Abstract
Polycystic ovary syndrome (PCOS) is indeed one of the most common gynecological endocrine disorders, affecting a significant number of females in their reproductive age. While the exact cause of PCOS is not fully understood, several factors are believed to contribute to its onset. The relationship between polycystic ovary syndrome (PCOS) and low-grade chronic inflammation is complex and not fully understood. While there is evidence to suggest an association between PCOS and inflammation, the exact cause and causal nature of this relationship are still under investigation. Several inflammatory markers, including IL-6 (interleukin-6), TNF-α (tumor necrosis factor-alpha), IL-17 (interleukin-17), CRP (C-reactive protein), NLR (neutrophil-to-lymphocyte ratio), and PLR (platelet-to-lymphocyte ratio), have been studied about PCOS. These markers are substances produced by the immune system in response to inflammation. Increased levels of IL-17, IL-1, and IL-8 were correlated with PCO. CRP to albumin ratio can be employed as a precise bio-marker for PCOS. The neutrophil-to-lymphocyte ratio (NLR) indicates poor cardiovascular health and metabolic syndrome (MS) and can be considered a negative regulator for FSH which indirectly stimulates testosterone production. Platelet/lymphocyte ratio (PLR) and mean platelet volume (MPV) are also recently found to be associated with PCOS. The literature explaining the underlying mechanisms with specific inflammatory markers and how inflammation relates to PCOS will be highlighted in this review article. It will also discuss the roles of inflammation and the association of different inflammatory markers in the pathogenesis of PCOS, which may usher in a new era in the treatment approach for PCOS.
Similar content being viewed by others
Introduction
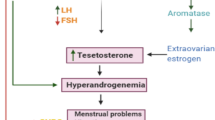
Today, polycystic ovary syndrome (PCOS) has become a recurring gynecological condition with a wide variety of different endocrine symptoms and is one of the leading causes of infertility in women of reproductive age [1, 2]. When other specific diagnoses, such as hyperprolactinemia and non-classical congenital adrenal hyperplasia, have been ruled out [3], PCOS is characterized by symptoms of ovarian dysfunction (oligo-ovulation and/or polycystic ovarian morphology, or PCOM) and androgen excess (hirsutism and/or hyperandrogenemia). Prevalence rates for PCOS among premenopausal women are higher than those among other women of childbearing age, ranging from 6% (using earlier, more restrictive standards) to 20% (using current, more thorough standard definitions) [4]. Menstrual irregularity and clinical or biochemical hyperandrogenism findings were used to diagnose PCOS in 1990, according to the National Institute of Health, after ruling out other androgen increases. The diagnosis of PCOS is determined if at least two of the symptoms are present after ruling out other endocrine disorders, such as oligo-amenorrhea, clinical and/or laboratory evidence of hyperandrogenism, and polycystic ovary appearance on ultrasonography [5]. The Androgen Excess Society (2006–2009) established criteria for diagnosing PCOS that take into account hyperandrogenism (hirsutism and/or hyperandrogenemia), ovulatory dysfunction (oligo-anovulation and/or polycystic ovary), and the elimination of other endocrinopathies that elevate androgen [3, 6]. Even if the diagnostic criteria are what they are, PCOS has long-lasting repercussions on women’s lives that last throughout their entire lifespans (Fig. 1).
Due to insulin resistance, PCOS has been linked to serious and persistent metabolic and cardiovascular diseases [5]. Additionally, chronic low-grade inflammation has been linked to it, and this is supposed to increase long-term cardiovascular illness [7, 8]. The fundamental mechanisms of PCOS are unknown, despite how common it is. A substantial amount of research links insulin resistance and androgen overproduction to the pathophysiology of the condition. According to several researches, hyperandrogenism in PCOS women is positively correlated with persistent low-grade inflammation. However, it is unclear if this link is causative [7, 9]. Subcutaneous adipose tissue (SAT) has a higher androgen production rate, which may be a factor in PCOS-related hyperandrogenemia [10, 11]. Emphasis on the inflammatory factors and their relationship to PCOS has increased as a result of the association between the prevalence of PCOS and chronic inflammation [12]. PCOS’s etiology may be influenced by the imbalance of pro-inflammatory and anti-inflammatory cytokines and cytokine gene polymorphisms [13]. As a result, inflammatory responses serve as mediators and aid in the emergence and exacerbation of PCOS’s metabolic characteristics. Polycystic ovary syndrome (PCOS) and insulin resistance are likely to be associated with obesity, and adipocytes (fat cells) that can contribute significantly to the synthesis of pro-inflammatory mediators, leading to chronic inflammation [14]. It has been uncovered that the activation of inflammatory pathways in adipocytes can impair triglyceride accumulation and contribute to insulin resistance. Inflammation within adipose tissue can disrupt the normal balance of lipid metabolism, leading to an increased release of free fatty acids (FFAs) from adipocytes [15]. Under normal conditions, adipocytes store excess energy as triglycerides. However, when inflammatory pathways are activated, certain pro-inflammatory cytokines (such as TNF-α and IL-6) can interfere with the process of storing triglycerides and promote the breakdown of stored fats. This results in the release of FFAs into the bloodstream. Elevated levels of FFAs, particularly saturated fatty acids, can have detrimental effects on insulin signaling and glucose metabolism. These FFAs can accumulate in non-adipose tissues such as the skeletal muscle, liver, and pancreatic beta cells. In these tissues, FFAs can interfere with insulin signaling pathways, leading to reduced insulin sensitivity or insulin resistance. The presence of excess FFAs can induce inflammation and activate intracellular signaling pathways, such as the serine kinases and nuclear factor kappa B (NF-κB), which can further promote insulin resistance. These pathways interfere with insulin receptor signaling and downstream glucose uptake and utilization. Furthermore, the accumulation of FFAs in the liver can lead to increased hepatic glucose production and contribute to hyperglycemia. The presence of high levels of FFAs can also affect pancreatic beta cells, impairing insulin secretion and further exacerbating insulin resistance. Overall, the inflammatory activation in adipocytes can disrupt normal triglyceride accumulation and increase the release of FFAs into the circulation. These FFAs can promote insulin resistance in various tissues, contributing to the development and progression of metabolic disorders such as type 2 diabetes and obesity-related insulin resistance. In addition to several other inflammatory markers, blood levels of C-reactive protein (CRP) are frequently used to evaluate low-grade chronic inflammation. Activated macrophages, as well as adipocytes, release interleukin-6 (IL-6) which eventually stimulates the liver to generate CRP, an acute-phase protein [16, 17]. Obese women having diabetes and cardiovascular complications show elevated serum CRP levels that are closely associated with long-term health hazards [18]. However, there are significant disputes regarding whether or not PCOS is an inflammatory process [19].
