis of chronic obstructive pulmonary disease (COPD) induced by cigarette smoke. J Thorac Dis 11(Suppl 17):S2129
Hussien NR, Al-Naimi MS, Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI (2018) Sulfonylurea and neuroprotection: the bright side of the moon. J Adv Pharm Technol Res 9(4):120–123
Hussien NR, Al-Niemi MS, Al-Kuraishy HM, Al-Gareeb AI (2021) Statins and Covid-19: the neglected front of bidirectional effects. J Pak Med Assoc 71(8):133
Iizuka Y, Inomata K, Nohara R, Miyawaki A, Watanabe Y (2021) Activation of intracellular antioxidant master gene Nrf2 by the extracts of Garcinia subelliptica, Ocimum gratissimum L., and Plectranthus ornatus. Int J Herb Med 9:54–60
Ishii T, Warabi E, Mann GE (2022) Mechanisms underlying Nrf2 nuclear translocation by non-lethal levels of hydrogen peroxide: p38 MAPK-dependent neutral sphingomyelinase2 membrane trafficking and ceramide/PKCζ/CK2 signaling. Free Radic Biol Med 191:191–202
Ivanciuc T, Sbrana E, Casola A, Garofalo RP (2018) Protective role of nuclear factor erythroid 2-related factor 2 against respiratory syncytial virus and human metapneumovirus infections. Front Immunol 9:854
Jia H, Zhang Y, Si X, Jin Y, Jiang D, Dai Z et al (2021) Quercetin alleviates oxidative damage by activating nuclear factor erythroid 2-related factor 2 signaling in porcine enterocytes. Nutrients 13(2):375
Jung K-A, Kwak M-K (2010) The Nrf2 system as a potential target for the development of indirect antioxidants. Molecules 15(10):7266–7291
Karam BS, Morris RS, Bramante CT, Puskarich M, Zolfaghari EJ, Lotfi-Emran S et al (2021) mTOR inhibition in COVID-19: a commentary and review of efficacy in RNA viruses. J Med Virol 93(4):1843–1846
Karan A, Bhakkiyalakshmi E, Jayasuriya R, Sarada D, Ramkumar KM (2020) The pivotal role of nuclear factor erythroid 2-related factor 2 in diabetes-induced endothelial dysfunction. Pharmacol Res 153:104601
Khalil HM, Eliwa HA, El-Shiekh RA, Al-Mokaddem AK, Hassan M, Tawfek AM et al (2021) Ashwagandha (Withania somnifera) root extract attenuates hepatic and cognitive deficits in thioacetamide-induced rat model of hepatic encephalopathy via induction of Nrf2/HO-1 and mitigation of NF-κB/MAPK signaling pathways. J Ethnopharmacol 277:114141
Khezri MR, Varzandeh R, Ghasemnejad-Berenji M (2022) The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell Mol Biol Lett 27(1):6
Kiser C, Gonul CP, Olcum M, Genc S (2021) Inhibitory effects of sulforaphane on NLRP3 inflammasome activation. Mol Immunol 140:175–185
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H et al (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7(1):11624
Korenori Y, Tanigawa S, Kumamoto T, Qin S, Daikoku Y, Miyamori K et al (2013) Modulation of N rf2/K eap1 system by W asabi 6-methylthiohexyl isothiocyanate in ARE-mediated NQO 1 expression. Mol Nutr Food Res 57(5):854–864
Lee S, Choi S-Y, Choo Y-Y, Kim O, Tran PT, Dao CT et al (2015) Sappanone A exhibits anti-inflammatory effects via modulation of Nrf2 and NF-κB. Int Immunopharmacol 28(1):328–336
Li L, Dong H, Song E, Xu X, Liu L, Song Y (2014) Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem Biol Interact 209:56–67
Li P, Lin B, Tang P, Ye Y, Wu Z, Gui S et al (2021) Aqueous extract of Whitmania pigra Whitman ameliorates ferric chloride-induced venous thrombosis in rats via antioxidation. J Thromb Thrombolysis 52:59–68
Liao M-T, Wu C-C, Wu S-FV, Lee M-C, Hu W-C, Tsai K-W et al (2021) Resveratrol as an adjunctive therapy for excessive oxidative stress in aging COVID-19 patients. Antioxidants 10(9):1440
