Abstract
Background
Trastuzumab and trastuzumab emtansine are specific antibody and antibody–drug conjugates used in the treatment of human epidermal growth factor receptor 2 (HER2) positive metastatic breast cancer. The aim of this study was to test their effect on the QTc interval duration and left ventricular ejection fraction (LVEF) in our patients, two parameters used in evaluation of cardiotoxicity. From May 2015 to October 2017, 26 patients with preserved LVEF were included in the study. All of them were previously treated with standard paclitaxel and cisplatin-based chemotherapy regimens. Electrocardiogram (ECG) was recorded just before each trastuzumab dose application and six months after the last dose. Echocardiography with LVEF measurement was performed several days before the application of the initial dose, and six months after the last cycle. Later, 24 patients with metastatic disease received additional treatment with trastuzumab emtansine after six months and the same ECG and echocardiography protocol was performed again. Due to reduction in LVEF, two patients were discontinued from additional treatment.
Results
A statistically significant QTc prolongation was found after each drug dose application, with an increase in mean QTc duration with every successive application, reaching the peak QTc values just before the fifth cycle of treatment. The QTc interval returned to its initial value six months after the last cycle (p < 0.001). These results were similar for both drugs. Mean LVEF before both treatment protocols was significantly higher compared to LVEF value after the treatment. LVEF before trastuzumab emtansine treatment was non-significantly higher than LVEF after trastuzumab treatment.
Conclusion
Trastuzumab and trastuzumab emtansine cardiotoxicity manifested as a significant and progressive QTc prolongation after successive drug applications, reaching the peak value just before the fifth cycle of both drugs. Both medications also caused statistically significant but asymptomatic LVEF reduction. Complete reversibility of cardiotoxic effects of both drugs was confirmed by QTc interval and LVEF normalisation after the treatment discontinuation.
Similar content being viewed by others
Background
Breast cancer is the most frequently diagnosed cancer in women worldwide, with the highest mortality rate among malignant diseases [1]. Twenty percent of breast cancers overexpress human epidermal growth factor receptor 2 (HER2), a transmembrane glycoprotein that serves as epidermal growth factor receptor (EGFR) with tyrosine-kinase activity. Therapies targeting HER2 are paramount in metastatic breast cancer management [2]. Improved survival in patients with metastatic HER2-positive breast cancer warrants their use in first-line and subsequent line treatment [3]. There are four HER2-directed agents: trastuzumab—a monoclonal antibody that binds the extracellular domain of HER2, ado-trastuzumab emtansine (T-DM1)—an antibody–drug conjugate composed of trastuzumab, a thioether linker, and a derivative of antimitotic agent maytansine, and also lapatinib and pertuzumab [4]. Randomized trials suggest non-inferiority of trastuzumab monotherapy in comparison to the combination of trastuzumab and chemotherapy [5,6,7]. Patients who relapse within six months of completing adjuvant trastuzumab therapy are eligible for ado-trastuzumab emtansine treatment [8]. Trastuzumab is associated with cardiotoxic risk, mostly manifested as an asymptomatic decrease in left ventricular ejection fraction (LVEF), and less commonly as symptomatic heart failure [9,10,11]. Two types of chemotherapy-related cardiac dysfunction have been described. Type I, in association with anthracycline use, manifests as partial myocyte destruction and clinical heart failure, while type II, associated with trastuzumab use, shows myocardial hibernation, probably not associated with myocyte death or clinical heart failure, and is therefore reversible [12]. Incidence of trastuzumab-related cardiotoxicity varies according to other comorbidities [13]. Important risk factors that contribute to trastuzumab-related cardiotoxicity are: age above 50 years, previous anthracycline use, and obesity [14,15,16,17,18]. Due to the high incidence rate of trastuzumab-related cardiotoxicity in patients with metastatic breast cancer, it is recommended to assess cardiac function (LVEF) before treatment, and in case of new onset heart failure symptoms [9, 11, 14, 19]. It is also recommended to obtain and analyze the electrocardiogram (ECG) before every trastuzumab and trastuzumab emtansine treatment. The long-term effect of trastuzumab on QT dispersion (QTd) was investigated in a pilot study showing significantly higher QTd in patients treated with trastuzumab after anthracycline-based regimen compared to patients treated with anthracycline-based regimen only (0.064 ± 0.023 s vs. 0.051 ± 0.016, p = 0.034) [20]. Also, mean increases in QTd and QTcd are significantly different in patients treated with paclitaxel-trastuzumab combination (0.021 ± 0.011 and 0.022 ± 0.014 s, respectively) compared to those treated with anthracycline-based regimen (0.005 ± 0.003 and 0.006 ± 0.008 s, respectively) in the same group (p = 0.0246) (20). Trastuzumab in long-term management can significantly prolong the QT interval, while a significant QT interval prolongation was not previously documented in trastuzumab emtansine treatment protocols [20, 21]. The aim of this study was to determine the effects of trastuzumab and trastuzumab emtansine on the QTc interval and LVEF.