Possible mechanism of inflammation in PCOS
A common symptom of PCOS is chronic inflammation, and new research suggests that people with PCOS have higher levels of inflammatory markers like C-reactive protein (CRP), leukocytes/white blood cells (WBCs), certain interleukins, and tumor necrosis factor (TNF) [20, 21]. Chronic low-grade inflammation has been associated with obesity and insulin resistance as well as endothelial dysfunction, atherosclerosis, and coronary heart disease [22]. Although the exact etiology of the chronic inflammation process associated with PCOS is not known, it is believed that patients who have already begun the inflammatory responses have a higher chance of developing insulin resistance, obesity, and cardiovascular problems. Although studies have linked PCOS with insulin resistance the underlying interconnected pathways causing these two conditions remain unknown [8]. Nearly 30% of the women with PCOS have visceral fat, or abdominal adiposity, which is closely associated with insulin resistance [23]. The body enters a pro-inflammatory condition because the adipose tissue is a primary source of cytokine release [24]. The exact mechanisms underlying inflammation in women with PCOS are not fully understood. However, there is a high prevalence of excess adiposity in PCOS, which is characterized by enlarged adipocytes and the presence of immune cells within adipose tissue. This leads to chronic low-grade inflammation [25]. However, the higher level of inflammation observed in both obese and non-obese PCOS patients must have some specific explanations. Experimental proofs pointed out the hereditary origin of such persistent low-grade inflammation in PCOS. Some studies have reported a connection between specific proinflammatory genotypes, such as those associated with TNF-α, TNF receptor (Type II), and IL-6, and PCOS [26, 27]. Inflammation of adipose tissue has also been linked to PCOS-related hyperandrogenism as a potential underlying cause. Deligeoroglou et al. [8] hypothesized that adipocyte hypertrophy induced by androgens leads to the narrowing of stromal arteries, which subsequently triggers inflammation due to tissue hypoxia. A recent meta-analysis data [18] revealed the appearance of PCOS-related chronic inflammation characterized by higher levels of circulatory CRP. A moderate rise in CRP levels of PCOS women not exceeding 5.0 mg/l indicates low-grade inflammation [18]. With little and contradictory data, the IL-6 and TNF-α-mediated inflammatory mechanisms involved in PCOS remain unclear. However, IL-6 and TNF-α are known to play a role in stimulating the production of C-reactive protein (CRP) in the liver. Therefore, the elevated CRP level can be used as an indirect marker for higher IL-6 and TNF levels. The same meta-analysis [18] further described that the appearance of PCOS amplifies with increasing CRP levels caused by obesity. Although the elevated levels of circulating CRP associated with PCOS are noticeable in women of normal weight, they are less significant among obese PCOS patients than in obese control subjects [28, 29]. Gonzalez made the hypothesis that obesity may make PCOS-related CRP rises harder to see [30]. It reveals that CRP level in obese PCOS patients, acts as a sensitive biomarker for chronic inflammation [30], but PCOS women had lower levels of adiponectin, which is considered a well-known anti-inflammatory protein [31]. Similar decreases have also been observed in pentraxin-3 (PTX3), a protein with structural similarities to CRP that regulates inflammation and may have anti-atherosclerotic activity that can reduce cardiovascular risks [32]. This abovementioned discussion pointed out a dual mechanism of prolonged inflammation in PCOS, with rising pro-inflammatory factors and falling anti-inflammatory protective ones. Additional evidence corroborating the idea that the severity of inflammation in PCOS is unrelated to obesity was established by a few research studies [33] that looked at how weight loss has been observed to impact inflammatory markers in both obese women with and without PCOS who lost weight experienced a positive reduction in inflammatory markers, the PCOS group’s response to weight loss was slower and less pronounced. These findings offer more proof in favor of the theory that PCOS disease, separate from obesity, is linked to chronic inflammation. Most of the research works link insulin resistance and androgen overproduction to PCOS etiology. Chronic inflammation has a substantial correlation with both excess testosterone and insulin resistance [18]. It is still unclear how precisely these three pathogenic entities interact and which role they play in the development of PCOS.
Chronic inflammation and obesity
Metabolic consequences are more likely to occur in obese people. The well-known trials of Dunaif et al. [34, 35] provided a clear illustration of the impact of obesity and PCOS. These studies demonstrated the independent and cumulative effects of PCOS and obesity. The main difference between the two, however, is obesity, which is linked to reduced insulin action in normal women compared to PCOS alone in normal-weight women [35]. Women with PCOS who are normal weight are comparatively more protected from developing impaired glucose tolerance than overweight and obese women since further obesity is linked to an increase in that risk [1, 2, 36]. Obesity can disrupt the usual progression of insulin resistance to diabetes by affecting normal time-related development. Initially, there is compensatory excessive secretion of insulin by cells, but over time, there is a decline in insulin production, leading to the development of glucose intolerance and fasting hyperglycemia. This is indicated by the elevated occurrence of glucose intolerance in obese adolescents with PCOS [1, 37]. As obesity increases, there is a corresponding increase in the prevalence of dyslipidemia, metabolic syndrome, and diabetes, which are all associated with obesity as related complications [1]. However, it is noteworthy that women with PCOS and a BMI under [1] who participated in a large multicenter experiment did not experience the onset of the metabolic syndrome. An individual with a normal waist circumference is typically associated with a normal metabolic profile and the absence of metabolic syndrome, including waist circumference-related issues, about PCOS [1]. Obesity has been linked to a higher risk of numerous malignancies, including endometrial and breast cancers, in the general population [38, 39]. The degree of epidemiological occurrence of breast cancer within the subpopulation of PCOS is lower; however, multiple epidemiological studies have provided evidence showing that women diagnosed with PCOS have a higher risk of developing certain types of cancers [40,41,42]. The majority of these studies lacked the statistical or long-term follow-up ability to examine the moderating effects of obesity on cancer risk in PCOS-affected women.
Understanding the pathophysiology of obesity and its associated conditions helps to clarify how obesity contributes significantly to metabolic syndrome via inflammatory adipokines. These comorbidities include endothelial dysfunction and hypertension, which are brought on by the activity of RAS-secreting adipokines, diabetes mellitus type 2, and dyslipidemia, which is brought on by hypercholesterolemia and hypertriglyceridemia. Insulin resistance is made worse by TNF-α and other inflammatory adipocyte secretagogues. The consequences of fatty acid lipotoxicity and these issues encourage atherogenesis, which includes coronary artery disease. The visceral white adipose tissue (WAT) inflammatory adipokines that trigger NF-κB hurt all of these illnesses. Chronic renal illness [43], obstructive sleep apnea [43], non-alcoholic fatty liver disease [44], and even reproductive dysfunctions [45] are additional diseases that can lead to metabolic syndrome. These diseases cause inflammation to spread, which improves the functionality of the affected tissue—adipose tissue, muscle, or vascular endothelium—or in several organs including the liver, heart, and kidneys [45]. In conclusion, these inflammatory adipokines have a significant impact on obesity and its comorbidities, such as atherosclerosis, making obesity the most avoidable public health issue in the USA and a menace that is fast spreading throughout the rest of the world [45].
