Abstract
Background
Recently, vitamin D was discovered to have an important role in female reproduction and IVF. However, there were no studies specifically addressed its role in polycystic ovarian syndrome (PCOS) patients undergoing ICSI cycles. Therefore, this cross-sectional study in a university hospital was conducted to evaluate the effect of serum level of vitamin D (VD) on the number of retrieved and fertilized oocytes, and chemical and clinical pregnancy rate in PCOS females undergoing ICSI cycles. The study included 80 PCOS cases undergoing ICSI cycles in the age from 20 to 39 years using antagonist protocol. Cases with severe male or tubal factors were excluded. Serum 25 (OH) D vitamin level was assessed by the ELIZA method on the day of oocyte retrieval. Correlation and regression analyses were used in the analysis.
Results
VD was positively correlated to both numbers of retrieved and fertilized oocytes (r = 0.35, 95% CI 0.15, 0.53, P = 0.001; r = 0.33; 95% CI 0.03, 0.57, P = 0.03, respectively). It was still significantly correlated to the number of oocytes (coefficient 0.47; 95% CI 0.1, 0.9; P = 0.018) and to the number of fertilized eggs (coefficient 0.3; 95% CI 0.02, 0.58; P = 0.03) after adjusting for age, BMI, and type of ovulation-triggering agent. This means with each 2 ng/mL increase in serum VD level, around one more egg will be retrieved. In addition, there will be one more fertilized oocyte with each 3 ng/mL increase in the vitamin level. However, no significant correlation was found between the vitamin level and the occurrence of chemical or clinical pregnancy.
Conclusions
Serum vitamin D level is positively correlated with the number of retrieved and fertilized oocytes in PCOS patients undergoing ICSI cycles.
Similar content being viewed by others
Background
Polycystic ovary syndrome (PCOS) is the commonest endocrine abnormality in women of reproductive age [1]. Moreover, it leads to many health problems throughout the woman’s life [1]. Worldwide, the prevalence of PCOS is 6–13% [2]. In Egypt, it is estimated that the prevalence of PCOS is 13% in fertile and 37.5% in secondary infertile patients [3].
IVF/ICSI is considered an important line of management for anovulatory infertility in PCOS if lifestyle modifications and usual ovulation induction had failed to achieve pregnancy or the patient has other factors of infertility requiring IVF, such as severe male factor of infertility or tubal occlusion [4, 5].
In the past decade, vitamin D was discovered to have an important role in many parts of the body in addition to the classical sites of the bone, kidney, and intestine [6]. In particular, vitamin D was found to be involved in the regulation of several hormones in the body including the anti-Müllerian hormone (AMH) [7], follicle-stimulating hormone, estradiol, and progesterone [8]. In addition, a correlation was suggested between vitamin D deficiency and PCOS. This relation may be due to the effects of vitamin D receptors on LH and sex hormone-binding globulin levels, the incidence of insulin resistance, testosterone levels, and aromatase gene expression [9,10,11,12,13,14].
There has been an increasing interest recently in the investigation of the role of vitamin D in IVF due to the effects of vitamin D receptors on LH and sex hormone-binding globulin levels, the incidence of insulin resistance, testosterone levels, and aromatase gene expression [9,10,11,12,13,14]. However, the results of such research studies were inconsistent [6]. Moreover, no studies evaluated the effect of VD status on IVF/ICSI outcomes in PCOS females who represent an important category of cases undergoing assisted reproduction.
Therefore, we investigated the relation between serum 25 (OH) D vitamin level and the outcome of ICSI cycles in PCOS cases.
Methods
This cross-sectional study was conducted in the IVF Unit of Cairo University from November 2016 to January 2018. It was approved by our institution’s research ethics committee. The ethical standards of the Declaration of Helsinki were followed, and informed consents were taken from the study participants. Collection and reporting of data followed the guidelines of the STROBE checklist for observational studies [15].