Methods
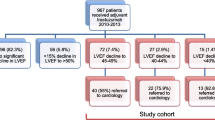
In this prospective cohort study, we investigated 65 women with metastatic (HER2+) breast cancer treated in our Department of Medical Oncology, University Hospital Centre Zagreb, Croatia. All patients underwent treatment with the standard chemotherapy regimen which included paclitaxel and cisplatin. Thirty-nine women were excluded from the second-line treatment due to poor clinical condition or fatal outcome. We gathered data from 26 patients who continued treatment with the specific antibody drug. The average age of our subjects was 57.96 years (± 7.48 years). Eligible patients were treated with six cycles of trastuzumab, as a first-line antibody drug treatment, followed by six cycles of trastuzumab emtansine as a second-line antibody drug treatment protocol, which is the standard in our institution. Both drugs were administered subcutaneously. Only 24 patients with preserved LVEF received the second-line treatment with the antibody drug, trastuzumab emtansine after six months since two patients had LVEF reduction, In addition, one patient developed left bundle branch block, and the other one reached the age limit (70 years). The electrocardiogram was obtained and analyzed before each drug application, and six months after the last application of trastuzumab and trastuzumab emtansine (mean heart rate 72 ± 12/min). Corrected QT values were calculated using Fridericia’s formula (QTc = QT interval/3√(60/heart rate). At the time of the ECG recordings, the patients were not on any antiarrhythmics, beta blockers, psychoactive drugs or antibiotics that could influence QT interval duration. All patients had normal serum sodium and potassium levels. Three women had diabetes and were treated with insulin, four women had arterial hypertension and were treated with the ACE inhibitor Ramipril and the Calcium channel blocker Amlodipine. The other patients had no comorbidities. Before the first application of trastuzumab and trastuzumab emtansine, as well as six months after the last cycle, an echocardiogram was performed in order to assess LVEF using the biplane Simpson’s method. Data was presented as frequencies, mean with standard deviation (SD) and median with 5th and 95th percentiles, as appropriate. For statistical analysis we used Student's t-test of paired samples to compare QTc intervals before each cycle and after the last application of both drugs. The Bayesian Pearson correlation test was used to examine the correlation between QTc intervals and LVEF. A statistically significant p-value of < 0.05 was used.
Results
ECG tracing was obtained before each drug application and six months after the last cycle in 26 patients treated with trastuzumab, and 24 patients treated with trastuzumab emtansine. The results of ECG analysis are shown below (Tables 1, 2, 3 and 4).
QTc interval duration showed progressive prolongation with each successive trastuzumab application, with insignificant shortening of the QTc6 interval, measured before the 6th trastuzumab application, while QTc values six months after the last application (QTc7) returned back to the initial values. QTc intervals also showed progressive prolongation with each successive trastuzumab emtansine application, with the exception of QTc6 interval shortening (obtained before the 6th trastuzumab emtansine application), while QTc values six months after the last application returned approximately to the initial values.