Chronic inflammation and insulin resistance
Up to 70% of PCOS women have been shown to have insulin resistance [46], and PCOS women also have 40% worse insulin sensitivity [47], despite obesity further compromising insulin metabolism. In both obesity and type 2 diabetes (T2D), the contribution of chronic inflammation in adipose tissue to the emergence of insulin resistance is well documented [48]. TNF-α and IL-6 are two of the main inflammatory cytokines produced by adipose tissue that have been linked to insulin resistance. The initial identification of TNF-α as the inflammatory cytokine associated with the onset of insulin resistance was primarily attributed to its release by macrophages residing in adipose tissue [11, 14, 49]. TNF-α levels and other indicators of insulin resistance have also a positive correlation [17]. Obese mice lacking TNF-α or its receptor exhibited inhibitory effects upon the onset of insulin resistance in rodents [15]. The primary method that TNF-α induces insulin resistance by promoting post-receptor serine phosphorylation of insulin receptor substrate-1 (IRS-1); although, TNF-α disrupts the insulin signaling pathway, as it promotes lipolysis and the release of free fatty acids, which contribute to increased hepatic glucose production [50]. Adiponectin, a hormone generated from adipocytes that helps to maintain peripheral glucose and lipid homeostasis, is similarly downregulated by it [51, 52]. Contrasting literature studies make it less obvious how IL-6 contributes to insulin resistance. According to several studies, the interaction between IL-6 and the metabolism of insulin varies depending on factors such as metabolic status, tissue type, and the duration of IL-6 elevation, whether it is transient or chronic [48]. For instance, a brief increase in IL-6 during exercise has both an anti-inflammatory and an increase in glucose absorption in the skeletal muscle [53]. The protein suppressor of cytokine signaling 3 (SOCS3), which binds to and blocks the insulin receptor, is upregulated by IL-6, which on the other hand has proinflammatory effects and causes insulin resistance in adipose tissue and the liver [54]. Additionally, it suppresses the transcription and phosphorylation of IRS-1 [55, 56]. Based on the aforementioned, there appears to be a vicious loop triangle between these three aspects of PCOS, even though the exact causal relationship between chronic inflammation, insulin resistance, and hyperandrogenism in PCOS is still not fully understood [57]. One potential explanation is that hyperandrogenism brought on by PCOS causes adipose tissue inflammation, which in turn causes dysregulated adipokine production and insulin resistance [57].
Chronic inflammation and hyperandrogenism
In PCOS, hyperandrogenism may be the root cause of low-grade chronic inflammation. By increasing mononuclear cell sensitivity, it also promotes glucose-induced inflammation [58]. On the other side, pro-inflammatory cytokines like TNF can increase androgen synthesis by upregulating steroidogenic enzymes and stimulating the growth of theca cells. Additionally, TNF is a modulator of insulin resistance, suggesting that the underlying mechanisms of insulin resistance in PCOS are likely diet-induced inflammation [59]. Women with PCOS have been found to have hypertrophic adipocytes due to exposure to high androgen. Inflammation was more likely to occur in hypertrophic adipocytes [60]. Adipose tissue adipocytes secreted compounds, some of which are inflammatory agents, in an autocrine/paracrine manner, which contributed to the low-grade inflammation associated with PCOS [24]. There may be a connection between PCOS’s low-grade chronic inflammation and hyperandrogenism and hypertrophic adipocyte [61]. Evidence from recent studies strongly suggests that adipose tissue plays a critical role in modulating chronic inflammation in obesity and diabetes [18]. Increased levels of circulating proinflammatory cytokines are primarily brought on by the hypertrophied adipocytes and immune cells that are located in adipose tissue (lymphocytes and macrophages) in obesity. Additionally, “metabolic inflammation”, which is associated with obesity, is thought to play a substantial role in the development of insulin resistance and T2D [18, 25]. Considering the established associations between obesity, type 2 diabetes (T2D), and PCOS, it is plausible to propose that inflammation within adipocytes could be a significant contributing factor in the development of PCOS. Previous studies have indicated that adipose tissue can be a potential source of elevated androgen production in women with PCOS [10, 11, 62, 63]. Additionally, several studies have reported a correlation between the hyperandrogenism observed in PCOS and the presence of persistent low-grade inflammation [64]. The precise relationship between chronic inflammation and increased androgen synthesis in adipose tissue in the context of PCOS remains largely unknown, primarily due to the limited availability of conflicting evidence in the existing literature [7,8,9]. As previously mentioned, Deligeoroglou et al. [8] demonstrated that inflammation could be caused by an excess of androgen, but according to the findings of González et al. [30], it has been revealed that inflammation triggered by food intake in women with PCOS can be directly contributed to the development of hyperandrogenism. Hence, further research is necessary to gain a deeper understanding of the interactions between hyperandrogenism and adipose tissue inflammation in PCOS. Earlier investigations that explored circulating markers of inflammation in women with PCOS did not specifically study adipose tissue as a source of inflammation [30]. Given that adipose tissue plays notable roles in the onset of prolonged inflammatory state and excessive synthesis and availability of androgen, future research must concentrate on the inflammatory state of adipose tissue in PCOS [65]. Additionally, genetic determinants of PCOS should also be studied to minimize the potential risks. Reports suggest that PCOS is very common among first-degree relatives and siblings with autosomal dominant inheritance. Moreover, monogenic causes of hirsutism and oligomenorrhea were identified in PCOS women. On the contrary, twin studies claimed that it is X-linked polygenic in nature [66] (Fig. 2).
Inflammatory consequences and possible markers related to polycystic ovary syndrome (PCOS): Considering the dietary influence, excessive intake of carbohydrates and fats causes obesity and hyperinsulinemia/insulin resistance (IR) in young women. Obesity and IR have a positive influence on the pathogenesis of PCOS. The obesity-induced hypertrophic adipocytes sharply increase the level of adiponectin, leptin, IL-6, and TNF-α which cumulatively contribute to the inflammation of adipose tissue. This hypertrophic adipose tissue resulted in androgen excess which might contribute to IR. The stimulated hepatocytes produce acute phase response protein CRP. The pro-inflammatory cytokines and leptins cause chronic low-grade inflammation via the activation of NF-κB. The excessive production of reactive oxygen species (ROS) and cortisol is responsible for the onset of stress-induced immune alterations as well as attenuated T cell response. Simultaneously, the increased macrophage activation, higher neutrophil-to-lymphocyte ratio (NLR), and mean platelet volume (MPV) are observed as the hallmarks of PCOS
Inflammatory markers related to PCOS
IL-6
One of the numerous cytokines that are generated by adipocytes from the body’s fat reserves is interleukin-6 (IL-6) [67]. There is notable evidence of increased IL-6 content in PCOS participants, which has been well discussed. However, the regulation of IL-6 levels is kept up by a close relationship with other cytokines, particularly mediated by a crucial regulator, NF-κB. To determine a likely cause for the underlying inflammatory condition in PCOS, the involvement of IL-6 in a wide range of other pathological ailments is also being studied. These problems include rheumatoid arthritis, cardiovascular diseases, asthma, colon cancer, and many more. IL-6 has opposing effects on the body due to its dual pro- and anti-inflammatory characteristics [68]. Under normal circumstances, IL-6 mediates vital processes like epithelial renewal and bolstering the body’s defensive system. In a recent study, it was found that insulin resistance may affect IL-6 production, and high levels of IL-6 in young PCOS women may also indicate an altered immune response and help to identify individuals with a higher risk of cardiovascular disease in the future [69]. However, persistent inflammation brought on by disorders like PCOS can be highly dangerous.
