Abstract
Background
Oxidative stress has an undeniable role in the impairment of sperm function and idiopathic male infertility. On the other hand, the local antioxidant system particularly glutathione peroxidase 3 (GPX3) as an extracellular enzyme protects male fertility from oxidative damages. Therefore, in the current study, we evaluated the association between two functional polymorphisms of the GPX3 gene with its levels in seminal fluid and subsequently with the risk of male infertility.
Result
We recruited 100 fertile and 100 infertile men for the study. Our results showed that the concentration of GPX3 was higher in the fertile group than infertile patients (p= <0.01), and there were positive correlations between GPX3 concentration in seminal fluid with sperm motility and morphology. The frequency of rs8177404 and rs3828599 genotypes and alleles was significantly different between the groups and we found that having the rs8177404 polymorphism (TC and CC genotypes) could increase the risk of idiopathic infertility more than 2-fold. On the other hand, the GG genotype (rs3828599) showed a protective effect against infertility. Our results demonstrated that men carrying CC genotype of rs8177404 polymorphism had significantly lower progressively motile sperm and higher immotile sperm compared with subjects carrying TT and TC genotypes. In the rs3828599 polymorphism, the GG carriers had significantly higher progressively motile and lower immotile sperm than AA carriers. Furthermore, men with genotypes of CC (rs8177404) and GG (rs3828599) had significantly lower and higher levels of GPX3 in the seminal fluid, respectively.
Conclusion
In conclusion, our results showed associations between sperm parameters with GPX3 levels and the gene polymorphisms. It seems rs8177404 and rs3828599 polymorphisms can affect GPX3 levels in seminal fluid and subsequently sperm parameters.
Similar content being viewed by others
Background
Statistics show that the prevalence of infertility in the world is about 10 to 15 %, of which approximately 45% is due to male factors [1]. More than 25% of infertile men show abnormal semen profiles with an unknown cause called idiopathic infertility [2]. However, oxidative stress has been introduced as one of the main reasons for idiopathic infertility [3] and it has been reported that the levels of reactive oxygen species (ROS) were elevated among 25% of infertile patients [4, 5].
Spermatozoa and white blood cells are the sources of ROS production in the semen [4, 5]. However, there is a balance between ROS production and elimination under physiological conditions and therefore its level is strictly controlled in sperm and seminal plasma. In oxidative stress, the balance is lost and a large amount of ROS accumulates in semen [6]. Oxidative stress can negatively affect sperm motility, acrosome reaction, and oocyte fusion and subsequently cause infertility. To prevent oxidative stress, there are enzymatic and non-enzymatic antioxidant defense systems in semen such as superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase, vitamins E and A, ascorbic acid, and glutathione [7].
The GPX, as one of the most important antioxidant enzymes, converts hydrogen peroxide (H2O2) into H2O and O2 [8]. Studies demonstrated that GPX 1, 3, 4, and 5 isoforms are present in the human reproductive system. Recent studies have indicated that GPX activity is associated with sperm DNA integrity as well as motility and count [9, 10]. The GPX3 isoform, also known as extracellular GPX, has been found in human seminal fluid. More interestingly, Giannattasio et al. [11] showed that GPX3 activity was ten-time higher in the seminal fluid of fertile than in infertile men.
Polymorphism of the genes which are involved in antioxidant defense or spermatogenesis could induce oxidative stress and male infertility [12, 13]. One of the factors that may affect the expression and activity of GPX3 in semen is the functional polymorphisms of its gene [14]. One of the most important polymorphisms of GPX3 which is located in the promoter region is rs8177404 (T to C). Studies showed that this polymorphism could reduce the expression of GPX3 and increase the risk of metabolic syndrome [15]. Another functional polymorphism in the promoter region of the GPX3 gene is rs3828599 (A to G). Studies indicated that the G allele could increase the expression of the GPX3 gene [14] and therefore the GG genotype could act as a protective factor against cancers [16].