The study population were 80 females who had PCOS and were scheduled to do ICSI cycles. They were in the age between 20 and 39 years and had a body mass index (BMI) between 20 and 29 kg/m2. Females who had medical disorders, concomitant tubal factor, endometriosis, or severe uterine factor of infertility (intrauterine adhesions) were excluded. In addition, women who were taking vitamin D supplements or whose husbands had severe male factor (severe oligo-atheno-teratospermia or azoospermia) were excluded. PCOS was diagnosed in our patients following the Rotterdam criteria [16]. The indication for ICSI was the failure of previous ovulation induction cycles to achieve pregnancy [4].
History and general and local examinations were done for all patients. Vaginal or cervical infections (if present) were treated first before the ICSI cycle. Basal (day 2 or 3 of menstruation or withdrawal bleeding in case of oligomenorrhea) hormonal profile (as a part of the diagnosis for PCOS if the criteria were not fulfilled by clinical picture alone) was assessed for all women. Screening for hepatitis B, hepatitis C, and HIV was performed before the start of the ICSI cycle.
Antagonist protocol was chosen for ovarian stimulation [4]. The details of the antagonist protocol followed our IVF unit protocol. Ovarian stimulation started on the second or third day of menstruation (or progestogen withdrawal) by 150 IU subcutaneous human menopausal gonadotropin (HMG) (Merional, IBSA, Lugano, Switzerland). The first folliculometry was done 5 days after the start of stimulation, then it was done every 2 to 3 days. Gonadotropin-releasing hormone antagonist (GnRH antagonist) (Cetrotide, Merck Serono, Darmstadt, Germany) was started in a fixed manner (0.25 mg daily) from the 6th day of ovarian stimulation. Ovulation trigger was done by intra-muscular 5000 IU of urinary human chorionic gonadotropin (HCG) (Choriomon, IBSA, Lugano, Switzerland) when 3 follicles reached 17 mm. According to our IVF unit’s policy, GnRH agonist triggering for ovulation (instead of HCG) and administration of dopamine agonist were done if serum estradiol was higher than 5000 pg/mL at the day of ovulation trigger. The GnRH agonist was in the form of subcutaneous 0.2 mg of GnRH analog (Decapeptyl, Ferring, Saint-Prex, Switzerland). In addition, dopamine agonist (Dostinex, Pfizer, New York, NY, USA) 0.5 mg/day orally for 8 days was given starting from the day of ovulation trigger. Oocyte retrieval was done 35–36 h after HCG (or GnRH agonist) injection.
Ultrasound-guided transfer of embryos was done on day 2 or 3 after oocyte retrieval (according to our IVF laboratory policy). Freezing of all embryos was done if 25 or more oocytes were retrieved [17]. Luteal phase support was started on the day of egg collection by twice daily 400 mg progesterone vaginal suppository intake (Cyclogest, Actavis, Barnstaple, UK). Daily 4 mg of estradiol valerate (the white tablets of Cyclo-Progenova, Bayer HealthCare Pharmaceuticals LLC, Berlin, Germany) was added to daily progesterone in case of GnRH agonist triggering for ovulation (according to our unit protocol). Luteal phase support continued for 15 days until serum quantitative pregnancy test (bHCG) and for further 8 weeks in case of positive pregnancy.
Pregnancy was considered positive (biochemical pregnancy) if serum bHCG was more than 50 mIU/mL. Clinical pregnancy was reported if a gestational sac (or more) with fetal cardiac activity was seen by ultrasound 2 weeks after a positive pregnancy test.