TNF alpha
Adipose tissue plays a significant role in the release of various molecules such as transforming necrosis factor-α (TNF-α), interleukin-6 (IL-6), and adiponectin. These molecules exhibit altered expression patterns in the context of obesity. The alterations in the expression patterns of adipokines in obesity can have an impact not only on adipose tissue itself but also on other tissues targeted by these adipokines. This can contribute to the establishment of a chronic, albeit mild, proinflammatory environment in individuals who are obese [70, 71]. While TNF-α is a proinflammatory cytokine and contributes to obesity-related systemic insulin resistance (IR) by blocking the tyrosine kinase of the insulin receptor in muscle and fat, adiponectin is an insulin sensitizer and anti-inflammatory molecule [72,73,74]. TNF-α has been linked to the pathophysiology of PCOS because it increases IR, results in hyperandrogenism (HA), and is involved in follicular formation. By reducing the insulin receptor’s tyrosine kinase activity, hyper-expression of TNF-α in muscle and adipose tissues is thought to contribute to the development of IR in humans [75, 76]. TNF-α has also been linked to chronic inflammatory disorders like ulcerative colitis, rheumatoid arthritis, and Crohn’s disease [77,78,79]. Previous research has shown that the expression of both the transcript and protein of adiponectin in adipose tissue can be reduced by TNF-α and interleukin-6. Conversely, other studies have indicated that TNF-α can increase the expression of adiponectin [73].
Nuclear factor (NF)-κB is a significant intracellular TNF-canonical pathway effector [73]. In numerous animal models, this transcription factor has been linked to insulin resistance along with a rise in blood levels of free fatty acids or other underlying causes of obesity [73].
A pro-inflammatory environment can also be observed in PCOS without having high body weight. The hyperandrogenic state present in the tissue may have positively influenced the macrophage quantity in the normal-weight-PCOS group. As seen in the ovaries of rats with hyperandrogenism, androgen is known to cause macrophages to produce more TNF-α, increasing inflammation [80]. Elevated levels of C-reactive protein (CRP) observed in women with PCOS, compared to age- and BMI-matched controls, indicate the presence of an inherent inflammatory process in PCOS [19, 81]. In non-obese women with PCOS, visceral adipocytes have been found to stimulate the release of free fatty acids into the bloodstream, potentially contributing to a mild proinflammatory environment in these individuals [73].
IL-17
IL-17, IL-17a, and IL-1Ra have recently been discovered to be strongly related to PCO [21, 82, 83]. Inflammatory and autoimmune illnesses are mostly impacted by IL-17a [82]. Patients with PCOS have significantly higher levels of IL-1Ra, which can reduce insulin resistance and blood glucose metabolism, resulting in obesity and metabolic syndrome [83]. Increased levels of inflammatory cytokines (IL-17a, IL-1a, IL-1b, IL-2, and IL-8) can interfere with ovarian follicle atresia, decrease apoptosis, and prevent oocyte maturation [83]. Additionally, PCOS patients with high anti-Müllerian hormone (AMH) levels experience a decline in the connection between the inflammatory factors [83]. Inflammatory factors are increased by an aberrant level of AMH, resulting in a persistently low concentration of systemic inflammation in the human body, which causes metabolic illnesses such as insulin resistance, improper glycolipid metabolism, and reproductive dysfunctions [83].
CRP
Emerging studies have brought attention to the role of persistent low-grade inflammation as a potential underlying factor responsible for the long-term consequences of PCOS [84,85,86]. Despite the epidemiology’s lack of clarity, certain research does point to a potential link between PCOS and cardiovascular (CV) morbidity and mortality [84,85,86]. Insulin resistance and obesity may mediate various cardiovascular abnormalities, including early ventricular abnormalities, endothelial dysfunction, and arterial stiffness, as well as the development of carotid and coronary atherosclerosis [85, 86]. The suggested mechanisms are linked to the detrimental consequences of insulin resistance, such as dyslipoproteinemia, hypertension, elevated oxidative stress, persistent low-grade inflammation, and disrupted homeostasis [84,85,86]. PCOS is characterized by a state of low-grade chronic inflammation, as evidenced by elevated levels of C-reactive protein (CRP), inflammatory cytokines like interleukin-6 and interleukin-18, and an increased count of white blood cells [86, 87]. Research has shown that C-reactive protein (CRP) is a dependable marker of inflammation and an accurate predictor of cardiovascular morbidity [29, 81, 86], particularly about lipid profile parameters [87].
Recently, Kalyan et al. [88] demonstrated that when compared to both free androgens and insulin resistance, the ratio of C-reactive protein (CRP) to albumin can serve as a more accurate and powerful indicator of inflammation in PCOS. In comparison to markers like insulin resistance and androgens [88], the CRP/albumin ratio demonstrated higher specificity and sensitivity for inflammation related to metabolic dysfunction in matched PCOS patients. Interestingly, patients with PCOS exhibited a higher CRP/albumin ratio regardless of their body mass index (BMI) [88]. This observation suggests that inflammation may play a significant pathophysiological role in PCOS independent of BMI and obesity. Furthermore, apart from environmental factors, variations in CRP concentrations could be attributed to genetic variability within the CRP gene [89]. Nevertheless, this polymorphism may be crucial for proving that CRP serves as a sensitive and reliable CVD predictor and for verifying the causal link between CRP and CV morbidity [89]. Theoretically, CRP could identify PCOS women who are more likely to develop type II diabetes and cardiovascular disease [86]. Only two studies that were published examined the CRP levels in PCOS patients and discovered that they were more likely to have high CRP levels than controls. These studies’ authors concluded that, in comparison to controls, women with PCOS had considerably higher CRP concentrations [81, 90]. They examined CRP levels in a larger cohort of PCOS patients in comparison to BMI-matched controls to either support or refute such a link.
Neutrophil-to-lymphocyte ratio (NLR)
In the body, lymphocytes and neutrophils serve as the initial line of defense against foreign invaders. The earliest markers for inflammation and regulation that are discovered in wounded tissues are neutrophils and lymphocytes, respectively. Major cell types implicated in both acute and chronic inflammation are activated. Today, several studies employ the neutrophil-to-lymphocyte ratio (NLR), which is determined by dividing the neutrophil count by the lymphocyte count, as a marker to assess the severity of inflammatory diseases [91,92,93,94]. Cardiovascular disease incidence has been linked to increased levels of systemic inflammatory markers [95, 96]. Numerous epidemiological studies have also shown that obesity [97], metabolic syndrome [98,99,100], diabetes mellitus [98], hypertension [98], and chronic low-grade inflammation are all linked. C-reactive protein and total leukocyte count have been comprehensively examined in several observational studies about various chronic diseases [101,102,103]. White blood cell (WBC) count measurements of low-grade inflammation have also been associated with the classic risk factors for chronic diseases, such as smoking, obesity, hypertension, and high triglyceride levels [98, 104, 105]. PCOS patients have a higher risk of developing cardiovascular illnesses because of their unusual hormonal pattern, which is marked by hyperandrogenism, insulin resistance, dyslipidemia, and an inflammatory state [106]. Besides the commonly recognized cardiovascular risk factors such as hypertension, diabetes, inflammation, and high cholesterol levels, the recent studies mentioned above draw attention to interesting similarities among these patients [107] and unmistakably show that PCOS may negatively affect or hasten the development of a negative cardiovascular risk profile and even of subclinical atherosclerosis signs [108]. Additionally discovered to be related to poor cardiovascular health was elevated neutrophil-to-lymphocyte ratio (NLR) values [98, 109]. Numerous cancer survival studies have revealed that NLR is a major predictor of patient survival overall and for each illness [94]. Given its affordability, accessibility, and ease of calculation, the neutrophil-to-lymphocyte ratio has the potential to be a key indicator of systemic inflammation. The risk of metabolic syndrome (MS) has been observed to increase when NLR grows, and NLR readings may be a valuable tool to anticipate the onset of MS [94]. Increased NLR was found to be an inflammation marker in PCOS patients [110, 111] and was found to be negatively correlated with FSH levels as well as positively correlated with free testosterone [112], androstenedione [112], sex hormone binding globulin [112], insulin resistance, and altered lipid parameters [22]. However, it was also found to occasionally not be associated with obesity [112, 113]. In addition, it was found that people with PCOS can have elevated NLR levels despite similar high-sensitivity C-reactive protein (hsCRP) levels [110, 113].