Student's t-test of paired samples was used to compare QTc interval values before each trastuzumab cycle and after the completion of therapy. A significant QT interval prolongation with every trastuzumab application was found, with a mean QTc1 duration of 449.4 ± 23.26 ms, QTc2 (before the second cycle) of 452.4 ± 20.73 ms, QTc3 (before the third cycle) 455.1 ms (± 21.08 ms), QTc4 (before the fourth cycle) 472.1 ms (± 32.08 ms), QTc5 (before the fifth cycle) 473 ms (± 27.79 ms) (p < 0,001). An insignificant shortening of the QTc6 interval before the sixth trastuzumab application was found, mean 471.3 ms (± 28.58 ms), while the mean QTc value six months after the last cycle (QTc7) returned almost to starting values 453 ms (± 20.42 ms) (p < 0,001) (Table 3).
Using the Bayesian Pearson correlation test with positive correlation presumption, we compared QTc1 interval values with LVEF values measured before trastuzumab treatment, and found no positive correlation; results show an insignificant negative correlation (r = − 0.125, BF10 = 0.162). In the comparison of QTc7 interval values with EF2 values measured after trastuzumab treatment, there was also no positive correlation found; results show an insignificant negative correlation (r = − 0.171, BF10 = 0.143). When comparing QTc1 interval values with EF1 values measured before trastuzumab emtansine treatment, results showed an insignificant negative correlation (r = − 0.108, BF10 = 0.286). Also, in comparison of QTc7 interval values with EF2 values measured after trastuzumab emtansine treatment, we found an insignificant negative correlation (r = − 0.170, BF10 = 0.341).
Discussion
Results of ECG analysis in 26 patients treated with trastuzumab and 24 patients who continued with trastuzumab emtansine treatment showed no significant effect of the given treatment on PQ and QRS interval values (Tables 1 and 2), similar to previous findings [22]. In addition, an earlier study on pertuzumab effects on PQ and QRS intervals also found no significant drug effect on the aforementioned intervals [23]. Other published data show discordant results regarding QTc interval prolongation after trastuzumab and trastuzumab emtansine treatment. Several studies found no clinically relevant effect on QTc in relation to trastuzumab treatment [22], while other publications found significant QT and QTc interval prolongation in patients with breast cancer, as a side effect of both acute and long-term treatment with trastuzumab [20, 24]. The risk of trastuzumab-related cardiac dysfunction was found to be higher in patients previously treated with anthracyclines [14,15,16,17,18]. It is important to emphasize that our patients were pretreated with paclitaxel and cisplatin before start of trastuzumab and trastuzumab emtansine. Studies have also shown an association between paclitaxel and ventricular arrhythmias, bradycardia, several degrees of atrioventricular conduction block, bundle branch block (effects mediated by paclitaxel vehicle Cremophor EL) as well as cardiac ischemia. In protocols involving the combination of doxorubicin and paclitaxel, cardiotoxicity was attributed to doxorubicin alone. Cisplatin has been shown to be associated with an increased risk of thrombotic events, deep vein thrombosis and pulmonary embolism, although specific cardiotoxicity is rarely reported [25]. In patients treated with doxorubicin and trastuzumab, results suggest a combined cardiotoxicity effect associated with the cumulative dose of doxorubicin; concurrent application of both drugs is expected to be safe if cumulative dose is limited to 180 mg/m2 or less [26,27,28,29]. Anthracycline induced-cardiotoxicity varies from 4% to over 36% in patients receiving a dose of 500–550 mg/m2. Main cardiotoxic mechanism is mediated by free radical formation, while apoptosis plays a significant role in myocardial cell loss. Cardiotoxicity can be categorized into acute (transient decline in myocardial contractility immediately after the infusion, incidence < 1%), early onset chronic progressive toxicity (within