Abstract
There is a dynamic interplay between pro- and anti-oxidant substances in human ejaculate. Excessive reactive oxygen species (ROS) generation can overwhelm protective mechanism and initiate changes in lipid and/or protein layers of sperm plasma membranes. Additionally, changes in DNA can be induced. The essential steps of lipid peroxidation have been listed as well as antioxidant substances of semen. A variety of detection techniques of lipid peroxidation have been summarized together with the lipid components of sperm membranes that can be subjected to stress. It is unsolved, a threshold for ROS levels that may induce functional sperm ability or may lead to male infertility.
Similar content being viewed by others
Introduction
Mammalian sperm cells present highly specific lipidic composition, high content of polyunsaturated fatty acids, plasmalogenes and sphingomyelins.
This unusual structure of sperm membrane is responsible for its flexibility and the functional ability of sperm cells. However, spermatozoa's lipids are the main substrates for peroxidation, what may provoke severe functional disorder of sperm. On the other hand, low (physiological) levels of lipid peroxidation reflect the influence of reactive oxygen species (ROS) on sperm metabolism enhancing the ability of human spermatozoa to interact with zona pellucida [1]. A reason for higher, pathological lipid peroxidation of sperm membranes can be unbalanced oxidative stress. In this review we will discuss the influence of reactive oxygen species on sperm function mainly in aspect of lipid peroxidation.
Free radicals
Free radicals are short-lived reactive chemical intermediates, which contain one or more electrons with unpaired spin (Table 1). They are highly reactive and oxidize lipids, amino acids and carbohydrates as well as causing DNA mutations. Reactive oxygen species therefore may have been implicated as an etiological factor of a very wide range of diseases [2–15]. Enhanced, pathological ROS generation in living organisms may be caused by several mechanisms like: ionizing radiation [16, 17], bioactivation of xenobiotics [18], inflammatory cells [19], increased cellular metabolism [20], decompartmentalisation of transition metal ions [21], activation of oxidases and oxygenases [22] and loss of antioxidant capacity [23, 24].
Membrane lipids
A characteristic feature of most, if not all, biological membranes is an asymmetrical arrangement of lipids within the bilayer. The lipid composition of plasma membrane of mammalian spermatozoa is markedly different from those of mammalian somatic cells. They have very high levels of phospholipids, sterols, saturated and polyunsaturated fatty acids therefore sperm cells are particularly susceptible to the damage induced by excessive ROS release (Table 2) [25–30].
Lipids are the major substances responsible for the fluidity of membrane lipid bilayers, and changes in composition of the plasma membranes of sperm cells from their epididymal maturation to their capacitation in the female reproductive tract. They are also involved as intermediates in the cell fusion [31–38].
Sperm cells undergo changes in lipid content during their passage through the epididymis. As a consequence of these changes the plasmalogenes (ether-linked lipids) become a major phospholipid component in the cauda epididymis and 2-fold increase of cholesterol/phospholipid molar ratio is observed during sperm migration from the seminiferous tubules [35].
Very high amounts of polyunsaturated fatty acids- especially docosahexaenoic (DHA) are found in the plasma membrane of human sperm (Table 2). DHA is thought to play a major role in regulating membrane fluidity in sperm and in the regulation of spermatogenesis [39–41]. DHA content is significantly higher in immature germ cells and immature spermatozoa as compared to mature sperm and indicates that that there is a net decrease in DHA content during the process of sperm maturation [41].
Another sperm lipid, phosphatidylserine, is known to translocate during capacitation. Before capacitation this phospholipid was found mainly in the midpiece but after capacitation, the localisation of phosphatidylserine was changed and it was identified also in the acrosomal region but never in the equatorial area [42].
Human sperm also contains desmosterol, which is lost during capacitation [43]. Other phospholipid, sphingomyelin in the sperm influences the rate of capacitation by slowing down the loss of sterols, and the exogenous sphingomyelinase accelerates capacitation promoting the loss of sterols and generating ceramide [44].