Given the role of GPX3 in protecting sperm against oxidative stress in seminal fluid, and also the existence of functional polymorphisms in the GPX3 gene which may be involved in male fertility, in this study, we investigated the possible association between rs3828599 and rs8177404 polymorphisms with the risk of male infertility. Moreover, we evaluated the relationship between these polymorphisms and GPX3 levels in seminal fluid and semen parameters in fertile and infertile men.
Methods
Study population and sample collection
In this study, a total of 200 men (100 idiopathic infertile and 100 fertile men) were recruited. The infertile men were selected from the patients who were referred to the infertility department of Al-Zahra Hospital or the Milad Infertility Clinic of Tabriz. Infertile men had no children after 1 year (or more) of unprotected sexual intercourse and had an abnormal semen profile according to World Health Organization (WHO) [2010] guidelines. Moreover, the cause of their infertility was unknown and patients with known infertility causes such as varicocele were excluded. Furthermore, men with severe oligozoospermia (<5 million/ml sperm) and azoospermia were not enrolled in this study. Fertile men were recruited from age-matched individuals who had children in the last 3 years without benefit from assisted reproductive technologies (ART). The persons who were taking medication, smoking cigarettes, or had diabetes, liver, kidney, and thyroid diseases were excluded from the study.
After 3 days of sexual abstinence, the semen samples were collected from all participants. After 40-min liquefaction, the semen parameters were analyzed according to the WHO criteria [WHO 2010] under a light microscope by a well-trained technician. Then, the samples were divided into sperm (pellet) and seminal fluid (supernatant) parts using centrifugation. The supernatant was kept at −20°C for measurement of GPX3 levels and the sperm were stored at −80°C for DNA isolation and gene analysis.
Enzyme-linked immunosorbent assay (ELISA)
The levels of GPX3 (ng/ml) were measured by a commercial human ELISA kit (Cat. Num. MBS765842 MyBioSource, San Diego, CA) as instructed by the company. This sandwich ELISA kit had a detection range of 1.56–100 ng/ml with a sensitivity of <0.938 ng/ml. The enzyme-substrate reaction was terminated by the provided stop solution and then the color change was spectrophotometrically measured at a wavelength of 450 nm. Finally, the enzyme levels in each sample were determined by comparing the O.D. of the samples to the standard curve.
Genotyping
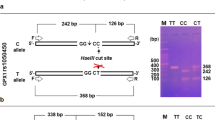
The genomic DNA was isolated from the sperm using a DNA extraction kit (BIORON, Germany). To analyze the quality and integrity of the extracted DNA sample, a 1.5% agarose gel electrophoresis was used. Moreover, the ratio of A260/A280 absorbance was obtained using NanoDrop 1000 (NanoDrop, Wilmington, USA) to evaluate the DNA purity. Tetra-primer ARMS-PCR was carried out to investigate the genotypes of rs8177404 and rs3828599 polymorphisms. For this purpose, we used the PCR master mix (Ampliqon, Denmark) and specific primers (listed in Table 1). After performing the PCR, the PCR product was run on 1.5% agarose gel and the size of products was evaluated after visualization under ultraviolet (UV) light. In the rs8177404 polymorphism, the existence of two bands with a length of 540bp and 370bp was assumed as the TT genotype, while the existence of bands with the length of 540bp and 223bp was considered as the CC genotype. Furthermore, the presence of three fragments with the length of 540bp, 370bp, and 223bp was referred to the TC genotype (Fig. 1a). As shown in Fig. 1b, the presence of two bands with the length of 494bp and 235bp, and length of 494bp and 316bp and three fragments (495bp, 316bp, and 235bp) were referred as the AA, GG, and AG genotypes of rs3828599 polymorphism, respectively.