Platelet/lymphocyte ratio (PLR), in addition to NLR, has been discovered to be an additional inflammatory marker for PCOS. All PCOS participants had considerably higher PLR than the BMI-matched controls. PLR increased in normal weight-PCOS even while obesity caused it to decline. These findings provide credence to the idea that PCOS is a chronic inflammatory condition distinct from obesity. In PCOS, mean platelet volume (MPV) levels were independently correlated with clinical pregnancy rate (CPR) [114]. PLR and various hormonal and metabolic markers appear to be connected.
Conclusion
PCOS is a gynecological disorder mostly affecting women at post-pubertal age worldwide. Interestingly, nowadays, the prevalence of PCOS is approximately 1 in every 10 women. The pathophysiology in terms of hormonal dysregulation has been well understood. Along with insulin resistance (IR) and hyperandrogenism (HA), there are influences of environmental factors, genetics, and epigenetics. Despite some clinical manifestations in correlation with the altered hormonal levels, there are no such definite tests for PCOS diagnosis to date. In this review, we have tried to correlate the pathogenesis of PCOS with its inflammatory consequences intending to develop novel markers for the precise and accurate diagnosis of PCOS. Moreover, those inflammatory parameters will help to predict the susceptibility of an individual to develop polycystic ovaries or ovulatory dysfunction. Though inflammatory aspects are important for oocyte growth higher counts of leukocytes, and increased concentration of CRP in the peripheral blood is also associated with the progression of PCOS. When focusing on the pro-inflammatory cytokines, it was observed that TNF-α had a positive correlation with IR. Moreover, IR may elevate the IL-6 level which is known to be a risk factor for cardiovascular disease in women. Increased levels of IL-17, IL-1, and IL-8 were correlated with PCO. Researchers suggest that a hyperandrogenic state during PCOS could stimulate the resident macrophages which contributed to the pro-inflammatory environment. As an obvious result, the higher amount of acute phase protein CRP is elevated in PCOS patients. Additionally, CRP to albumin ratio can be employed as a precise bio-marker for PCOS. Inflammatory cells like lymphocytes and neutrophils could be increased during PCOS and can be depicted as the earliest marker. Evidence suggested that the neutrophil-to-lymphocyte ratio (NLR) indicated poor cardiovascular health and metabolic syndrome (MS). This is to be further noted that NLR is a negative regulator for FSH which indirectly stimulates testosterone production. Therefore, NLR could serve as another inflammatory marker for PCOS diagnosis. On the other hand, the platelet/lymphocyte ratio (PLR) is also helpful in assessing the prognosis of PCOS. For better clinical correction, further research should be done to reveal the mechanistic aspect of those inflammatory markers in PCOS. Taking together all the facts, it can be inferred that the establishment of such important inflammatory biomarkers is beneficial for the early detection and management of PCOS patients.
Availability of data and materials
None.
References
Bhattacharya K, Sengupta P, Dutta S, Chaudhuri P, Das Mukhopadhyay L, Syamal AK (2021) Waist-to-height ratio and BMI as predictive markers for insulin resistance in women with PCOS in Kolkata. India Endocrine 72(1):86–95
Bhattacharya K, Saha I, Sen D, Bose C, Ray Chaudhuri G, Datta S, Sengupta S, Bhattacharya S, Barman SS, Syamal AK (2022) Role of anti-Mullerian hormone in polycystic ovary syndrome. Middle East Fertil Soc J 27:32
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF (2009) Task force on the phenotype of the polycystic ovary syndrome of the Androgen excess and PCOS society. The androgen excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril 91(2):456–88
Escobar-Morreale HF (2018) Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol 14(5):270–284
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril 81(1):19–25
Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, Janssen OE, Legro RS, Norman RJ, Taylor AE, Witchel SF, Androgen Excess Society (2006) Positions statement: criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab 91(11):4237–45
Repaci A, Gambineri A, Pasquali R (2011) The role of low-grade inflammation in the polycystic ovary syndrome. Mol Cell Endocrinol 335(1):30–41
Deligeoroglou E, Vrachnis N, Athanasopoulos N, Iliodromiti Z, Sifakis S, Iliodromiti S, Siristatidis C, Creatsas G (2012) Mediators of chronic inflammation in polycystic ovarian syndrome. Gynecol Endocrinol 28(12):974–978
Çıtar Dazıroğlu ME, Acar TN (2023) The effect on inflammation of adherence to the Mediterranean diet in polycystic ovary syndrome. Curr Nutr Rep 12(1):191–202
Amer SA, Alzanati NG, Warren A, Tarbox R, Khan R. Excess androgen production in subcutaneous adipose tissue of women with polycystic ovarian syndrome is not related to insulin or LH. J Endocrinol. 2019:JOE-18-0674.R1. https://doi.org/10.1530/JOE-18-0674.
O’Reilly MW, Kempegowda P, Jenkinson C, Taylor AE, Quanson JL, Storbeck KH, Arlt W (2017) 11-oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J Clin Endocrinol Metab 102(3):840–848
Rudnicka E, Suchta K, Grymowicz M, Calik-Ksepka A, Smolarczyk K, Duszewska AM, Smolarczyk R, Meczekalski B (2021) Chronic low grade inflammation in pathogenesis of PCOS. Int J Mol Sci 22(7):3789
Wu H, Yu K, Yang Z (2015) Associations between TNF-α and interleukin gene polymorphisms with polycystic ovary syndrome risk: a systematic review and meta-analysis. J Assist Reprod Genet 32(4):625–634
Tilg H, Moschen AR (2008) Inflammatory mechanisms in the regulation of insulin resistance. Mol Med 14(3–4):222–31
Guilherme A, Virbasius JV, Puri V, Czech MP (2008) Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9(5):367–377
Pepys MB, Hirschfield GM (2003) C-reactive protein: a critical update. J Clin Invest 111(12):1805–1812. https://doi.org/10.1172/JCI18921. Erratum in: J Clin Invest. 2003 Jul;112(2):299.