the first year from the completion of treatment, incidence 1.6–2.1%) or late onset chronic progressive toxicity (presenting as dilated cardiomyopathy (CMP) at least one year following the completion of therapy) [25]. The incidence of trastuzumab-related heart failure has been found to be 2–7%, with an increase to 27% with prior treatment with anthracyclines, if trastuzumab is used concurrently with anthracycline plus cyclophosphamide. Trastuzumab toxicity is not dose-dependent and is frequently reversible, in contrast to anthracycline cardiotoxicity [25]. There is currently not enough data on cardiotoxicity pattern of trastuzumab emtansine, not even from a trial including a large number of patients However, more adverse events were associated with trastuzumab emtansine than with trastuzumab alone. [30]
Our study found a significant QTc interval prolongation with every cycle of trastuzuma (Table 3, Fig. 1). An insignificant shortening of the QTc6 interval, before the sixth trastuzumab treatment, was found, while QTc values six months after the last cycle returned to almost starting values (mean QTc7 453 ms ± 20.42 ms), (Table 3, Fig. 1). A significant QTc interval prolongation was found after the very first trastuzumab cycle, and the QTc interval continued to prolong after each new dose, reaching the peak value before the fifth application (mean QTc5 473 ± 27.79 ms), (Table 3, Fig. 1). According to previous research, the mechanism of trastuzumab cardiotoxicity is presumed to be type II toxicity, which is not associated with myocyte death. Therefore, it was expected for QTc7 interval values (six months after the last dose of trastuzumab) to return to the initial values. We confirmed these findings from the previous studies regarding reversibility of trastuzumab-related cardiotoxic effects [12]. The results of one multicenter study on the safety and pharmacokinetic characteristics of trastuzumab emtansine in female patients with HER2-positive metastatic breast cancer showed a clinically irrelevant effect on QTc interval [21]. Our study, on the other hand, showed a progressive prolongation of the QTc interval duration with each trastuzumab emtansine cycle reaching the highest values before the fifth cycle with a mean of 474.5 ms (± 28.95 ms). This was followed by shortening of the QTc6 interval before the sixth trastuzumab emtansine application to a mean of 471.9 ms (± 25.56 ms). Finally the QTc values six months after the last application returned approximately to the basal values of QTc7 with a mean of 454.4 ms (± 16.91 ms) (Table 4, Fig. 1). These results are similar to the previously mentioned data for trastuzumab alone (Fig. 1). The QTc interval after trastuzumab emtansine administration significantly increased after the first drug treatment and then continued to do so with each successive drug cycle, reaching the peak value before the fifth cycle and then significantly decreasing before the sixth cycle, which is in contrast to the same cycle of trastuzumab alone. Six months posttreatment, the QTc interval values also returned to approximately same starting values (Fig. 1). Based on these results, we presume that trastuzumab emtansine has the same type II cardiotoxicity effect as trastuzumab, without myocyte death in pathogenesis [12]. Many studies have shown an asymptomatic decrease in LVEF as the most common trastuzumab-related cardiotoxicity effect, less often presenting as clinical heart failure [9,10,11]. Our results show statistically significant LVEF decrease after the trastuzumab treatment, in comparison to initial pretreatment values (EF1 mean 64.69 ± 4.84 vs EF2 mean 60.58 ± 6.58, t = 4.96, df = 25, p < 0.001), but this reduction was also asymptomatic in our patient cohort (Tables 5 and 6, Fig. 2). A similar cardiotoxic effect was found after trastuzumab emtansine treatment, with a statistically significantly lower LVEF following the protocol, in comparison to initial values (EF1 mean 61.75, SD 3.85, EF2 mean 59.54, SD 4.12, t = 7.65, df = 23, p < 0.001) (Tables 5 and 6, Fig. 2 but with