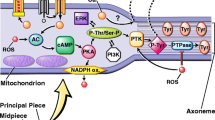
Cholesterol is known to regulate the fluidity and the permeability of cell membrane. Cholesterol efflux during capacitation enables the massive influx of extracellular Ca2+. Increased intracellular Ca2+ concentration plays an important role in the acrosome reaction [45–47]. The acrosome reaction is a crucial step during gamete interaction in all species, including man. It allows spermatozoa to penetrate the zona pellucida and fuse with the oocyte membrane. Spermatozoa unable to undergo the acrosome reaction will not fertilize intact oocytes. Zona pellucida binds to at least two different receptors in the plasma membrane. One is a Gi-coupled receptor that activates phospholipase C beta 1. The other one is a tyrosine kinase receptor coupled to phospholipase C gamma. Binding to the receptor would regulate adenylyl cyclase leading to elevation of cAMP and protein kinase activation. The protein kinase activates a voltage-dependent Ca2+ channel in the outer acrosomal membrane which releases Ca2+ from the interior of the acrosome to the cytosol. This is the first, relatively small rise in Ca2+ which leads to activation of the phospholipase C gamma. The products of phosphatidyl-inositol bisphosphate hydrolysis by phospholipase C diacylglycerol and inositol-trisphosphate will lead to protein kinase translocation to the plasma membrane and its activation. Protein kinase opens a voltage-dependent Ca2+ channel in the plasma membrane, leading to the second increase in Ca2+. The Gi or tyrosine kinase can also activate an Na+/H+ exchanger leading to alkalization of the cytosol. Protein kinase also activates phospholipase A2 to generate arachidonic acid from membrane phospholipids.
Arachidonic acid will be converted to prostaglandins and leukotriens by the enzymes, cyclooxygenase and lipoxygenase, respectively. The increase in Ca2+ and pH leads to membrane fusion and acrosomal exocytosis [48]. Acrosome reaction takes place in the anterior region of the sperm head. Acrosomal plasma membrane is probably prevented from fusion with the acrosomal outer membrane by its high concentration of anti-fusogenic sterols.
Sperm membrane lipids play also an important role regulating the polarized migration of sperm surface antigens during developmental processes such as maturation and capacitation. Moreover, lipid regionalization may also lead to protein regionalization by virtue of the preferential solubility of the proteins at different sites [49–51].
Furthermore, sperm membrane lipids are involved in gamete interactions. One of them is sulfogalactosylglycerolipid [52] and another one lysophosphatidylcholine [53], which stimulate the fertilizing ability of spermatozoa and induce the changes in composition of zona pellucida, and in oolemma promoting sperm-egg fusion.
In summary, the all-lipid components located in the sperm membranes are involved in regulation of sperm maturation, spermatogenesis, capacitation, acrosome reaction and eventually in membrane fusion. Obviously, peroxidation of sperm lipids may also disturb all the mentioned sperm functions, and in extreme cases even completely inhibit spermatogenesis.
Antioxidants
Every human ejaculate is contaminated with potential secretors of ROS, such as activated leukocytes, precursor germ cells or morphologically abnormal sperm cells. On the other hand, every human ejaculate has intra- and extracellular antioxidants of enzymatic and non-enzymatic systems. Enzymic and low-molecular weight antioxidants exist in human semen to scavenge free radicals as self-protection mechanisms [29, 54–56].
The most important antioxidants in human semen
Enzymic antioxidants:
Superoxide dismutase
Catalase
Glutathione peroxidase
Low molecular weight antioxidants:
α-tocopherol
β-carotene
ascorbate
urate
Transition-metal chelators
transferrin
lactoferrin
caeruloplasmin
In some pathological conditions (e.g., genital tract inflammation), the excessive ROS generation leads to seminal oxidative stress which may exhaust antioxidant activity. The final effect of peroxidation is observed among seminal lipids also by their elevated levels [57–59].