Lau DC, Dhillon B, Yan H, Szmitko PE, Verma S (2005) Adipokines: molecular links between obesity and atheroslcerosis. Am J Physiol Heart Circ Physiol 288(5):H2031–H2041
Aboeldalyl S, James C, Seyam E, Ibrahim EM, Shawki HE, Amer S (2021) The role of chronic inflammation in polycystic ovarian syndrome-a systematic review and meta-analysis. Int J Mol Sci 22(5):2734
Duleba AJ, Dokras A (2012) Is PCOS an inflammatory process? Fertil Steril 97(1):7–12
Rudnicka E, Kunicki M, Suchta K, Machura P, Grymowicz M, Smolarczyk R (2020) Inflammatory markers in women with polycystic ovary syndrome. Biomed Res Int 4(2020):4092470
Foroozanfard F, Soleimani A, Arbab E, Samimi M, Tamadon MR (2017) Relationship between IL-17 serum level and ambulatory blood pressure in women with polycystic ovary syndrome. J Nephropathol 6(1):15–24
Özay AC, Özay ÖE (2021) The importance of inflammation markers in polycystic ovary syndrome. Rev Assoc Med Bras (1992) 67(3):411–417
Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, Di Fede G, Rini G (2007) Abdominal fat quantity and distribution in women with polycystic ovary syndrome and extent of its relation to insulin resistance. J Clin Endocrinol Metab 92(7):2500–2505
Chait A, den Hartigh LJ (2020) Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front Cardiovasc Med 7:22
Gregor MF, Hotamisligil GS (2011) Inflammatory mechanisms in obesity. Annu Rev Immunol 29:415–445
Escobar-Morreale HF, Calvo RM, Villuendas G, Sancho J, San Millán JL (2003) Association of polymorphisms in the interleukin 6 receptor complex with obesity and hyperandrogenism. Obes Res 11(8):987–996
Peral B, San Millán JL, Castello R, Moghetti P, Escobar-Morreale HF (2002) Comment: the methionine 196 arginine polymorphism in exon 6 of the TNF receptor 2 gene (TNFRSF1B) is associated with the polycystic ovary syndrome and hyperandrogenism. J Clin Endocrinol Metab 87(8):3977–3983
Tarkun I, Arslan BC, Cantürk Z, Türemen E, Sahin T, Duman C (2004) Endothelial dysfunction in young women with polycystic ovary syndrome: relationship with insulin resistance and low-grade chronic inflammation. J Clin Endocrinol Metab 89(11):5592–5596
Boulman N, Levy Y, Leiba R, Shachar S, Linn R, Zinder O, Blumenfeld Z (2004) Increased C-reactive protein levels in the polycystic ovary syndrome: a marker of cardiovascular disease. J Clin Endocrinol Metab 89(5):2160–2165
González F (2012) Inflammation in polycystic ovary syndrome: underpinning of insulin resistance and ovarian dysfunction. Steroids 77(4):300–305
Polito R, Monda V, Nigro E, Messina A, Di Maio G, Giuliano MT, Orrù S, Imperlini E, Calcagno G, Mosca L, Mollica MP, Trinchese G, Scarinci A, Sessa F, Salerno M, Marsala G, Buono P, Mancini A, Monda M, Daniele A, Messina G (2020) The important role of adiponectin and orexin-A, two key proteins improving healthy status: focus on physical activity. Front Physiol 11:356
Ristagno G, Fumagalli F, Bottazzi B, Mantovani A, Olivari D, Novelli D, Latini R (2019) Pentraxin 3 in cardiovascular disease. Front Immunol 17(10):823
Abiad F, Khalife D, Safadi B, Alami R, Awwad J, Khalifeh F, Ghazeeri G (2018) The effect of bariatric surgery on inflammatory markers in women with polycystic ovarian syndrome. Diabetes Metab Syndr 12(6):999–1005
Dunaif A, Segal KR, Futterweit W, Dobrjansky A (1989) Profound peripheral insulin resistance, independent of obesity, in polycystic ovary syndrome. Diabetes 38(9):1165–1174
Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T (1992) Evidence for distinctive and intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes 41(10):1257–1266
Gambineri A, Pelusi C, Manicardi E, Vicennati V, Cacciari M, Morselli-Labate AM, Pagotto U, Pasquali R (2004) Glucose intolerance in a large cohort of Mediterranean women with polycystic ovary syndrome: phenotype and associated factors. Diabetes 53(9):2353–2358
Bhattacharya SM (2010) Prevalence of metabolic syndrome in women with polycystic ovary syndrome, using two proposed definitions. Gynecol Endocrinol 26(7):516–520
Kaaks R, Lukanova A, Kurzer MS (2002) Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 11(12):1531–1543
Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 348(17):1625–38
Schildkraut JM, Schwingl PJ, Bastos E, Evanoff A, Hughes C (1996) Epithelial ovarian cancer risk among women with polycystic ovary syndrome. Obstet Gynecol 88(4 Pt 1):554–559
Schmeler KM, Soliman PT, Sun CC, Slomovitz BM, Gershenson DM, Lu KH (2005) Endometrial cancer in young, normal-weight women. Gynecol Oncol 99(2):388–392
Secreto G, Zumoff B (1994) Abnormal production of androgens in women with breast cancer. Anticancer Res 14(5B):2113–7
Hall JE, Crook ED, Jones DW, Wofford MR, Dubbert PM (2002) Mechanisms of obesity-associated cardiovascular and renal disease. Am J Med Sci 324(3):127–137
Fabbrini E, Sullivan S, Klein S (2010) Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications. Hepatology 51(2):679–689
Bhattacharya K, Sengupta P, Datta S, Bhattacharya S (2020) pathophysiology of obesity: endocrine, inflammatory and neural regulators. Research J Pharm And Tech 13(9):4469–4478
Legro RS, Castracane VD, Kauffman RP (2004) Detecting insulin resistance in polycystic ovary syndrome: purposes and pitfalls. Obstet Gynecol Surv 59(2):141–154
O’Driscoll JB, Mamtora H, Higginson J, Pollock A, Kane J, Anderson DC (1994) A prospective study of the prevalence of clear-cut endocrine disorders and polycystic ovaries in 350 patients presenting with hirsutism or androgenic alopecia. Clin Endocrinol (Oxf) 41(2):231–236
Makki K, Froguel P, Wolowczuk I (2013) Adipose tissue in obesity-related inflammation and insulin resistance: cells, cytokines, and chemokines. ISRN Inflamm 22(2013):139239
DeUgarte CM, Bartolucci AA, Azziz R (2005) Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil Steril 83(5):1454–1460
Nehir Aytan A, Bastu E, Demiral I, Bulut H, Dogan M, Buyru F (2016) Relationship between hyperandrogenism, obesity, inflammation and polycystic ovary syndrome. Gynecol Endocrinol 32(9):709–713
Ün B, Dolapçıoğlu KS, Güler Okyay A, Şahin H, Beyazıt A (2016) Evaluation of hs-CRP and visseral adiposity index in patients with policystic ovary syndrome by clinical and laboratory findings. Eur J Obstet Gynecol Reprod Biol 204:16–20