no clinical repercussions). In our study, we have shown statistically significant effect on LVEF reduction related to both drugs (Fig. 2). One randomized trial of first-line trastuzumab emtansine versus trastuzumab and docetaxel reported an asymptomatic decline in LVEF [31]. Our results also demonstrate a significantly higher LVEF before trastuzumab than before trastuzumab emtansine treatment, and an insignificantly higher LVEF after trastuzumab than after trastuzumab emtansine treatment, possibly reflecting a cumulative cardiotoxic effect of these drugs. As LVEF values before trastuzumab emtansine were insignificantly higher than LVEF after trastuzumab treatment (LVEF2 T mean 60.58, SD 6.58, LVEF1 TE mean 61.75, SD 3.85, p = 0.29), we could speculate some degree of LVEF recovery after the trastuzumab treatment (Table 6, Fig. 2). In addition, many studies have shown trastuzumab-related toxicity to be mostly reversible [10, 17, 32,33,34]. As expected, our results did not show any positive correlation between QT interval and LVEF, before or after both drug protocols. An insignificant negative correlation was found instead. Finally, it is important to notice that most of our patients with metastatic breast cancer, considering their clinical condition, also received other symptomatic therapy, such as antidepressants (amitriptyline, desipramine, imipramine, fluoxetin), antipsychotics (sertindole, ziprasidone, risperidone, citalopram) or antibiotics (quinolone: levofloxacin, moxifloxacin), macrolides (erythromycin, clarithromycin), antimalarials (quinine), antiprotozoal (pentamidine) or antifungal (azole group) medications for acute infections. Some of these medications might also cause QTc interval prolongation which may increase the risk of sudden cardiac death (SCD) due to polymorphic tachycardia, also known as torsades de pointes (TdP) [35]. Ventricular repolarization prolongation often leads to oscillations of the membrane potential called early after-depolarization (EAD), which, in case it reaches a critical threshold in a large myocardial area, can promote ectopic activity [35, 36]. Those ectopic beats are usually followed by a long pause, with a subsequent sinus beat showing marked QTc prolongation. Timing of the ventricular premature contraction (VPC) that occurs on the T-wave of a preceding QRS complex, can trigger an episode of TdP. This pattern of onset of a short-long-short cycle is typical for drug-induced TdP, also known as pause-dependant TdP [35, 37]. This is a pattern where TdP often follows a sudden adrenergic surge, such as exercise or arousal. It is usually not sustained and terminates spontaneously. However, successive events can degenerate into ventricular fibrillation and result in SCD [38].
LVEF values measured before and after both antibody drug protocols (LVEF1 T Left ventricular ejection fraction before trastuzumab, LVEF2 T Left ventricular ejection fraction after trastuzumab, LVEF1 ET Left ventricular ejection fraction before trastuzumab emtansine, LVEF2 ET Left ventricular ejection fraction after trastuzumab emtansine)
Conclusion
In conclusion, trastuzumab and trastuzumab emtansine had no effect on PQ and QRS interval duration, but have shown a significant effect on QTc interval duration. There was a significant QTc interval prolongation, already seen with first application of both drugs, with further significant QTc prolongation after each successive drug cycle, reaching the peak value before the fifth cycle. Effects of both drugs on QTc interval prolongation were reversible, and posttreatment values returned to approximately starting levels in both protocols. Our results also confirm clinically silent but statistically significant LVEF reduction after both drug protocols, with a partial LVEF recovery after the trastuzumab treatment. There was no correlation between QTc interval duration and LVEF, measured before or after treatment with both drug protocols (Additional files 1, 2 and 3).
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article (and its additional file).