The PCR products of rs8177404 and rs3828599 GPX3 gene polymorphisms on 1.5% agarose gel using tetra-primer ARMS-PCR. a The presence of two bands with a length of 540bp and 370bp, and length of 540bp and 223bp and three fragments (540bp, 370bp, and 223bp) were referred as the TT, CC, and TC genotypes of rs8177404 polymorphism, respectively. b The presence of two bands with a length of 494bp and 235bp, and length of 494bp and 316bp and three fragments (495bp, 316bp, and 235bp) were referred as the AA, GG, and AG genotypes of rs3828599 polymorphism, respectively. L, DNA ladder
Statistical analysis
The results were expressed as mean ± standard deviation. The normal distribution of data was confirmed by the one-sample Kolmogorov-Smirnov test. The semen parameters and GPX3 levels were compared between the infertile and fertile groups and among the genotypes by t-test and One-way ANOVA analysis, respectively. Tukey post hoc test was also applied as a follow-up test to evaluate the quantitative data between two genotypes. The genotype distribution was checked for Hardy-Weinberg equilibrium. The frequencies of allele and genotype of the polymorphisms were compared between the groups using the chi-square test. To the relative risk of the genotypes/alleles on male infertility, logistic regression analysis was used. The p<0.05 level was considered as the statistical significance and the data were analyzed using SPSS V.16 software.
Results
There was no significant difference in age (24.25±6.19 vs. 33.69±5.24 years, p=0.491) and body mass index (BMI) (26.37±3.03 vs. 25.50±3.76 kg/m2, p=0.077) between fertile and infertile men. We found that the concentration of GPX3 in the seminal fluid of fertile men was significantly higher than in infertile patients (2.62±0.89 vs. 1.8±0.80 ng/ml, respectively; p= <0.01). The sperm parameters of fertile and infertile men are summarized in Table 2. The range of sperm concentration in fertile and infertile groups was 45–80 and 8–65 million/ml, respectively. We found that there were significant differences in concentration, motility, and morphology of sperm between the groups (p<0.05).
As demonstrated in Table 3, there was a moderate positive correlation between GPX3 concentration in seminal fluid and the percentage of progressively motile sperm in both groups (p<0.05). Moreover, we observed a moderate positive correlation between GPX concentration and the number of sperm with normal morphology in the fertile group (r=0.428 and p=0.047).
Our results indicated that the frequency of rs8177404 and rs3828599 genotypes and alleles was significantly different between the fertile and infertile groups as shown in Tables 4 and 5, respectively. In this regard, we found a higher frequency of TT genotype (or T allele) of rs8177404 polymorphism in fertile men compared to infertile individuals (p=0.034). In rs3828599 polymorphism, infertile men carried a higher frequency of the wild-type genotype (AA) when compared with fertile persons (p=0.025). Logistic regression analysis showed that having the rs8177404 polymorphism (TC and CC genotypes) could increase the risk of idiopathic infertility more than 2-fold (Table 4). On the other hand, the genotype GG (rs3828599) showed a protective effect against infertility that can decrease the risk of male infertility about 3 (1/0.338) times (Table 5).
Our results demonstrated that the fertile men carrying CC genotype had significantly lower progressively motile sperm and higher immotile sperm compared with subjects carrying TT and TC genotypes (Table 6). Moreover, men with the CC genotype had statistically lower sperm with normal morphology in comparison with TT carriers. In the infertile group, the individuals with TT genotype had higher levels of progressively motile sperm and sperm concentration compared to the CC carriers. The rs3828599 polymorphism did not show a significant association with sperm parameters. However, GG carriers had significantly higher progressively motile sperm than AA carriers in the fertile group. In the infertile group, individuals with the GG genotype had lower immotile sperm than AA genotype carriers.
As shown in Table 7, the fertile men with genotypes of CC (rs8177404) and GG (rs3828599) had significantly lower and higher levels of GPX3 in the seminal fluid, respectively. In the infertile group, the CC genotype was also associated with lower GPX3 levels.
Discussion
Given the role of oxidative stress in abnormal sperm function [6, 7] and the importance of the local antioxidant system particularly GPX3 in the protection of male fertility [9, 10], in the current study, we evaluated the association between rs3828599 and rs8177404 polymorphisms of GPX3 gene with its levels in seminal fluid and subsequently with the risk of male infertility.