Escobar-Morreale HF, Luque-Ramírez M, González F (2011) Circulating inflammatory markers in polycystic ovary syndrome: a systematic review and metaanalysis. Fertil Steril 95(3):1048–58.e1-2
Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK (2003) Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J 17(8):884–886
Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, Mooney RA (2003) Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem 278(16):13740–13746
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316(2):129–139
Torres-Leal FL, Fonseca-Alaniz MH, Rogero MM, Tirapegui J (2010) The role of inflamed adipose tissue in the insulin resistance. Cell Biochem Funct 28(8):623–631
Shorakae S, Ranasinha S, Abell S, Lambert G, Lambert E, de Courten B, Teede H (2018) Inter-related effects of insulin resistance, hyperandrogenism, sympathetic dysfunction and chronic inflammation in PCOS. Clin Endocrinol (Oxf) 89(5):628–633
González F, Nair KS, Daniels JK, Basal E, Schimke JM (2012) Hyperandrogenism sensitizes mononuclear cells to promote glucose-induced inflammation in lean reproductive-age women. Am J Physiol Endocrinol Metab 302(3):E297-306
Rojas J, Chávez M, Olivar L, Rojas M, Morillo J, Mejías J, Calvo M, Bermúdez V (2014) Polycystic ovary syndrome, insulin resistance, and obesity: navigating the pathophysiologic labyrinth. Int J Reprod Med 2014:719050
Henninger AM, Eliasson B, Jenndahl LE, Hammarstedt A (2014) Adipocyte hypertrophy, inflammation and fibrosis characterize subcutaneous adipose tissue of healthy, non-obese subjects predisposed to type 2 diabetes. PLoS ONE 9(8):e105262
Spritzer PM, Lecke SB, Satler F, Morsch DM (2015) Adipose tissue dysfunction, adipokines, and low-grade chronic inflammation in polycystic ovary syndrome. Reproduction 149(5):R219–R227
Ostinelli G, Laforest S, Denham SG, Gauthier MF, Drolet-Labelle V, Scott E, Hould FS, Marceau S, Homer NZM, Bégin C, Andrew R, Tchernof A (2022) Increased adipose tissue indices of androgen catabolism and aromatization in women with metabolic dysfunction. J Clin Endocrinol Metab 107(8):e3330–e3342
Wang L, Li S, Zhao A, Tao T, Mao X, Zhang P, Liu W (2012) The expression of sex steroid synthesis and inactivation enzymes in subcutaneous adipose tissue of PCOS patients. J Steroid Biochem Mol Biol 132(1–2):120–126
Daskalopoulos G, Karkanaki A, Piouka A, Prapas N, Panidis D, Gkeleris P, Athyros VG (2015) Excess metabolic and cardiovascular risk is not manifested in all phenotypes of polycystic ovary syndrome: implications for diagnosis and treatment. Curr Vasc Pharmacol 13(6):788–800
Wisse BE (2004) The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol 15(11):2792–2800
Khan MJ, Ullah A, Basit S (2019) Genetic basis of polycystic ovary syndrome (PCOS): current perspectives. Appl Clin Genet 24(12):249–260
Kistner TM, Pedersen BK, Lieberman DE (2022) Interleukin 6 as an energy allocator in muscle tissue. Nat Metab 4(2):170–179
Covarrubias AJ, Horng T (2014) IL-6 strikes a balance in metabolic inflammation. Cell Metab 19(6):898–899
Fulghesu AM, Sanna F, Uda S, Magnini R, Portoghese E, Batetta B (2011) IL-6 serum levels and production is related to an altered immune response in polycystic ovary syndrome girls with insulin resistance. Mediators Inflamm 2011:389317
Mitchell M, Armstrong DT, Robker RL, Norman RJ (2005) Adipokines: implications for female fertility and obesity. Reproduction 130(5):583–597
Cao H (2014) Adipocytokines in obesity and metabolic disease. J Endocrinol 220(2):T47-59
Hotamisligil GS, Shargill NS, Spiegelman BM (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259(5091):87–91
Oróstica L, Astorga I, Plaza-Parrochia F, Vera C, García V, Carvajal R, Gabler F, Romero C, Vega M (2016) Proinflammatory environment and role of TNF-α in endometrial function of obese women having polycystic ovarian syndrome. Int J Obes (Lond) 40(11):1715–1722
Peraldi P, Hotamisligil GS, Buurman WA, White MF, Spiegelman BM (1996) Tumor necrosis factor (TNF)-alpha inhibits insulin signaling through stimulation of the p55 TNF receptor and activation of sphingomyelinase. J Biol Chem 271(22):13018–13022
Thathapudi S, Kodati V, Erukkambattu J, Katragadda A, Addepally U, Hasan Q (2014) Tumor necrosis factor-alpha and polycystic ovarian syndrome: a clinical, biochemical, and molecular genetic study. Genet Test Mol Biomarkers 18(9):605–609
Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL (2001) TNF-alpha and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab 86(8):3761–3767
Gareb B, Otten AT, Frijlink HW, Dijkstra G, Kosterink JGW (2020) Review: local tumor necrosis factor-α inhibition in inflammatory bowel disease. Pharmaceutics 12(6):539
Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH (2021) The role of tumor necrosis factor alpha (TNF-α) in autoimmune disease and current TNF-α inhibitors in therapeutics. Int J Mol Sci 22(5):2719
Pagnini C, Cominelli F (2021) Tumor necrosis factor’s pathway in Crohn’s disease: potential for intervention. Int J Mol Sci 22(19):10273
Figueroa F, Motta A, Acosta M, Mohamed F, Oliveros L, Forneris M (2015) Role of macrophage secretions on rat polycystic ovary: its effect on apoptosis. Reproduction 150(5):437–448
Kelly CC, Lyall H, Petrie JR, Gould GW, Connell JM, Sattar N (2001) Low grade chronic inflammation in women with polycystic ovarian syndrome. J Clin Endocrinol Metab 86(6):2453–2455
Özçaka Ö, Buduneli N, Ceyhan BO, Akcali A, Hannah V, Nile C, Lappin DF (2013) Is interleukin-17 involved in the interaction between polycystic ovary syndrome and gingival inflammation? J Periodontol 84(12):1827–1837
Kuang H, Duan Y, Li D, Xu Y, Ai W, Li W, Wang Y, Liu S, Li M, Liu X, Shao M (2020) The role of serum inflammatory cytokines and berberine in the insulin signaling pathway among women with polycystic ovary syndrome. PLoS One 15(8):e0235404
Yildiz BO, Bozdag G, Yapici Z, Esinler I, Yarali H (2012) Prevalence, phenotype and cardiometabolic risk of polycystic ovary syndrome under different diagnostic criteria. Hum Reprod 27(10):3067–3073
Bates GW Jr (2019) Polycystic ovary syndrome: a reproductive and metabolic web of risk, comorbidities, and disease. Fertil Steril 111(3):471–472
Blumenfeld Z (2019) The possible practical implication of high CRP levels in PCOS. Clin Med Insights Reprod Health 22(13):1179558119861936
Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342(12):836–843