Peroxidation of membrane lipids
Peroxidation of polyunsaturated fatty acids (PUFAs) in sperm cell membranes is an autocatalytic, self-propagating reaction, which can give a rise to cell dysfunction associated with loss of membrane function and integrity. The first step in the peroxidation process, called – initiation – is the abstraction of a hydrogen atom from an unsaturated fatty acid. The second step – propagation – is the formation of a lipid alkyl radical followed then by its rapid reaction with oxygen to form a lipid peroxyl radical. The peroxyl radical is capable of abstracting a hydrogen atom from an unsaturated fatty acid with the concomitant formation of a lipid radical and lipid hydroperoxide. Since the peroxyl and alkyl radicals are regenerated, the cycle of propagation could continue indefinitely or until one of the substrates is consumed or terminated in the radical-radical reaction (Figure. 1).
Lipid peroxidation products
Peroxidation of polyunsaturated fatty acids has been implicated in a wide variety of pathological conditions including infertility, cardiac and cerebral ischaemic-reperfusion injury, and inflammatory joint diseases amongst others. The most popular (but not the most important) product of lipid peroxidation is malondialdehyde (MDA). There are a lot of other products of lipid peroxidation such as: conjugated dienes, and secondary peroxidation products, which include saturated and unsaturated aldehydes, ketones, oxo- and hydroxy acids, and saturated and unsaturated hydrocarbons (e.g. ethane, pentane).
Lipid peroxidation in biological membranes causes impairment of membrane functioning, decreased fluidity, inactivation of membrane-bound receptors and enzymes, and increased non-specific permeability to ions. Moreover, lipid hydroperoxides decompose upon exposure to copper while iron chelates the other factors including metals as haem, haemoglobin or myoglobin. Cytotoxic aldehydes are formed as a consequence of lipid hydroperoxide degradation. Malondialdehyde (MDA) and 4-hydroxynonenal are hydrophilic, and are released from low density lipoproteins (LDL) into aqueous surroundings. Hydroxynonenal is biologically active and can cause severe cell dysfunction both on protein and DNA levels. Hydroxynonenal is chemotactic for polymorphonuclear leukocytes (PMNs) at picomolar concentrations, inhibits cell proliferation and is mutagenic [60–63].
In contrast to MDA and hydroxynonenal, the other aldehyde products of lipid peroxidation are hydrophobic and remain closely associated with LDL accumulating to milimolar concentrations. Aldehydes at these elevated levels react with the protein portion of the LDL molecule, called apolipoprotein B (apoB). Consequently, the protein takes a negative charge and its complete structural rearrangement results in the formation of ox-LDL. Ox-LDL is no longer recognized by the LDL receptor, and has several proinflammatory properties.
Methods used to detect and to measure lipid peroxidation [64]
TBA test
The spectrophotometric thiobarbituric acid (TBA) test has been frequently used for many years as an indicator of the peroxidation of polyunsatured fatty acids. This test involves the reaction of aldehydes with TBA at 100°C under acidic conditions to produce a pink-colored chromogen, which strongly absorbs light at a wavelength of 532 nm.
Fatty acids analysis by GLC or high-performance liquid chromatography (HPLC)
The analysis of fatty acids by GLC or HPLC is very useful for assessment of lipid peroxidation stimulated by different metal complexes, that give different distribution. In this test the loss of unsaturated fatty acids is measured.
Oxygen electrode
Concentration of dissolved oxygen is defined by oxygen electrode when measuring uptake of oxygen by carbon-centered radicals and during peroxide decomposition reactions.
Gluthatione peroxidase test
Glutathione peroxidase reacts with hydrogen peroxide oxidizing GSH to GSSG. Addition of glutathione reductase and NADPH to reduce GSSG to GSH results in consumption of NADPH which can be related to the peroxide content.
Cyclooxygenase activity test
Stimulation of cyclooxygenase activity can be used to measure trace amounts of peroxide in biological fluids.
GLC/mass spectrometry assay
Lipid peroxides are measured by extraction, reduction to alcohols, separation by GLC and identification by mass spectrometry.
Hydrocarbon gases assay
This assay can be used as a non-invasive in vivo detection of peroxidation. Pentane and ethane are formed during lipid peroxide decomposition and can be measered using GC method.