Vitamin D measurement
Blood samples were collected at the day of oocyte collection. Two milliliters of blood was obtained and allowed to clot at room temperature. The blood tube was then centrifuged for 15 min at and was stored below 20 °C until assayed. ELISA test (25 OH vitamin D3/D2, ORGENTEC Diagnostika GmbH, Mainz, Germany) was used to measure serum 25 (OH) D vitamin level. Measurement of serum vitamin D followed the manufacturer’s instructions in the kit’s leaflet. One hundred microliters of patient samples, calibrators, and controls was pipetted into wells and incubated for 30 min at room temperature (20–30 °C). Then, the contents of the micro-wells were discarded and washed 3 times with 300 μl of wash solution. Thereafter, 100 μl of the enzyme conjugate was dispensed into each well and incubated for 15 min at room temperature. After another washing, 100 μl of enzyme-substrate solution was dispensed into each well and incubated for 15 min at room temperature. Afterwards, 100 μl of a stop solution was added to each well of the molecules and incubated for 5 min. The optical density at 450 nm was read, and the serum 25 (OH) vitamin D level was interpreted accordingly in nanograms/milliliter.
The primary outcomes of the study were correlations of the serum vitamin D level to the number of retrieved oocytes and number of fertilized embryos resulting from ICSI cycle, while the secondary outcomes were correlation of serum vitamin D at the day of oocyte retrieval to the occurrence of chemical and clinical pregnancy in our study cohort.
Sample size calculation
Calculation was based on the correlation between the serum level of vitamin D and the number of good-quality embryos (or the number of fertilized oocytes) as the study primary outcome. Setting the power of study at 80% and alpha at 0.05 (2 tailed), we needed 80 cases to detect a minimally clinically significant correlation coefficient of 0.3. Sample size calculation was done using the IBM SPSS SamplePower software, release 3.0.1 (IBM Corp., Armonk, NY, USA).
Statistical analysis
The normal distribution of continuous data was assessed by the Anderson-Darling test (in addition to Q-Q plot). In turn, the description of data was as mean (SD) or median (range). Qualitative data were described in the form of count (percentage). Correlation between vitamin D level and the number of retrieved number of fertilized eggs, number of grade one embryos, and number of implanted embryos was done using Pearson correlation. Afterwards, multivariable regression analysis was done to calculate the coefficient of the relation between serum 25 (OH) D vitamin level and the number of retrieved oocytes adjusting for possible confounders, namely, age, BMI, and type of ovulation-triggering agent (HCG vs GnRH agonist). Another multivariable regression analysis was done to assess the relation between vitamin level and the number of fertilized oocytes adjusting for the same previous confounding factors. In addition, logistic regression was performed to measure the odds ratio of occurrence of chemical and clinical pregnancy in relation to the vitamin level. 95% confidence intervals (CI) were described for all applicable statistics. P value <ȉ0.05 was considered significant. Statistical analysis was done using the SPSS software, version 23 (IBM Corp., Armonk, NY, USA).
Results
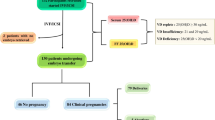
A flow chart of the study population is presented in (Fig. 1). A total of 113 PCOS patients in the age of 20–39 were assessed for eligibility. Eighty women were eligible and accepted to participate. For all cases, the primary outcomes (number of retrieved and fertilized oocytes) were recorded.
The median (range) of age and BMI were 32 (20, 32) years and 27 (24, 29), respectively. Most of our study cases had primary infertility (n = 74; 92.5%) and irregular menstruation (n = 48, 60%) (Table 1).
As regards the ICSI cycle characteristics, most of the patients took HCG for triggering ovulation (n = 71, 78.8%), while the remaining proportion had GnRH agonist (n = 9, 11.3%). The medians (range) of the number of retrieved and fertilized oocytes were 12 (4, 35) and 7 (1, 20), respectively, with a fertilization rate of 80% (13%, 100%). No transfer with freezing of all embryos occurred in 6 cases (8%) while the remaining women had their embryo transfer at day 3, n = 69 (93.4%), and day 2, n = 5 (6.8%). Sixty-five percent of the cases had a transfer of 3 grade 1 embryos, while others had less number of grade 1 embryos. Biochemical pregnancy occurred in 31 out of 74 cases who did an embryo transfer (41.9%). However, gestational sac with a fetal pulsation was detected in 27 patients (36.5%). Most women had one gestational sac, and 3 cases (11.1%) had two sacs. Only one case developed moderate OHSS which resolved on follow-up without a need for hospital admission (Table 2).