Lim A, Radujkovic A, Weigand MA, Merle U (2021) Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann Intensive Care 11:1–13
Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu P et al (2017a) Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid Redox Signal 26(1):28–43
Liu Q, Lv H, Wen Z, Ci X, Peng L (2017b) Isoliquiritigenin activates nuclear factor erythroid-2 related factor 2 to suppress the NOD-like receptor protein 3 inflammasome and inhibits the NF-κB pathway in macrophages and in acute lung injury. Front Immunol 8:1518
Luo Z, Aslam S, Welch WJ, Wilcox CS (2015) Activation of nuclear factor erythroid 2–related factor 2 coordinates dimethylarginine dimethylaminohydrolase/PPAR-γ/endothelial nitric oxide synthase pathways that enhance nitric oxide generation in human glomerular endothelial cells. Hypertension 65(4):896–902
Macabrey D, Longchamp A, MacArthur MR, Lambelet M, Urfer S, Deglise S et al (2022) Sodium thiosulfate acts as a hydrogen sulfide mimetic to prevent intimal hyperplasia via inhibition of tubulin polymerisation. EBioMedicine 78:103954
Mantero V, Abate L, Basilico P, Balgera R, Salmaggi A, Nourbakhsh B et al (2021) COVID-19 in dimethyl fumarate-treated patients with multiple sclerosis. J Neurol 268(6):2023–2025
Marutani E, Yamada M, Ida T, Tokuda K, Ikeda K, Kai S et al (2015) Thiosulfate mediates cytoprotective effects of hydrogen sulfide against neuronal ischemia. J Am Heart Assoc 4(11):e002125
McCord JM, Hybertson BM, Cota-Gomez A, Geraci KP, Gao B (2020) Nrf2 activator PB125® as a potential therapeutic agent against COVID-19. Antioxidants 9(6):518
McFee R (2020) COVID-19 medical management including World Health Organization (WHO) suggested management strategies. Dis Mon 66(9):101068
Mendonca P, Soliman KF (2020) Flavonoids activation of the transcription factor Nrf2 as a hypothesis approach for the prevention and modulation of SARS-CoV-2 infection severity. Antioxidants 9(8):659
Mimura J, Inose-Maruyama A, Taniuchi S, Kosaka K, Yoshida H, Yamazaki H et al (2019) Concomitant Nrf2-and ATF4-activation by carnosic acid cooperatively induces expression of cytoprotective genes. Int J Mol Sci 20(7):1706
Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Jeandet P, Saad HM, Batiha GE-S (2022a) A raising dawn of pentoxifylline in management of inflammatory disorders in Covid-19. Inflammopharmacology 1–11
Mostafa-Hedeab G, Al-Kuraishy HM, Al-Gareeb AI, Welson NN, Batiha GE-S, Conte-Junior CA (2022b) Selinexor and COVID-19: the neglected warden. Front Pharmacol 13:884228. https://doi.org/10.3389/fphar.2022.884228
Motterlini R, Nikam A, Manin S, Ollivier A, Wilson JL, Djouadi S et al (2019) HYCO-3, a dual CO-releaser/Nrf2 activator, reduces tissue inflammation in mice challenged with lipopolysaccharide. Redox Biol 20:334–348
Moubarak M, Kasozi KI, Hetta HF, Shaheen HM, Rauf A, Al-Kuraishy HM et al (2021) The rise of SARS-CoV-2 variants and the role of convalescent plasma therapy for management of infections. Life 11(8):734
Müller S, Behnen M, Bieber K, Möller S, Hellberg L, Witte M et al (2016) Dimethylfumarate impairs neutrophil functions. J Invest Dermatol 136(1):117–126
Nadwa EH, Al-Kuraishy HM, Al-Gareeb AI, Elekhnawy E, Albogami SM, Alorabi M et al (2023) Cholinergic dysfunction in COVID-19: frantic search and hoping for the best. Naunyn-Schmiedeberg’s Arch Pharmacol 396(3):453–468