Light emission
Peroxyl radicals can be produced as a self-reaction of excited carbonyls and singlet oxygen. Singlet oxygen and excited carbonyls emit light as they decay to the resting state. Heam moiety of proteins can decompose lipid peroxides with concomitant formation of reactive intermediates which can react with producing luminol light. The light emission can be measured in chemiluminescent assays.
Fluorescence
Aldehydes such as malondialdehyde (MDA) can react at acidic pH with amino groups to form Schiff bases. At neutral pH, fluorescent dihydropyridines may be formed. Aldehydes can also polymerize to produce fluorescent products in the absence of amino groups.
HPLC/antibody techniques
Cytotoxic aldehydes (e.g. 4-hydroxynonenal) can be measured by HPLC. Moreover, several techniques have been developed using antibodies to detect proteins modified by lipid peroxidation products.
Lipid peroxidation – detrimental effects on sperm functions
• Excessive generation of ROS in semen, mainly by neutrophils but also by abnormal spermatozoa, could be a cause for infertility [64]. High concentrations of hydrogen peroxide induce lipid peroxidation and result in cell death.
•Increased ROS production by spermatozoa is associated with a decreased mitochondrial membrane potential (MMP). The patients with abnormal semen parameters had a significantly lower MMP [65].
• Infertile men have decreased sperm variables induced by higher ROS levels in semen. A positive relationship exists between increased sperm damage by ROS and higher levels of cytochrome c, and caspases 9 and 3, which indicate apoptosis in patients with 'male factor' of infertility [66].
• Excess of free radical generation frequently involves an error in spermiogenesis resulting in the release of spermatozoa from the germinal epithelium exhibiting abnormally high levels of cytoplasmic retention. Redundant cytoplasm contains enzymes that fuel further generation of ROS by the spermatozoa's plasma membrane redox systems. The consequences of such oxidative stress include a loss of motility and fertilizing potential, and the induction of DNA damage in the sperm nucleus. The loss of sperm function is due to the peroxidation of unsaturated fatty acids in the sperm plasma membrane, as a consequence of which the latter loses its fluidity and the cells lose their function [67].
• H2O2 directly affects sperm functions critical at fertilization process in a dose- and time-dependent fashion. Low concentrations maintain capacitation, whereas high concentrations have deleterious effects, as determined by the end points of the capacitation process. These effects are probably dependent on modifications of plasma membrane and intracellular homeostasis by the oxidative process [68].
• The sublethal effects of oxidative stress on motility parameters are significantly associated with membrane translocation of phosphatidylserine in sperm cells membrane [69].
• Oxidative stress induced by white blood cells has a damaging effect on the polyunsaturated fatty acids of sperm phospholipids which may result, among the other effects, in decreased membrane fluidity [70].
Biopositive effects of free radicals
The generation of ROS occurs physiologically during normal cell metabolism. Mitochondrial respiration is the main biological source of superoxide anion radicals under physiological conditions. During the tetravalent reduction of oxygen to water by the mitochondrial cytochrome c oxidase, these radicals can leak to the cell. At low concentrations reactive oxidants have a biopositive effect and act selectively [71]. They act on the metabolism of prostanoids, in gene regulation or in the regulation of cellular growth, intracellular signaling, and the other types of signal transduction. Moreover, oxygen free radicals play an important role in regulation of vasotonus and in antimicrobial defense. Limited amounts of ROS can also interfere physiologically in the regulation of sperm functions. It has been observed that low amounts of free radicals in human semen enhance spermatozoa ability to bind zona pellucida. In addition, the incubation of sperm cells with low concentrations of hydrogen peroxide was found to stimulate sperm capacitation, hyperactivation, acrosome reaction and oocyte fusion [72–75].
Future directions
Future research efforts should be directed towards understanding the role of particular components of sperm membrane and its transformations. Unfortunately, it is still not clear if sperm membrane peroxidated products are indispensable or detrimental in this process. If sperm membrane lipid peroxidation can be considered as useful, the inhibition of endogenous antioxidant activity would be advantageous in special, physiologic conditions. In such situation the administration of exogenous antioxidants would be inappropriate. On the other hand, if sperm lipid peroxidation inhibits or alters the physiological processes of, for instance, sperm maturation, the therapeutic intervention would be beneficial. Determining the structure and functional changes in sperm membrane lipids during the process of peroxidation may be useful in understanding the role of lipid metabolism in spermatozoa physiology and may help to develop novel therapeutic strategies for male infertility.