Giannattasio et al. [11] evaluated the GPX3 enzyme in seminal fluid and showed that its activity was ten-times higher in fertile men compared to the infertile patients. In accordance, we also found a higher concentration of GPX3 in the seminal fluid of fertile men than of infertile patients. Although, Giannattasio et al. [11] did not measure the levels of the enzyme, given the current study findings, part of the elevated activity in fertile men might be due to higher levels of the GPX3 enzyme in the seminal fluid of fertile individuals than the infertile group. In supporting the role of seminal fluid GPX3 in sperm function, we observed positive correlations between GPX3 concentration with sperm motility and morphology. Spermatozoa is very susceptible to the oxidative stress due to abundant polyunsaturated fatty acids in the membrane and also being transcriptionally inactive (reviewed in Ref. [3]). Therefore, low levels of GPX3 in the seminal plasma could result in excessive hydrogen peroxide and consequently oxidative stress. More interestingly, it has been shown that hydrogen peroxide is the major ROS responsible for impairing the motility of spermatozoa [17]. Crisol et al. [18] also reported lower activity of GPX (the total activity of isoforms) in samples with severe asthenozoospermia, oligozoospermia, and teratozoospermia compared with normal samples. Moreover, it has been indicated that the intracellular levels of GSH were lower when sperm morphology was severely impaired [19]. In contrast, Macanovic and colleagues [20] reported negative correlations between GPX activity with sperm morphology and motility. Such a contradiction could be due to, firstly, assessment of total GPX instead of isoform 3 alone, which is the most important isoform in the seminal fluid; secondly, the low sample size of Macanovic’s study; and thirdly, recruiting patients who were normozoospermic but applied for reproductive technology procedures for fertility treatment.
To investigate one of the possible reasons underlying low levels of GPX3 in the seminal fluid of infertile patients, we analyzed the frequency of rs8177404 and rs3828599 polymorphisms which are located at the promoter region of the GPX3 gene and can potentially affect the gene expression and consequently GPX3 levels [14, 15]. Our results represented that the frequency of the TT genotype (rs8177404) was higher in fertile men than infertile patients and having the C allele (both TC and CC genotypes) could increase the risk of male infertility. As shown in the current study, the CC genotype was negatively associated with sperm motility and morphology. On the other hand, the association between male infertility with abnormal morphology and motility of sperm has been well-documented [21,22,23]. A previous study showed that T to C substitution at promotor of GPX3 gene in rs8177404 polymorphism could reduce the gene expression [15] that can result in low levels of GPX3 enzyme; we also found lower levels of GPX3 in the seminal fluid of individuals with CC genotype compared to other genotypes. Therefore, it can be hypothesized that rs8177404 polymorphism can negatively affect sperm motility and morphology by reducing the levels of GPX3 and consequently increasing ROS in the seminal fluid. Huang et al. [24] also reported an association between this oxidative stress-related genetic variant and arterial ischemic stroke.
In rs3828599 polymorphism, the frequency of wild genotype (AA) was higher among infertile than fertile groups and the GG genotype demonstrated a protective effect against male infertility. Moreover, we found a positive association between the GG genotype and sperm motility. Previous studies showed that the GG genotype could increase the expression of the GPX3 gene and act as a protective factor [14, 16]. In parallel with previous findings, we also found higher levels of GPX3 in the seminal fluid of men carrying the GG genotype compared to other genotypes (AA and GA). Therefore, the GG genotype could increase the gene expression and levels of GPX3 enzyme in the seminal fluid and consequently protect sperm from oxidative stress.
For the first time, this study evaluated GPX3 gene polymorphisms in fertile and infertile men and their association with levels of GPX3 enzyme and sperm parameters. However, several limitations should be noted with this study, including relatively low sample size and lack of data regarding ROS status in the semen or sperm. Therefore, further studies are required to investigate the association between GPX3 polymorphisms and oxidative stress-related damages of sperm.