Serum 25 (OH) D vitamin level was mostly in the deficient category, n = 72 (90%), while the remaining patients, n = 8 (10%), had insufficient levels of the vitamin. None of our PCOS cases had replete vitamin D status (Table 3).
Regarding the study primary outcomes; vitamin D was found to be correlated to both the number of retrieved and fertilized oocytes (r = 0.35, 95% CI 0.15, 0.53, P = 0.001; r = 0.33, 95% CI 0.03, 0.57, P = 0.03, respectively). After adjusting for age, BMI, and type of ovulation-triggering agent in regression analysis, serum vitamin D level was still significantly correlated to the number of oocytes (coefficient 0.47; 95% CI 0.1, 0.9; P = 0.018) and to the number of fertilized eggs (coefficient 0.3; 95% CI 0.02, 0.58; P = 0.03). This means with each 2 ng/mL increase in serum 25 (OH) D vitamin level, around one more egg will be retrieved. In addition, there will be one more additional fertilized oocyte with each 3 ng/mL in the vitamin level (Table 4, Figs. 2 and 3). However, there was no significant correlation between the vitamin level and the occurrence of chemical or clinical pregnancy (Table 4).
Discussion
The present study demonstrated that 72 (90%) of our PCOS patients undergoing ICSI trials had deficient status of serum 25 (OH) D vitamin. This percentage was slightly higher than another study done by Thomson et al. who observed that up to 85% of ladies with PCOS have serum level of vitamin D that is below 20 ng/mL [18]. However, not all studies have shown a correlation between derangement in vitamin D level and the incidence of PCOS [19]. That correlation between vitamin D deficiency and PCOS may be due to the effects of vitamin D receptors on LH and sex hormone-binding globulin levels, the incidence of insulin resistance, testosterone levels, and aromatase gene expression [9,10,11,12,13,14]. Moreover, the vitamin D status in Egypt was recently investigated in the general female population [20], and it was found that the prevalence of VD deficiency is 72% in childbearing age. This may be due to different lifestyle factors (sunlight exposure, sunscreen application, and deficient diet) making VD deficiency endemic in our country.
As regards the study primary outcomes, the number of retrieved oocytes and fertilized ones was positively correlated with serum VD level. That relation was still significant after adjusting for possible confounders signifying the independence of VD in the affection of the number of collected and fertilized oocytes. The plausible explanation may be that VD acts as a transcription factor to regulate the expression of the CYP19 gene, which encodes aromatase, an essential enzyme in the production of estrogen [21]. In addition, vitamin D is involved in the regulation of anti-Müllerian hormone (AMH) [7] and follicle-stimulating hormone [8]. Moreover, the association between VD deficiency and various metabolic disturbances in PCOS including insulin resistance may explain (at least partly) the impaired follicular growth [9,10,11,12,13,14, 22,23,24,25,26,27]. In accordance with our results, vitamin D deficiency (25 nmol/L or, 10 ng/mL) was found to be associated with lower rates of follicle development and pregnancy after ovarian stimulation with 50 mg clomiphene citrate [28].
It has been suggested that VD plays a role in the decidual function and embryo implantation [29]. Therefore, in the last few years, due to emerging evidence of the role of VD in female human reproduction including implantation, there was an increasing interest in investigating the correlation between vitamin D deficiency and in vitro fertilization outcome [30,31,32]. Some investigators have found better IVF outcomes in cases of sufficient VD status [33,34,35], whereas other studies failed to show any correlation between serum or follicular fluid VD level and pregnancy outcome after IVF/ICSI [29, 36,37,38,39]. However, no similar study assessed the effect of derangement of serum VD level on ICSI outcome in the particular category of PCOS females.