Abbreviations
- QTc:
-
Corrected QT interval
- LVEF:
-
Left ventricular ejection fraction
- HER2+:
-
Human epidermal growth factor receptor 2 positive
- ECG:
-
Electrocardiogram
- EGFR:
-
Epidermal growth factor receptor
- T-DM1:
-
Trastuzumab emtansine
- T :
-
Test statistic
- Df:
-
Degrees of freedom
- P :
-
Statistical significance
- SD:
-
Standard deviation
- R :
-
Rho coefficient
- BF10:
-
Bayesian factor 10
- CMP:
-
Cardiomyopathy
- SCD:
-
Sudden cardiac death
- TdP:
-
Torsade de pointes
- EAD:
-
Early after-depolarization
- VPC:
-
Ventricular premature contraction
References
Tevaarwerk AJ, Gray RJ, Schneider BP et al (2013) Survival in patients with metastatic recurrent breast cancer after adjuvant chemotherapy: little evidence of improvement over the past 30 years. Cancer 119:1140. https://doi.org/10.1002/cncr.27819
Dawood S, Broglio K, Buzdar AU et al (2010) Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 28:92. https://doi.org/10.1200/JCO.2008.19.9844
Balduzzi S, Mantarro S, Guarneri V et al (2014) Trastuzumab-containing regimens for metastatic breast cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD006242.pub2
Isakoff SJ, Baselga J (2011) Trastuzumab-DM1: building a chemotherapy-free road in the treatment of human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 29:351. https://doi.org/10.1200/JCO.2010.31.6679
Hamberg P, Bos MM, Braun HJ et al (2011) Randomized phase II study comparing efficacy and safety of ombination-therapy trastuzumab and docetaxel vs. sequential therapy of trastuzumab followed by docetaxel alone at progression as first-line chemotherapy in patients with HER2+ metastatic breast cancer: HERTAX trial. Clin Breast Cancer 11:103. https://doi.org/10.1016/j.clbc.2011.03.003
Pagani O, Klingbiel D, Ruhstaller T et al (2017) Do all patients with advanced HER2 positive breast cancer need upfront-chemo when receiving trastuzumab? Randomized phase III trial SAKK 22/99. Ann Oncol 28:305. https://doi.org/10.1093/annonc/mdw622
Inoue K, Nakagami K, Mizutani M et al (2010) Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 trial group. Breast Cancer Res Treat 119:127. https://doi.org/10.1007/s10549-009-0498-7
FDA approves new treatment for late-stage breast cancer (2013). http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm340704.htm. Accessed 22 Feb 2013
Keefe DL (2002) Trastuzumab-associated cardiotoxicity. Cancer 95:1592. https://doi.org/10.1002/cncr.10854
Perez EA, Rodeheffer R (2004) Clinical cardiac tolerability of trastuzumab. J Clin Oncol 22:322. https://doi.org/10.1200/JCO.2004.01.120
Fluza M (2009) Cardiotoxicity associated with trastuzumab treatment of HER2+ breast cancer. Adv Ther 26(Suppl 1):S9. https://doi.org/10.1007/s12325-009-0048-z
Ewer MS, Lippman SM (2005) Type II chemotherapy-related cardiac dysfunction: time to recognize a new entity. J Clin Oncol 23:2900. https://doi.org/10.1200/JCO.2005.05.827
Henry ML, Niu J, Zhang N et al (2018) Cardiotoxicity and cardiac monitoring among chemotherapy-treated breast cancer patients. JACC Cardiovasc Imaging 11:1084. https://doi.org/10.1016/j.jcmg.2018.06.005
Ewer SM, Ewer MS (2008) Cardiotoxicity profile of trastuzumab. Drug Saf 31:459. https://doi.org/10.2165/00002018-200831060-00002
Guenancia C, Lefebvre A, Cardinale D et al (2016) Obesity as a risk factor for anthracyclines and trastuzumab cardiotoxicity in breast cancer: a systematic review and meta-analysis. J Clin Oncol 34:3157. https://doi.org/10.1200/JCO.2016.67.4846
Suter TM, Procter M, van Veldhuisen DJ et al (2007) Trastuzumab-associated cardiac adverse effects in herceptin adjuvant trial. J Clin Oncol 25:3859. https://doi.org/10.1200/JCO.2006.09.1611
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783. https://doi.org/10.1056/NEJM200103153441101
Bowels EJ, Wellman R, Feigelson HS et al (2012) Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 104:1293. https://doi.org/10.1093/jnci/djs317