Conclusions
In conclusion, our results showed that GPX3 levels were significantly lower in the seminal fluid of infertile men compared to fertile individuals and its levels were significantly associated with sperm parameters, particularly motility and morphology. Moreover, we found positive and negative associations between rs8177404 and rs3828599 GPX3 gene polymorphisms and male infertility. It can be noted that these polymorphisms can affect GPX3 levels in seminal fluid and subsequently sperm parameters.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- ART:
-
Assisted reproductive technologies
- BMI:
-
Body mass index
- GPX:
-
Glutathione peroxidase
- H2O2 :
-
Hydrogen peroxide
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
References
Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA (2012) National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 9(12):e1001356. https://doi.org/10.1371/journal.pmed.1001356
Kothandaraman N, Agarwal A, Abu-Elmagd M, Al-Qahtani MH (2016) Pathogenic landscape of idiopathic male infertility: new insight towards its regulatory networks. NPJ Genomic Med 1(1):1–9
Alahmar AT (2019) Role of oxidative stress in male infertility: an updated review. J Hum Reprod Sci 12(1):4–18. https://doi.org/10.4103/jhrs.JHRS_150_18
Sanocka D, Kurpisz M (2004) Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2(1):1–7
Zalata A, Christophe A, Depuydt C, F SCHOONJANS, and F Comhaire (1998) White blood cells cause oxidative damage to the fatty acid composition of phospholipids of human spermatozoa. Int J Androl 21(3):154–162. https://doi.org/10.1111/j.1365-2605.1998.00112.x
Agarwal A, Virk G, Ong C, Du Plessis SS (2014) Effect of oxidative stress on male reproduction. World J Mens Health 32(1):1–17. https://doi.org/10.5534/wjmh.2014.32.1.1
Huang C, Cao X, Pang D, Li C, Luo Q, Zou Y, Feng B, Li L, Cheng A, Chen Z (2018) Is male infertility associated with increased oxidative stress in seminal plasma? A-meta analysis. Oncotarget 9(36):24494–24513. https://doi.org/10.18632/oncotarget.25075
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta (BBA) Gen Sub 1830(5):3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020
Chabory E, Damon C, Lenoir A, Henry-Berger J, Vernet P, Cadet R, Saez F, Drevet J (2010) Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. J Anim Sci 88(4):1321–1331. https://doi.org/10.2527/jas.2009-2583
Drevet JR (2006) The antioxidant glutathione peroxidase family and spermatozoa: a complex story. Mol Cell Endocrinol 250(1-2):70–79. https://doi.org/10.1016/j.mce.2005.12.027
Giannattasio A, De Rosa M, Smeraglia R, Zarrilli S, Cimmino A, Di Rosario B, Ruggiero R, Colao A, Lombardi G (2002) Glutathione peroxidase (GPX) activity in seminal plasma of healthy and infertile males. J Endocrinol Invest 25(11):983–986. https://doi.org/10.1007/BF03344072
Tavilani H, Fattahi A, Esfahani M, Khodadadi I, Karimi J, Bahrayni E, Vatannejad A, Vaisi-Raygani A, Ghorbani M, Latifi Z (2014) Genotype and phenotype frequencies of paraoxonase 1 in fertile and infertile men. Syst Biol Reprod Med 60(6):361–366. https://doi.org/10.3109/19396368.2014.960624
Ghasemi H, Khodadadi I, Fattahi A, Moghimbeigi A, Tavilani H (2017) Polymorphisms of DNA repair genes XRCC1 and LIG4 and idiopathic male infertility. Syst Biol Reprod Med 63(6):382–390. https://doi.org/10.1080/19396368.2017.1374488
Lin J-C, Kuo W-R, Chiang F-Y, Hsiao P-J, Lee K-W, Wu C-W, Juo S-HH (2009) Glutathione peroxidase 3 gene polymorphisms and risk of differentiated thyroid cancer. Surgery 145(5):508–513. https://doi.org/10.1016/j.surg.2008.12.008