References
Aitken RJ, Clarkson JS, Fishel S: Generation of reactive oxygen species, lipid peroxidation, and human sperm function. Biol Reprod. 1989, 41: 183-197.
Rowley A, Gutteridge JMC, Blake DR, Farr M, Halliwell B: Lipid peroxidation in rheumatoid arthritis: thiobarbituric acid reactive material and catalytic iron salts in synovial fluid from rheumatoid patients. Clin Sci. 1984, 66: 691-695.
White CR, Brock TA, Chang LY, Crapo J, Briscoe P, Ku D, Bradley WA, Gianturco SH, Gore J, Freeman BA, et al: Superoxide and peroxynitrite in atherosclerosis. Proc Natl Acad Sci. 1994, 91: 1004-1048.
Beresewicz A, Horackowa M: Alterations on electrical and contractile behavior of isolated cardiomyocytes by hydrogen peroxide: possible ionic mechanisms. J Mol Cell Cardiol. 1991, 23: 899-918. 10.1016/0022-2828(91)90133-7.
Manning AS, Hearse DJ: Reperfusion-induced arrhythmias: mechanism and prevention. J Moll Cell Cardiol. 1984, 16: 497-518.
Reeves JP, Bailey CA, Hales CC: Redox modification of sodium-calcium exchange activity in cardiac sarcolemmal vesicles. J Biol Chem. 1986, 261: 4948-4955.
Andorn AC, Britton RS, Bacon BR: Evidence that lipid peroxidation and total iron are increased in Alzheimer's brain. Neurobiol Aging. 1990, 11: 316-320.
Floyd RA: Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990, 4: 2587-2597.
Braughler JM, Hall ED: Central nervous system trauma and stroke. I. Biochemical considerations for oxygen radical formation and lipid peroxidation. Free Rad Biol Med. 1989, 6: 289-301. 10.1016/0891-5849(89)90056-7.
Ben-Shackar D, Eshel G, Riederer P, Youdim MBH: Role of iron and iron chelation in dopaminergic-induced neurodegeneration: implications for Parkinson's disease. Ann Neurol. 1992, 32: S105-S110.
Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng HX, et al: Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993, 362: 59-62. 10.1038/362059a0.
Thorniley MS, Lane NJ, Manek S, Green CJ: Non-invasive measurement of respiratory chain dysfunction following hypothermic renal storage and transplantation. Kidney Int. 1994, 45: 1489-1496.
Chin JH, Azhar S, Hoffman BB: Inactivation of endothelial derived relaxing factor by oxidized lipoproteins. J Clin Invest. 1992, 89: 10-18.
Otamiri T, Sjodahl R: Increased lipid peroxidation in malignant tissues of patients with colorectal cancer. Cancer. 1989, 64: 422-425.
Walker PM: Ischaemia/reperfusion injury in skeletal muscle. Ann Vasc Surg. 1991, 5: 399-402.
Sadani GR, Nadkarni GD: Changes in lipid peroxide levels and the activity of reactive oxygen scavenging systems in thyroid tissue after exposure to radioactive iodine in rats. Thyroid. 1997, 7: 937-941.
Zhang H, Zheng RL, Wei ZQ, Li WJ, Gao QX, Chen WQ, Wang ZH, He J, Liang JP, Han GW, Huang T, et al: Effects of pre-exposure of mouse testis with low-dose (16)O8+ ions or 60Co gamma-rays on sperm shape abnormalities, lipid peroxidation and superoxide dismutase (SOD) activity induced by subsequent high-dose irradiation. Int J Radiat Biol. 1998, 73: 163-167. 10.1080/095530098142545.
Akiyama M: [In vivo scavenging effect of ethylcysteine on reactive oxygen species in human semen]. Nippon Hinyokika Gakkai Zasshi. 1999, 90: 421-8.