Fox KF (2006) The evaluation of left ventricular function for patients being considered for, or receiving trastuzumab (Herceptin) therapy. Br J Cancer 95:1454. https://doi.org/10.1038/sj.bjc.6603340
Tanriverdi O, Meydan N, Barutca S (2012) Long-term effect of trastuzumab on QT dispersion in adjuvant treatment for patients with Her2 receptor positive breast cancer a pilot study. Med Oncol 29(5):3265–3271. https://doi.org/10.1007/s12032-012-0291-z
Gupta M, Wang B, Carrothers TJ, LoRusso PM, Chu YW, Shih T et al (2013) Effects of trastuzumab emtansine (T-DM1) on qt interval and safety of pertuzumab plus T-DM1 in patients with previously treated human epidermal growth factor receptor 2-positive metastatic breast cancer. Clin Pharmacol Drug Dev 2(1):11–24. https://doi.org/10.1002/cpdd.9
Xu N, Redfern CH, Gordon M, Eppler S, Lum BL, Trudeau C (2014) Trastuzumab, in combination with carboplatin and docetaxel, does not prolong the QT interval of patients with HER2-positive metastatic or locally advanced inoperable solid tumors: results from a phase Ib study. Cancer Chemother Pharmacol 74(6):1251–1260. https://doi.org/10.1007/s00280-014-2603-9
Garg A, Li J, Clark E, Knott A, Carrothers TJ, Marier JF, Cortes J et al (2013) Exposure-response analysis of pertuzumab in HER2-positive metastatic breast cancer: absence of effect on QTc prolongation and other ECG parameters. Cancer Chemother Pharmacol 72(5):1133–1141. https://doi.org/10.1007/s00280-013-2279-6
Patane S (2014) Cardiotoxicity: trastuzumab and cancer survivors. Int J Cardiol 177(2):554–556. https://doi.org/10.1016/j.ijcard.2014.08.117
Bovelli D, Plataniotis G, Roila F (2010) On behalf of the ESMO guidelines working group, cardiotoxicity of chemotherapeutic agents and radiotherapy-related heart disease: ESMO clinical practice guidelines. Ann Oncol 21(5):v277–v282. https://doi.org/10.1093/annonc/mdq200
Gianni L, Eiermann W, Semiglazov V et al (2010) Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet 375:377. https://doi.org/10.1016/S0140-6736(09)61964-4
Bianchi G, Albanell J, Eiermann W et al (2003) Pilot trial of trastuzumab starting with or after the doxorubicin component of a doxorubicin plus paclitaxel regimen for women with HER2-positive advanced breast cancer. Clin Cancer Res 9:5944
Gianni L, Semiglova V, Manikhas GM et al (2007) Neoadjuvant trastuzumab in locally advanced breast cancer (NOAH): antitumour and safety analysis (abstract). J Clin Oncol 25:10s. https://doi.org/10.1200/jco.2007.25.18_suppl.532
Joensuu H, Bono P, Kataja V et al (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with of without trastuzumab, as adjuvant treatments of breast cancer: fina results of the FinHer trial. J Clin Oncol 27:5685. https://doi.org/10.1200/JCO.2008.21.4577
Von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M et al (2019) Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 380:617–628. https://doi.org/10.1056/NEJMoa1814017
Hurvitz SA, Dirix L, Kocsis J et al (2013) Phase II randomised study of trastuzumab emtansine versus trastuzumab plus docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer. J Clin Oncol 31:1157. https://doi.org/10.1200/JCO.2012.44.9694
Rastogi P, Jeoung J, Geyer CE et al (2007) Five-year update of cardiac dysfunction on NSABP B-31, a randomized trial of sequential doxorubicin/cyclophosphamide (AC)-paclitaxel compared to AC-T with trastuzumab (abstract). J Clin Oncol 25:6s
Ewer MS, Vooletich MT, Durand JB et al (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23:7820. https://doi.org/10.1200/JCO.2005.13.300
Guarneri V, Lenihan DJ, Valero V et al (2006) Long-term cardiac tolerability of trastuzumab in metastatic breast cancer: the M.D. Anderson Cancer Center experience. J Clin Oncol 24:4107. https://doi.org/10.1200/JCO.2005.04.9551