However, our study has its limitations. Despite the power of the study was enough for the study’s primary aims, namely correlation of serum 25 (OH) D vitamin level with a number of retrieved and fertilized oocytes, it was not enough to detect a statistically significant difference in pregnancy rates (as secondary outcomes). Although the absence of such correlations was in agreement with other studies [29, 36,37,38,39], we could not exclude the probable effect of VD on implantation and pregnancy as these outcomes were secondary outcomes. Moreover, those studies that failed to prove this correlation assessed VD level in the general IVF population (not specifically PCOS patients) and compared the pregnancy in different categories of VD status. It may be that the absolute level of VD affects the pregnancy outcome. In turn, statistically, dealing with VD as a continuous predictor variable of IVF outcome (as we did in the present study) may elucidate the actual effect of vitamin D on different cycle parameters. Therefore, further studies with adequate power to detect an effect on pregnancy rate are needed. In addition, studies are needed to evaluate the effect of vitamin D supplementation on IVF/ICSI pregnancy outcomes in those who are deficient or insufficient.
Conclusion
In conclusion, based on the study results, VD was positively correlated with the number of retrieved and the number of fertilized oocytes in PCOS patients undergoing ICSI cycles. According to the regression analysis adjusting for possible confounders, with each increase of serum 25 (OH) of 2 ng/mL, there was one more retrieved oocyte. In addition, there was an addition of one fertilized oocyte with each 3 ng/mL increase in serum VD level.
Availability of data and materials
The authors confirm the availability of data and materials.
Abbreviations
- AMH:
-
Anti-Müllerian hormone
- BMI:
-
Body mass index
- CI:
-
Confidence intervals
- GnRH:
-
Gonadotropin-releasing hormone antagonist
- HCG:
-
Human chorionic gonadotropin
- HMG:
-
Human menopausal gonadotropin
- OHSS:
-
Ovarian hyperstimulation syndrome
- PCOS:
-
Polycystic ovary syndrome
- VD:
-
Vitamin D
References
Bellver J, Rodriguez-Tabernero L, Robles A et al (2018) Polycystic ovary syndrome throughout a woman’s life. J Assist Reprod Genet 35(1):25–39
Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO (2016) The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod 31(12):2841–2855
Sanad AS (2014) Prevalence of polycystic ovary syndrome among fertile and infertile women in Minia Governorate, Egypt. Int J Gynaecol Obstet 125(1):81–82
Thessaloniki ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2008) Consensus on infertility treatment related to polycystic ovary syndrome. Hum Reprod 23(3):462–477
Balen AH, Morley LC, Misso M et al (2016) The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update 22(6):687–708
Nandi A, Sinha N, Ong E, Sonmez H, Poretsky L (2016) Is there a role for vitamin D in human reproduction? Horm Mol Biol Clin Invest 25(1):15–28
Dennis NA, Houghton LA, Jones GT, van Rij AM, Morgan K, McLennan IS (2012) The level of serum anti-Mullerian hormone correlates with vitamin D status in men and women but not in boys. J Clin Endocrinol Metab 97(7):2450–2455
Irani M, Merhi Z (2014) Role of vitamin D in ovarian physiology and its implication in reproduction: a systematic review. Fertil Steril 102(2):460–468 e463
Yildizhan R, Kurdoglu M, Adali E et al (2009) Serum 25-hydroxyvitamin D concentrations in obese and non-obese women with polycystic ovary syndrome. Arch Gynecol Obstet 280(4):559–563
Kotsa K, Yavropoulou MP, Anastasiou O, Yovos JG (2009) Role of vitamin D treatment in glucose metabolism in polycystic ovary syndrome. Fertil Steril 92(3):1053–1058