Nguyen V, Zhang Y, Gao C, Cao X, Tian Y, Carver W et al (2022) The spike protein of sars-cov-2 impairs lipid metabolism and increases susceptibility to lipotoxicity: Implication for a role of nrf2. Cells 11(12):1916
Onohuean H, Al-Kuraishy HM, Al-Gareeb AI, Qusti S, Alshammari EM, Batiha GE-S (2021) Covid-19 and development of heart failure: mystery and truth. Naunyn-Schmiedeberg’s Arch Pharmacol 394:2013–2021
Pall ML, Levine S (2015) Nrf2, a master regulator of detoxification and also antioxidant, anti-inflammatory and other cytoprotective mechanisms, is raised by health promoting factors. Sheng Li Xue Bao 67(1):1–18
Paparo L, Maglio MA, Cortese M, Bruno C, Capasso M, Punzo E et al (2022) A new butyrate releaser exerts a protective action against SARS-CoV-2 infection in human intestine. Molecules 27(3):862
Parsamanesh N, Asghari A, Sardari S, Tasbandi A, Jamialahmadi T, Xu S et al (2021) Resveratrol and endothelial function: a literature review. Pharmacol Res 170:105725
Pepe G, Basilicata MG, Carrizzo A, Adesso S, Ostacolo C, Sala M et al (2019) β-Lactoglobulin heptapeptide reduces oxidative stress in intestinal epithelial cells and angiotensin II-induced vasoconstriction on mouse mesenteric arteries by induction of nuclear factor erythroid 2-related factor 2 (Nrf2) translocation. Oxid Med Cell Longev 2019:1616239. https://doi.org/10.1155/2019/1616239
Di Pierro F, Derosa G, Maffioli P, Bertuccioli A, Togni S, Riva A et al (2021) Possible therapeutic effects of adjuvant quercetin supplementation against early-stage COVID-19 infection: a prospective, randomized, controlled, and open-label study. Int J Gen Med 14:2359–2366. https://doi.org/10.2147/IJGM.S318720
Qin S, Hou DX (2016) Multiple regulations of Keap1/Nrf2 system by dietary phytochemicals. Mol Nutr Food Res 60(8):1731–1755
Qu Y, Haas de Mello A, Morris DR, Jones-Hall YL, Ivanciuc T, Sattler RA et al (2023) SARS-CoV-2 inhibits NRF2-mediated antioxidant responses in airway epithelial cells and in the lung of a murine model of infection. Microbiol Spect 11(3):e0037823. https://doi.org/10.1128/spectrum.00378-23
Ramezani A, Nahad MP, Faghihloo E (2018) The role of Nrf2 transcription factor in viral infection. J Cell Biochem 119(8):6366–6382
Rasheed HA, Al-Kuraishy HM, Al-Gareeb AI, Hussien NR, Al-Nami MS (2019) Effects of diabetic pharmacotherapy on prolactin hormone in patients with type 2 diabetes mellitus: bane or boon. J Adv Pharm Technol Res 10(4):163
Razliqi RN, Ahangarpour A, Mard SA, Khorsandi L (2023) Gentisic acid protects against diabetic nephropathy in nicotinamide-streptozotocin administered male mice by attenuating oxidative stress and inflammation: the role of miR-200a/Keap1/Nrf2 pathway, renin-angiotensin system (RAS) and NF-кB. Chem Biol Interact 380:110507
Reddy PVB, Agudelo M, Atluri VS, Nair MP (2012) Inhibition of nuclear factor erythroid 2-related factor 2 exacerbates HIV-1 gp120-induced oxidative and inflammatory response: role in HIV associated neurocognitive disorder. Neurochem Res 37:1697–1706
Reddy NM, Tamatam CM, Aparna A, Reddy SP (2022) Nrf2 is required for optimal alveolar-macrophage-mediated apoptotic neutrophil clearance after oxidant injury. Antioxidants 11(2):212
Ren L, Zhan P, Wang Q, Wang C, Liu Y, Yu Z et al (2019) Curcumin upregulates the Nrf2 system by repressing inflammatory signaling-mediated Keap1 expression in insulin-resistant conditions. Biochem Biophys Res Commun 514(3):691–698
Rhee SG, Bae SH (2015) The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1. Free Radic Biol Med 88:205–211