Nachimuthu S, Assar MD, Schussler JM (2012) Drug-induced QT interval prolongation: mechanisms and clinical management. Ther Adv Drug Saf 3(5):241–253. https://doi.org/10.1177/2042098612454283
January C, Riddle J (1989) Early after depolarizations: mechanism of induction and block. A role for L-type Ca2+ current. Circ Res 64:977–990. https://doi.org/10.1161/01.res.64.5.977
El-Sherif N, Caref E, Chinushi M, Restivo M (1999) Mechanism of arrhythmogenicity of the short-long cardiac sequence that precedes ventricular tachyarrhythmias in the long QT syndrome. J Am Coll Cardiol 33:1415–1423. https://doi.org/10.1016/s0735-1097(98)00700-1
Passman R, Kadish A (2001) Polymorphic ventricular tachycardia, long Q-T syndrome, and torsades de pointes. Med Clin North Am 85:321–341. https://doi.org/10.1016/s0025-7125(05)70318-7
Acknowledgements
Not applicable.
Funding
Data used in this research was gathered by using findings after performing routine electrocardiography and echocardiography examination for women treated with trastuzumab and trastuzumab emtansine in our institution.
Author information
Authors and Affiliations
Contributions
RL has made a substantial contributions to the conception and design of the work, analysis and interpretation of data for the work and drafting the work. MLB has made a substantial contributions to the concept and design of the work, drafting the work and revising it critically for important intellectual content. IIV has made a substantial contribution by drafting the work and revising it critically for important intellectual content. LB has made a substantial contribution by drafting the work and revising it critically for important intellectual content. MB has made a substantial contributions to the design of the work and the acquisition of the work. JJ has made a substantial contributions to the design of the work and the acquisition of the work. NDP has made a substantial contribution by drafting the work and revising it critically for important intellectual content. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All patients signed informed consent before the testing and data analysis has been approved by the Ethics committee of Clinical Hospital Center Zagreb. The research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
Consent for publication
Patients signed informed consent regarding publishing their data.
Competing interests
The authors declare that there are no competing interests regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1. Table 1.
The table shows the values of PQ, QRS and QTc intervals before each cycle (PQ1-6, QRS1-6, QTc1-6) as well as the values of PQ7, QRS7 and QTc7 after the last cycle of trastuzumab for each individual patient. Average heart rate values for each individual patient as well as the age of each patient at the time of trastuzumab therapy are also shown.
Additional file 2. Table 1.
The table shows the values of PQ, QRS and QTc intervals before each cycle (PQ1-6, QRS1-6, QTc1-6) as well as the values of PQ7, QRS7 and QTc7 after the last cycle of trastuzumab emtansine for each individual patient. Average heart rate values for each individual patient as well as the age of each patient at the time of trastuzumab emtansine therapy are also shown.
Additional file 3. Table 1.
The table shows the values of ejection systolic function for each patient before (LVEF1) and after (LVEF2) administration of transtuzumab and before and after administration of trastuzumab emtansine (LVEF1, LVEF2).
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Levicki, R., Lovrić Benčić, M., Ivanac Vranešić, I. et al. Effects of trastuzumab and trastuzumab emtansine on corrected QT interval and left ventricular ejection fraction in patients with metastatic (HER2+) breast cancer. Egypt Heart J 75, 11 (2023). https://doi.org/10.1186/s43044-023-00331-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43044-023-00331-y