Abstract
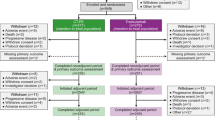
We evaluated the efficacy and safety of sequential therapy with trastuzumab monotherapy (H-mono) followed by H plus docetaxel (D) after disease progression (H → H + D) versus combination therapy with H + D as first-line therapy. Patients with human epidermal growth factor receptor type 2 (HER2)-positive metastatic breast cancer (MBC) and left ventricular ejection fraction >50% were randomly assigned to either (a) H → H + D [H, once weekly 2 mg/kg (loading dose, 4 mg/kg); D, once every 3 weeks 60 mg/m2] or (b) H + D. Primary endpoints were progression-free survival (PFS) for the H-mono stage of the H → H + D group and H + D group and overall survival (OS) for both groups. Secondary endpoints were overall response rate, time to treatment failure, second PFS and safety. The planned number of patients was 160 patients in total. Of 112 patients enrolled, 107 were eligible. After 112 patients were enrolled, the Independent Data Monitoring Committee recommended stopping enrollment because PFS and OS were greater in the H + D group than the H → H + D group. Median PFS was 445 days in the H + D group versus 114 days for H-mono in the H → H + D group [hazard ratio (HR), 4.24; P < 0.01]. OS was significantly longer in the H + D group (HR, 2.72; P = 0.04). H + D therapy is significantly superior to H → H + D therapy as first-line therapy in patients with HER2-positive MBC, especially in terms of OS.





Similar content being viewed by others
References
Hortobagyi GN (1998) Treatment of breast cancer. N Engl J Med 339:974–984
Piccart MJ (2001) Proposed treatment guidelines for HER2-positive metastatic breast cancer in Europe. Ann Oncol 12(Suppl.1):S89–S94
NCCN Clinical Practice Guidelines in Oncology version 1 (2009) Breast cancer. http://www.nccn.org/professionals/physician_gls/PDF/breast.pdf
Slamon DJ, Leyland-Jones B, Shak S et al (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344:783–792
Marty M, Cognetti F, Maraninchi D et al (2005) Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol 23:4265–4274
Vogel CL, Cobleigh MA, Tripathy D et al (2002) Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J Clin Oncol 20:719–726
Fountzilas G, Razis E, Tsavdaridis D et al (2003) Continuation of trastuzumab beyond disease progression is feasible and safe in patients with metastatic breast cancer: a retrospective analysis of 80 cases by the Hellenic cooperative oncology group. Clin Breast Cancer 4:120–125
Gelmon KA, Mackey J, Verma S et al (2004) Use of trastuzumab beyond disease progression: observations from a retrospective review of case histories. Clin Breast Cancer 5:52–58
Antoine EC, Extra JM, Vincent-Salomon A et al (2007) Multiple lines of trastuzumab provide a survival benefit for women with metastatic breast cancer: results from the Hermine cohort study [abstract]. Eur J Cancer 5(suppl):213 Abstract 2099
Mackey J, Gelmon KA, Verma S et al (2002) Continued use of Herceptin after disease progression in women with HER2-positive (HER2+) metastatic breast cancer (MBC): results from a retrospective analysis of 105 cases [abstract]. J Clin Oncol 21:52a Abstract 207
Von Minckwitz G, Zielinski C, Maarteense E et al (2008) Capecitabine vs. capecitabine + trastuzumab in patients with HER2-positive metastatic breast cancer progressing during trastuzumab treatment: the TBP phase III study (GBG 26/BIG 3–05) [abstract]. J Clin Oncol 26(suppl):47s Abstract 1025
O’Shaughnessy J, Blackwell KL, Burstein H et al (2008) A randomized study of lapatinib alone or in combination with trastuzumab in heavily pretreated HER2+ metastatic breast cancer progressing on trastuzumab therapy [abstract]. J Clin Oncol 26(suppl):44s Abstract 1015
Therasse P, Arbuck SG, Eisenhauer EA et al (2000) New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, National cancer institute of the United States, National cancer institute of Canada. J Natl Cancer Inst 92:205–216
Bontenbal M, Seynaeve C, Stouthard J et al (2008) Randomized study comparing efficacy/toxicity of monotherapy trastuzumab followed by monotherapy docetaxel at progression, and combination trastuzumab/docetaxel as first-line chemotherapy in HER2-neu positive, metastatic breast cancer (MBC) (HERTAX study) [abstract]. J Clin Oncol 26(suppl):44s Abstract 1014
Baselga J (2001) Herceptin alone or in combination with chemotherapy in the treatment of HER2-positive metastatic breast cancer: pivotal trial. Oncology 61(suppl 2):14–21
Tripathy D, Slamon D, Leyland-Jones B et al (2000) Treatment beyond progression in the Herceptin pivotal combination chemotherapy trial [abstract]. Breast Cancer Res Treat 64:32 Abstract 25
Bullock K, Blackwell K (2008) Clinical efficacy of taxane–trastuzumab combination regimens for HER-2-positive metastatic breast cancer. Oncologist 13:515–525
Acknowledgments
We thank the patients who participated in this trial and their families; the medical, nursing, and research staff at the institutions; the independent data and safety monitoring committee; the monitors, data managers, statisticians, and programmers at Chugai Pharmaceutical and EPS. This study was sponsored and funded by Chugai Pharmaceutical.