Baez-Duarte BG, Mendoza-Carrera F, García-Zapién A, Flores-Martínez SE, Sánchez-Corona J, Zamora-Ginez I, Torres-Rasgado E, León-Chávez BA, Pérez-Fuentes R, M R G o D o t I M d S Social (2014) Glutathione peroxidase 3 serum levels and GPX3 gene polymorphisms in subjects with metabolic syndrome. Arch Med Res 45(5):375–382. https://doi.org/10.1016/j.arcmed.2014.05.001
Wang J-Y, Yang I-P, Wu D-C, S-w H, Wu J-Y, Juo S-HH (2010) Functional glutathione peroxidase 3 polymorphisms associated with increased risk of Taiwanese patients with gastric cancer. Clin Chim Acta 411(19-20):1432–1436. https://doi.org/10.1016/j.cca.2010.05.026
Baumber J, Ball BA, GRAVANCE CG, Medina V, Davies-Morel MC (2000) The effect of reactive oxygen species on equine sperm motility, viability, acrosomal integrity, mitochondrial membrane potential, and membrane lipid peroxidation. J Androl 21(6):895–902
Crisol L, Matorras R, Aspichueta F, Expósito A, Hernández ML, Ruiz-Larrea MB, Mendoza R, Ruiz-Sanz JI (2012) Glutathione peroxidase activity in seminal plasma and its relationship to classical sperm parameters and in vitro fertilization-intracytoplasmic sperm injection outcome. Fertil Steril 97(4):852–857 e1
Garrido N, Meseguer M, Alvarez J, Simón C, Pellicer A, Remohí J (2004) Relationship among standard semen parameters, glutathione peroxidase/glutathione reductase activity, and mRNA expression and reduced glutathione content in ejaculated spermatozoa from fertile and infertile men. Fertil Steril 82:1059–1066
Macanovic B, Vucetic M, Jankovic A, Stancic A, Buzadzic B, Garalejic E, Korac A, Korac B, Otasevic V (2015) Correlation between sperm parameters and protein expression of antioxidative defense enzymes in seminal plasma: a pilot study. Dis Markers 2015:1–5. https://doi.org/10.1155/2015/436236.
Guzick DS, Overstreet JW, Factor-Litvak P, Brazil CK, Nakajima ST, Coutifaris C, Carson SA, Cisneros P, Steinkampf MP, Hill JA (2001) Sperm morphology, motility, and concentration in fertile and infertile men. N Engl J Med 345(19):1388–1393. https://doi.org/10.1056/NEJMoa003005
GilanI ZS, Gilani MAS (1998) The correlation between sperm morphology and motility in fertile and infertile men. Med J Islamic Rep Iran (MJIRI) 11(4):319–324
Milardi D, Grande G, Sacchini D, Astorri AL, Pompa G, Giampietro A, De Marinis L, Pontecorvi A, Spagnolo AG, Marana R (2012) Male fertility and reduction in semen parameters: a single tertiary-care center experience. Int J Endocrinol 2012:1–6. https://doi.org/10.1155/2012/649149.
K-m H, Zhao Z, Chen W-x (2009) The correlation between rs8177404 rs8177412 rs8177412 of promoter polymorphisms in the plasma glutathione peroxidase (GPX-3) gene and arterial ischemic stroke. J Apoplexy Nerv Dis 2:12
Acknowledgements
We thank staff of Al-Zahra Hospital and the Milad Infertility Clinic of Tabriz for preparing the samples and cooperation.
Funding
No specific funding was received for the study.
Author information
Authors and Affiliations
Contributions
Design and critical revisions: AF and MN; Laboratory analysis: MP, SG, and TG; literature review and manuscript drafting: AF, MP, and SG; All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Patients were given written informed consent after the full explanation of the study, and the study procedure was approved by the ethical committee of Ahar Branch, Islamic Azad University (approval cod: 22030503952005).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pournasir, M., Ghorbian, S., Ghasemnejad, T. et al. Glutathione peroxidase 3 (extracellular isoform) levels and functional polymorphisms in fertile and infertile men. Middle East Fertil Soc J 26, 13 (2021). https://doi.org/10.1186/s43043-021-00057-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-021-00057-4