Kalyan S, Goshtesabi A, Sarray S, Joannou A, Almawi WY (2018) Assessing C reactive protein/albumin ratio as a new biomarker for polycystic ovary syndrome: a case-control study of women from Bahraini medical clinics. BMJ Open 8(10):e021860
Hage FG, Szalai AJ (2009) The role of C-reactive protein polymorphisms in inflammation and cardiovascular risk. Curr Atheroscler Rep 11(2):124–130
Lejman-Larysz K, Pietrzyk D, Ćwiertnia A, Kozłowski M, Kwiatkowski S, Szydłowska I, Nawrocka-Rutkowska J, Brodowski J, Sowińska-Przepiera E, Cymbaluk-Płoska A, Brodowska A (2023) Influence of hsCRP parameter on the occurrence of metabolic syndrome in patients with polycystic ovary syndrome. Biomedicines 11(7):1953
Kolaczkowska E, Kubes P (2013) Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 13(3):159–175
Oncel RC, Ucar M, Karakas MS, Akdemir B, Yanikoglu A, Gulcan AR, Altekin RE, Demir I (2015) Relation of neutrophil-to-lymphocyte ratio with GRACE risk score to in-hospital cardiac events in patients with ST-segment elevated myocardial infarction. Clin Appl Thromb Hemost 21(4):383–388
Karakas MS, Korucuk N, Tosun V, Altekin RE, Koç F, Ozbek SC, Ozel D, Ermis C (2016) Red cell distribution width and neutrophil-to-lymphocyte ratio predict left ventricular dysfunction in acute anterior ST-segment elevation myocardial infarction. J Saudi Heart Assoc 28(3):152–158
Liu CC, Ko HJ, Liu WS, Hung CL, Hu KC, Yu LY, Shih SC (2019) Neutrophil-to-lymphocyte ratio as a predictive marker of metabolic syndrome. Medicine (Baltimore) 98(43):e17537
Liu Z, Xv Y, Liu X, Zhou X (2022) Associations of systemic inflammatory markers with the risks of chronic heart failure: a case-control study. Clinics (Sao Paulo) 14(77):100056
Dabass A, Talbott EO, Rager JR, Marsh GM, Venkat A, Holguin F, Sharma RK (2018) Systemic inflammatory markers associated with cardiovascular disease and acute and chronic exposure to fine particulate matter air pollution (PM2.5) among US NHANES adults with metabolic syndrome. Environ Res 161:485–491
Brooks GC, Blaha MJ, Blumenthal RS (2010) Relation of C-reactive protein to abdominal adiposity. Am J Cardiol 106(1):56–61
Imtiaz F, Shafique K, Mirza SS, Ayoob Z, Vart P, Rao S (2012) Neutrophil lymphocyte ratio as a measure of systemic inflammation in prevalent chronic diseases in Asian population. Int Arch Med 5(1):2
van den Brink W, van Bilsen J, Salic K, Hoevenaars FPM, Verschuren L, Kleemann R, Bouwman J, Ronnett GV, van Ommen B, Wopereis S (2019) Current and future nutritional strategies to modulate inflammatory dynamics in metabolic disorders. Front Nutr 26(6):129
Tylutka A, Morawin B, Walas Ł, Michałek M, Gwara A, Zembron-Lacny A (2023) Assessment of metabolic syndrome predictors in relation to inflammation and visceral fat tissue in older adults. Sci Rep 13(1):89
Saito K, Kihara K (2010) Role of C-reactive protein as a biomarker for renal cell carcinoma. Expert Rev Anticancer Ther 10(12):1979–1989
Lee S, Choe JW, Kim HK, Sung J (2011) High-sensitivity C-reactive protein and cancer. J Epidemiol 21(3):161–168
Dudda J, Schupp T, Rusnak J, Weidner K, Abumayyaleh M, Ruka M, Egner-Walter S, Forner J, Müller J, Bertsch T, Kittel M, Akin I, Behnes M (2023) C-reactive protein and white blood cell count in cardiogenic shock. J Clin Med 12(3):965
Chung PS, Tsai KZ, Lin YP, Lin YK, Lin GM (2020) Association between leukocyte counts and physical fitness in male military members: the CHIEF study. Sci Rep 10(1):6082
Farhangi MA, Keshavarz SA, Eshraghian M, Ostadrahimi A, Saboor-Yaraghi AA (2013) White blood cell count in women: relation to inflammatory biomarkers, haematological profiles, visceral adiposity, and other cardiovascular risk factors. J Health Popul Nutr 31(1):58–64
Patel S (2018) Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J Steroid Biochem Mol Biol 182:27–36
Petrie JR, Guzik TJ, Touyz RM (2018) Diabetes, hypertension, and cardiovascular disease: clinical insights and vascular mechanisms. Can J Cardiol 34(5):575–584
Garoufi A, Pagoni A, Papadaki M, Marmarinos A, Karapostolakis G, Michala L, Soldatou A (2021) Cardiovascular risk factors and subclinical atherosclerosis in Greek adolescents with polycystic ovary syndrome: its relationship with body mass index. Children (Basel) 9(1):4
Angkananard T, Anothaisintawee T, McEvoy M, Attia J, Thakkinstian A (2018) Neutrophil lymphocyte ratio and cardiovascular disease risk: a systematic review and meta-analysis. Biomed Res Int 11(2018):2703518
Liu W, Li S, Lou X, Li D, Wang F, Zhang Z (2022) Assessment of neutrophil to lymphocyte ratio, C-reactive protein, mean platelet volume in obese, and nonobese patients with polycystic ovary syndrome. Medicine (Baltimore) 101(29):e29678
Almaeen AH, Alduraywish AA, Nabi M, Shah NN, Shaik R, Tantry BA (2022) Quantitative changes in white blood cells: correlation with the hallmarks of polycystic ovary syndrome. Medicina (Kaunas) 58(4):535
Pergialiotis V, Trakakis E, Parthenis C, Hatziagelaki E, Chrelias C, Thomakos N, Papantoniou N. Correlation of platelet to lymphocyte and neutrophil to lymphocyte ratio with hormonal and metabolic parameters in women with PCOS. Horm Mol Biol Clin Investig. 2018;34(3):/j/hmbci.2018.34.issue-3/hmbci-2017-0073/hmbci-2017-0073.xml. https://doi.org/10.1515/hmbci-2017-0073.
Taşkömür AT, Erten Ö (2022) Relationship of inflammatory and metabolic parameters in adolescents with PCOS: BMI matched case-control study. Arch Endocrinol Metab 66(3):372–381
Li L, Yu J, Zhou Z (2022) Mean platelet volume and polycystic ovary syndrome: a systematic review and meta-analysis. J Int Med Res 50(1):3000605211067316
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
KB and RD participated in the conception and design of the study. KB, RD, GRC, NP, and AKS participated in literature search and extraction. AKB, RB, MPP, CB, RB, NS, and SSR contributed to writing the manuscript. KB, RD, AKB, and AKS contributed to revising and finalizing the article.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dey, R., Bhattacharya, K., Basak, A.K. et al. Inflammatory perspectives of polycystic ovary syndrome: role of specific mediators and markers. Middle East Fertil Soc J 28, 33 (2023). https://doi.org/10.1186/s43043-023-00158-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-023-00158-2