Ruiz S, Pergola PE, Zager RA, Vaziri ND (2013) Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83(6):1029–1041
Rushworth SA, Shah S, MacEwan DJ (2011) TNF mediates the sustained activation of Nrf2 in human monocytes. J Immunol 187(2):702–707
Russo C, Valle MS, Malaguarnera L, Romano IR, Malaguarnera L (2023) Comparison of vitamin D and resveratrol performances in COVID-19. Nutrients 15(11):2639
Ryan DG, Knatko EV, Casey AM, Hukelmann JL, Naidu SD, Brenes AJ et al (2022) Nrf2 activation reprograms macrophage intermediary metabolism and suppresses the type I interferon response. Iscience 25(2):103827. https://doi.org/10.1016/j.isci.2022.103827
Saha S, Buttari B, Panieri E, Profumo E, Saso L (2020) An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 25(22):5474
Schulte B, König M, Escher BI, Wittenburg S, Proj M, Wolf V et al (2022) Andrographolide derivatives target the KEAP1/NRF2 axis and possess potent anti-SARS-CoV-2 activity. ChemMedChem 17(5):e202100732
Simões JLB, de Araújo JB, Bagatini MD (2021) Anti-inflammatory therapy by cholinergic and purinergic modulation in multiple sclerosis associated with SARS-CoV-2 infection. Mol Neurobiol 58:5090–5111
Sindhu RK, Verma R, Salgotra T, Rahman MH, Shah M, Akter R et al (2021) Impacting the remedial potential of nano delivery-based flavonoids for breast cancer treatment. Molecules 26(17):5163
Singh E, Matada GSP, Abbas N, Dhiwar PS, Ghara A, Das A (2021) Management of COVID-19-induced cytokine storm by Keap1-Nrf2 system: a r eview. Inflammopharmacology 29(5):1347–1355
Song P, Li W, Xie J, Hou Y, You C (2020) Cytokine storm induced by SARS-CoV-2. Clin Chim Acta 509:280–287
Sugimoto M, Ko R, Goshima H, Koike A, Shibano M, Fujimori K (2021) Formononetin attenuates H2O2-induced cell death through decreasing ROS level by PI3K/Akt-Nrf2-activated antioxidant gene expression and suppressing MAPK-regulated apoptosis in neuronal SH-SY5Y cells. Neurotoxicology 85:186–200
Takahashi T, Nakano T, Katoh G, Shinoda Y, Yamamoto C, Yoshida E et al (2020) Nuclear factor erythroid 2-related factor 2 (NRF2) is a negative regulator of tissue plasminogen activator synthesis in cultured human vascular endothelial EA. hy926 cells. J Toxicol Sci 45(4):237–43
Thimmulappa RK, Scollick C, Traore K, Yates M, Trush MA, Liby KT et al (2006) Nrf2-dependent protection from LPS induced inflammatory response and mortality by CDDO-Imidazolide. Biochem Biophys Res Commun 351(4):883–889
Timpani CA, Rybalka E (2020) Calming the (Cytokine) storm: dimethyl fumarate as a therapeutic candidate for COVID-19. Pharmaceuticals 14(1):15
Uddin MJ, Kim EH, Hannan MA, Ha H (2021) Pharmacotherapy against oxidative stress in chronic kidney disease: promising small molecule natural products targeting nrf2-ho-1 signaling. Antioxidants 10(2):258
Vomund S, Schäfer A, Parnham MJ, Brüne B, Von Knethen A (2017) Nrf2, the master regulator of anti-oxidative responses. Int J Mol Sci 18(12):2772
Wang Y, Wang X, Zhang L, Zhang R (2018) Alleviation of acute lung injury in rats with sepsis by resveratrol via the phosphatidylinositol 3-kinase/nuclear factor-erythroid 2 related factor 2/heme oxygenase-1 (PI3K/Nrf2/HO-1) pathway. Med Sci Monit: Int Med J Exp Clin Res 24:3604
Wang Y, Chen D, Xie H, Jia M, Sun X, Peng F et al (2022) AUF1 protects against ferroptosis to alleviate sepsis-induced acute lung injury by regulating NRF2 and ATF3. Cell Mol Life Sci 79(5):228