Author information
Authors and Affiliations
Corresponding author
Additional information
List of contributions are given in Appendix.
Appendix
Appendix
Contributions
Conception and design: Yasuo Ohashi, Masashi Ando, Toru Watanabe.
Financial support: Chugai Pharmaceutical Co., Ltd.
The following investigators and institutions also participated in the trial:
Junichiro Watanabe, Shizuoka Cancer Center, Shizuoka, Japan; Yoshinori Ito, The Cancer Institute Hospital of Japanese Foundation for Cancer Research, Tokyo, Japan; Keisei Anan, Kitakyushu Municipal Medical Center, Fukuoka, Japan; Yuichi Takatsuka, Kansai Rosai Hospital, Hyogo, Japan; Hitoshi Arioka, Yokohama Rosai Hospital, Kanagawa, Japan; Kenichi Watanabe, Hokkaido Cancer Center, Hokkaido, Japan; Reiki Nishimura, Kumamoto Municipal Hospital, Kumamoto, Japan; Kenjiro Aogi, National Hospital Organization Shikoku Cancer Center, Ehime, Japan; Seigo Nakamura, St. Luke’s International Hospital, Tokyo, Japan; Nobuaki Sato, Niigata Cancer Center Hospital, Niigata, Japan; Masato Koseki, National Hospital Organization Kure Medical Center and Chugoku Cancer Center, Hiroshima, Japan; Yutaka Tokuda, Tokai University Hospital, Kanagawa, Japan; Shinji Ohno, National Kyushu Cancer Center, Fukuoka, Japan; Shigeru Murakami, Hiroshima University Hospital, Hiroshima, Japan; Akihiko Chiba, Kanagawa Cancer Center, Kanagawa, Japan; Hideo Inaji, Osaka Medical Center for Cancer and Cardiovascular Diseases, Osaka, Japan; Shintaro Takao, Hyogo Cancer Center, Hyogo, Japan; Hiroko Yamashita, Nagoya City University Hospital, Aichi, Japan; Syouji Oura. Wakayama Medical University Hospital, Wakayama, Japan.
Data analysis and interpretation: Yasuo Ohashi, Shigeto Miura, Kenji Eguchi, Tetsu Shinkai.
Manuscript writing: Kenichi Inoue, Kazuhiko Nakagami, Mitsuhiro Mizutani, Yasuo Hozumi, Yasuhiro Fujiwara, Norikazu Masuda, Fumine Tsukamoto, Mitsue Saito, Shigeto Miura, Kenji Eguchi, Tetsu Shinkai, Masashi Ando, Toru Watanabe, Noriyuki Masuda, Yasuo Ohashi, Muneaki Sano, Shinzaburo Noguchi. Editorial assistance was provided by Medi-Kelsey Limited. All authors contributed materially to drafts and revisions and approved the final manuscript.
Final approval of manuscript: Kenichi Inoue, Yasuo Ohashi, Shinzaburo Noguchi.
Rights and permissions
About this article
Cite this article
Inoue, K., Nakagami, K., Mizutani, M. et al. Randomized phase III trial of trastuzumab monotherapy followed by trastuzumab plus docetaxel versus trastuzumab plus docetaxel as first-line therapy in patients with HER2-positive metastatic breast cancer: the JO17360 Trial Group. Breast Cancer Res Treat 119, 127–136 (2010). https://doi.org/10.1007/s10549-009-0498-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-009-0498-7