Villegas J, Kehr K, Soto L, Henkel R, Miska W, Sanchez R: Reactive oxygen species induce reversible capacitation in human spermatozoa. Andrologia. 2003, 35: 227-232. 10.1046/j.1439-0272.2003.00564.x.
Hollan S: Membrane fluidity of blood cells. Haematologia (Budap). 1996, 27: 109-127.
Huang YL, Tseng WC, Lin TH: In vitro effects of metal ions (Fe2+, Mn2+, Pb2+) on sperm motility and lipid peroxidation in human semen. J Toxicol Environ Health A. 2001, 62: 259-67. 10.1080/009841001459414.
Davydov DR: Microsomal monooxygenase in apoptosis: another target for cytochrome c signaling?. Trends Biochem Sci. 2001, 26: 155-160. 10.1016/S0968-0004(00)01749-7.
Aitken RJ, Sawyer D: The human spermatozoon – not waving but drowning. Adv Exp Med Biol. 2003, 518: 85-98.
Hsu PC, Liu MY, Hsu CC, Chen LY, Guo YL: Effects of vitamin E and/or C on reactive oxygen species-related lead toxicity in the rat sperm. Toxicology. 1998, 128: 169-79. 10.1016/S0300-483X(98)00068-7.
Alvarez JG, Storey BT: Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol Reprod Dev. 1995, 42: 334-346.
Mack SR, Everingham J, Zaneveld LJD: Isolation and partial characterization of the plasma membrane from human spermatozoa. J Exp Zool. 1986, 240: 127-136.
Poulos A, White IG: The phospholipid composition of human spermatozoa and seminal plasma. J Reprod Fertil. 1973, 35: 265-272.
Jones R, Mann T, Sherins RJ: Peroxidative breakdown of phospholipids in human spermatozoa, spermicidal properties of fatty acid peroxides and protective action of seminal plasma. Fertil Steril. 1979, 31: 531-537.
Alvarez JG, Touchstone JC, Blasco L, Storey BT: Spontaneus lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: superoxide dismutase as major enzyme protectant against oxygen toxicity. J Androl. 1987, 8: 338-348.
Aitken RJ, Clarkson JS: Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J Reprod Fertil. 1987, 81: 459-469. 10.1530/jrf.0.0810459.
Tesarik J, Flechon JE: Distribution of sterols and anionic lipids in human sperm plasma membrane: effects of in vitro capacitation. J Ultrastruct Mol Struct Res. 1986, 97: 227-237.
Benoff S: Preliminares to fertilization: the role of cholesterol during capacitation of human spermatozoa. Hum Reprod. 1993, 8: 2001-2008.
Yeagle PL: Lipids and lipid-intermediate structures in the fusion of biological membranes. Curr Top Membr. 1994, 4: 197-214.
Parks JE, Hammerstedt RH: Developmental changes occuring in the lipids of ram epididymal spermatozoa plasma membrane. Biol Reprod. 1985, 32: 653-658.
Aveldano MI, Rotstein NP, Vermouth NT: Lipid remodeling during epididymal maturation of rat spermatozoa. Enrichment in plasmenylcholines containing long-chain polyenoic fatty acids of the n-9 series. Biochem J. 1992, 283: 235-241.
Stubbs CD, Smith AD: The modification of polyunsaturated fatty acids composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984, 779: 89-137. 10.1016/0304-4157(84)90005-4.
Schlegel RA, Hammerstedt R, Cofer CP, Kozarsky K: Changes in the organization of the lipid bilayer of the plasma membrane during spermatogenesis and epididymal maturation. Biol Reprod. 1986, 34: 379-391.
Paltauf F: Ether lipids in biomembranes. Chem Phys Lipids. 1994, 74: 101-139. 10.1016/0009-3084(94)90054-X.
Haidl G, Opper C: Changes in lipids and membrane anisotropy in human spermatozoa during epididymal maturation. Hum Reprod. 1997, 12: 2720-2723. 10.1093/humrep/12.12.2720.