Wu A-G, Yong Y-Y, Pan Y-R, Zhang L, Wu J-M, Zhang Y et al (2022) Targeting Nrf2-mediated oxidative stress response in traumatic brain injury: therapeutic perspectives of phytochemicals. Oxid Med Cell Longev 2022. https://doi.org/10.1155/2022/1015791
Wyler E, Franke V, Menegatti J, Kocks C, Boltengagen A, Praktiknjo S et al (2019) Single-cell RNA-sequencing of herpes simplex virus 1-infected cells connects NRF2 activation to an antiviral program. Nat Commun 10(1):4878
Xingyue L, Shuang L, Qiang W, Jinjuan F, Yongjian Y (2021) Chrysin ameliorates sepsis-induced cardiac dysfunction through upregulating Nfr2/Heme oxygenase 1 pathway. J Cardiovasc Pharmacol 77(4):491–500
Yagishita Y, Fahey JW, Dinkova-Kostova AT, Kensler TW (2019) Broccoli or sulforaphane: is it the source or dose that matters? Molecules 24(19):3593
Yan J, Li J, Zhang L, Sun Y, Jiang J, Huang Y et al (2018) Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radic Biol Med 121:78–85
Yu C, Xiao J-H (2021) The Keap1-Nrf2 system: a mediator between oxidative stress and aging. Oxid Med Cell Longev 2021:1–16
Yu Y, Yang Y, Yang M, Wang C, Xie K, Yu Y (2019) Hydrogen gas reduces HMGB1 release in lung tissues of septic mice in an Nrf2/HO-1-dependent pathway. Int Immunopharmacol 69:11–18
Zhang S, Wang J, Wang L, Aliyari S, Cheng G (2022) SARS-CoV-2 virus NSP14 Impairs NRF2/HMOX1 activation by targeting Sirtuin 1. Cell Mol Immunol 19(8):872–882
Zhong M, Lynch A, Muellers SN, Jehle S, Luo L, Hall DR et al (2019) Interaction energetics and druggability of the protein–protein interaction between Kelch-like ECH-Associated Protein 1 (KEAP1) and Nuclear Factor Erythroid 2 Like 2 (Nrf2). Biochemistry 59(4):563–581
Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X et al (2016) Sulforaphane protects against rotenone-induced neurotoxicity in vivo: involvement of the mTOR, Nrf2 and autophagy pathways. Sci Rep 6(1):32206
Zhu D-D, Tan X-M, Lu L-Q, Yu S-J, Jian R-L, Liang X-F et al (2021) Interplay between nuclear factor erythroid 2-related factor 2 and inflammatory mediators in COVID-19-related liver injury. World J Gastroenterol 27(22):2944
Zinovkin R, Grebenchikov O (2020) Transcription factor Nrf2 as a potential therapeutic target for prevention of cytokine storm in COVID-19 patients. Biochem Mosc 85:833–837
Acknowledgements
This work was supported by the Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, Saudi Arabia [Grant No. GRANT4,132].
Funding
Open Access funding enabled and organized by Projekt DEAL. Open Access funding enabled and organized by Projekt DEAL. This work was supported by the University of Witten-Herdecke Germany.
Author information
Authors and Affiliations
Contributions
RSH and HMA-K conceptualized the manuscript, wrote, edited and reviewed the main text, and approved the final edition of the manuscript. AA, MP, EAA, and GE-SB wrote, corrected, amended, and approved the final edition of the manuscript. HMS prepared the figures, wrote, corrected, amended, and approved the final edition of the manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors consent for publication.
Conflict of interest
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hamad, R.S., Al-kuraishy, H.M., Alexiou, A. et al. SARS-CoV-2 infection and dysregulation of nuclear factor erythroid-2-related factor 2 (Nrf2) pathway. Cell Stress and Chaperones 28, 657–673 (2023). https://doi.org/10.1007/s12192-023-01379-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12192-023-01379-0