Hall JC, Hadley J, Doman T: Correlation between changes in rat sperm membrane lipids, protein, and the membrane physical state during epididymal maturation. J Androl. 1991, 12: 76-87.
Ollero M, Powers RD, Alvarez JG: Variation of docosahexaenoic acid content in subsets of human spermatozoa at different stages of maturation: implications for sperm lipoperoxidative damage. Mol Reprod Develop. 2000, 55: 326-334. 10.1002/(SICI)1098-2795(200003)55:3<326::AID-MRD11>3.3.CO;2-1.
Kotwicka M, Jendraszak M, Warchol JB: Plasma membrane translocation of phosphatidylserine in human spermatozoa. Folia Histochem Cytobiol. 2002, 40: 111-112.
Cross NL: Decrease in order of human sperm lipids during capacitation. Biol Reprod. 2003, 69: 529-534.
Cross NL: Sphingomyelin modulates capacitation of human sperm in vitro. Biol Reprod. 2000, 63: 1129-1134.
Zaneveld LJD, De Jonge CJ, Anderson RA, Mack SR: Human sperm capacitation and the acrosome reaction. Hum Reprod. 1991, 6: 1265-1274.
Schroeder F, Jefferson JR, Kier AB, Knittel J, Scallen TJ, Wood WG, Hapala I: Membrane cholesterol dynamics: cholesterol domains and kinetics pools. Proc Soc Exp Biol Med. 1991, 196: 235-252.
Langlais J, Zollinger M, Plante L, Chapdelaine A, Bleau G, Roberts KD: Localization of cholesterol sulfate in human spermatozoa in support of a hypothesis for the mechanism of capacitation. Proc Natl Acad Sci USA. 1981, 78: 7266-7270.
Breitbart H, Spungin B: The biochemistry of the acrosome reaction. Mol Hum Reprod. 1997, 3: 195-202. 10.1093/molehr/3.3.195.
Hinkovska VT, Dimitrov GP, Koumanov KS: Phospholipid composition and phospholipid asymmetry of ram spermatozoa plasma membranes. Int J Biochem. 1986, 18: 1115-1121. 10.1016/0020-711X(86)90085-6.
Arts E, Kuiken J, Jager S, Hoekstra D: Fusion of artificial membranes with mammalian spermatozoa. Specific involvement of the equatorial segment after acrosome reaction. Eur J Biochem. 1993, 217: 1001-1009.
Martinez P, Morros A: Membrane lipid dynamics during human sperm capacitation. Front Biosci. 1996, 1: d103-117.
Weerachatyanukul W, Rattanachaiyanont M, Carmona E, Furimsky A, Mai A, Shoushtarian A, Sirichotiyakul S, Ballakier H, Leader A, Tanphaichitr N: Sulfogalactosylglycerolipid is involved in human gamete interaction. Mol Reprod Dev. 2001, 60: 569-578. 10.1002/mrd.1122.
Riffo MS, Parraga M: Role of phospholipase A2 in mammalian sperm-egg fusion: development of hamster oolemma fusibility by lysophosphatidylcholine. J Exp Zool. 1997, 279: 81-88. 10.1002/(SICI)1097-010X(19970901)279:1<81::AID-JEZ8>3.3.CO;2-V.
Fraga GG, Motchnik PA, Shigenega MK: Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc Natl Acad Sci USA. 1991, 88: 11003-11006.
Molianen J, Hovatta O, Lindroth L: Vitamin E levels in seminal plasma can be elevated by oral administration of vitamin E in infertile men. Int J Androl. 1993, 16: 165-166.
Lewis SEM, Boyle PM, McKinney KA: Total antioxidant capacity of seminal plasma is different in fertile and infertile men. Fertil Steril. 1995, 64: 868-870.
Sanocka D, Miesel R, Jedrzejczak P, Kurpisz M: Oxidative stress and male infertility. J Androl. 1996, 17: 449-454.
Miesel R, Jedrzejczak P, Sanocka D, Kurpisz M: Severe antioxidant deficiency in human semen samples with pathological spermiogram parameters. Andrologia (Deu). 1997, 29: 77-83.
Sanocka D, Miesel R, Jedrzejczak P, Chelmonska-Soyta A, Kurpisz M: Effect of reactive oxygen species and the activity of antioxidant systems in human semen; association with male infertility. Int J Androl. 1997, 20: 255-264. 10.1046/j.1365-2605.1997.00050.x.
Aitken RJ, Harkness D, Buckingham DW: Analysis of lipid peroxidation mechanisms in human spermatozoa. Mol Reprod Dev. 1993, 35: 302-315.
Jones R, Mann T, Sherins R: Peroxidative breakdown of phospholipids in human spermatozoa. Spermicidal properties of fatty acid peroxides, and protective action of seminal plasma. Fertil Steril. 1979, 31: 531-537.
Esterbauer H, Zollner H, Schaur RJ: Hydroxyalkenals:cytotoxic products of lipid peroxidation. Atlas of science: Biochemistry. 1988, 1: 311-319.
Esterbauer H, Rotheneder-Dieber M, Waeg G, Striegl G, Jurgens G: Biochemical, structrural, and functional properties of oxidized low-density lipoprotein. Chem Res Toxicol. 1990, 3: 77-92.
de Lamirande E, Gagnon C: Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum Reprod. 1995, 10 (Suppl 1): 15-21.
Wang X, Sharma RK, Gupta A, George V, Thomas AJ, Falcone T, Agarwal A: Alterations in mitochondria membrane potential and oxidative stress in infertile men: a prospective observational study. Fertil Steril. 2003, Suppl 2: 844-850. 10.1016/S0015-0282(03)00983-X.
Wang X, Sharma RK, Sikka SC, Thomas AJ, Falcone T, Agarwal A: Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil Steril. 2003, 80: 531-535. 10.1016/S0015-0282(03)00756-8.
Aitken RJ, Sawyer D: The human spermatozoon – not waving but drowning. Adv Exp Med Biol. 2003, 518: 85-98.
Oehninger S, Blackmore P, Mahony M, Hodgen G: Effects of hydrogen peroxide on human spermatozoa. J Assist Reprod Genet. 1995, 12: 41-47.
Kemal Duru N, Morshedi M, Oehninger S: Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil Steril. 2000, 74: 1200-1207. 10.1016/S0015-0282(00)01591-0.
Zalata AA, Christophe AB, Depuydt CE, Schoonjans F, Comhaire FH: White blood cells cause oxidative damage to the fatty acid composition of phospholipids of human spermatozoa. Int J Andro. 1998, 21: 154-162. 10.1046/j.1365-2605.1998.00112.x.
de Lamirande E, Jiang H, Zini A, Kodama H, Gagnon C: Reactive oxygen species and sperm physiology. Rev Reprod. 1997, 2: 48-54. 10.1530/ror.0.0020048.
Griveau JF, Le Lannou D: Reactive oxygen species and human spermatozoa: physiology and pathology. Int J Androl. 1997, 20: 61-69. 10.1046/j.1365-2605.1997.00044.x.
de Lamirande E, Gagnon C: A positive role for the superoxide anion in triggering hyperactivation and capacitation of human spermatozoa. Int J Androl. 1993, 16: 21-25.
de Lamirande E, Harakat A, Gagnon C: Human sperm capacitation induced by biological fluids and progesterone, but not by NADH or NADPH, is associated with the production of superoxide anion. J Androl. 1998, 19: 215-225.
Saran M, Bors W: Oxygen radicals acting as chemical messengers: a hypothesis. Free Radic Res Commun. 1989, 7: 213-220.
Author information
Authors and Affiliations
Corresponding author
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
This article is published under an open access license. Please check the 'Copyright Information' section either on this page or in the PDF for details of this license and what re-use is permitted. If your intended use exceeds what is permitted by the license or if you are unable to locate the licence and re-use information, please contact the Rights and Permissions team.
About this article
Cite this article
Sanocka, D., Kurpisz, M. Reactive oxygen species and sperm cells. Reprod Biol Endocrinol 2, 12 (2004). https://doi.org/10.1186/1477-7827-2-12
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1477-7827-2-12