Selimoglu H, Duran C, Kiyici S et al (2010) The effect of vitamin D replacement therapy on insulin resistance and androgen levels in women with polycystic ovary syndrome. J Endocrinol Investig 33(4):234–238
Zadeh-Vakili A, Ramezani Tehrani F, Daneshpour MS, Zarkesh M, Saadat N, Azizi F (2013) Genetic polymorphism of vitamin D receptor gene affects the phenotype of PCOS. Gene. 515(1):193–196
Irani M, Minkoff H, Seifer DB, Merhi Z (2014) Vitamin D increases serum levels of the soluble receptor for advanced glycation end products in women with PCOS. J Clin Endocrinol Metab 99(5):E886–E890
Figurova J, Dravecka I, Javorsky M, Petrikova J, Lazurova I (2016) Prevalence of vitamin D deficiency in Slovak women with polycystic ovary syndrome and its relation to metabolic and reproductive abnormalities. Wien Klin Wochenschr 128(17-18):641–648
(2008) STROBE statement--checklist of items that should be included in reports of observational studies (STROBE initiative). Int J Public Health 53(1):3–4
Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group (2004) Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod 19(1):41–47
Practice Committee of the American Society for Reproductive Medicine (2016) Prevention and treatment of moderate and severe ovarian hyperstimulation syndrome: a guideline. Fertil Steril 106(7):1634–1647
Thomson RL, Spedding S, Buckley JD (2012) Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol 77(3):343–350
Moini A, Shirzad N, Ahmadzadeh M, Hosseini R, Hosseini L, Sadatmahalleh SJ (2015) Comparison of 25-hydroxyvitamin D and calcium levels between polycystic ovarian syndrome and normal women. Int J Fertility Steril 9(1):1–8
Botros RM, Sabry IM, Abdelbaky RS, Eid YM, Nasr MS, Hendawy LM (2015) Vitamin D deficiency among healthy Egyptian females. Endocrinol Nutr 62(7):314–321
Kinuta K, Tanaka H, Moriwake T, Aya K, Kato S, Seino Y (2000) Vitamin D is an important factor in estrogen biosynthesis of both female and male gonads. Endocrinology. 141(4):1317–1324
Van Belle TL, Gysemans C, Mathieu C (2013) Vitamin D and diabetes: the odd couple. Trends Endocrinol Metab 24(11):561–568
Mathieu C (2015) Vitamin D and diabetes: where do we stand? Diabetes Res Clin Pract 108(2):201–209
Colonese F, Laganà AS, Colonese E, Sofo V, Salmeri FM, Granese R, Triolo O (2015) The pleiotropic effects of vitamin D in gynaecological and obstetric diseases: an overview on a hot topic. Biomed Res Int 2015:986281
Teegarden D, Donkin SS (2009) Vitamin D: emerging new roles in insulin sensitivity. Nutr Res Rev 22(1):82–92
Muscogiuri G, Policola C, Prioletta A et al (2012) Low levels of 25(OH)D and insulin-resistance: 2 unrelated features or a cause-effect in PCOS? Clin Nutr 31(4):476–480
Jia XZ, Wang YM, Zhang N et al (2015) Effect of vitamin D on clinical and biochemical parameters in polycystic ovary syndrome women: a meta-analysis. J Obstet Gynaecol Res 41(11):1791–1802
Ott J, Wattar L, Kurz C et al (2012) Parameters for calcium metabolism in women with polycystic ovary syndrome who undergo clomiphene citrate stimulation: a prospective cohort study. Eur J Endocrinol 166(5):897–902
Pacis MM, Fortin CN, Zarek SM, Mumford SL, Segars JH (2015) Vitamin D and assisted reproduction: should vitamin D be routinely screened and repleted prior to ART? A systematic review. J Assist Reprod Genet 32(3):323–335
Vanni VS, Vigano P, Somigliana E et al (2014) Vitamin D and assisted reproduction technologies: current concepts. Reprod Biol Endocrinol 12:47
Paffoni A, Ferrari S, Vigano P et al (2014) Vitamin D deficiency and infertility: insights from in vitro fertilization cycles. J Clin Endocrinol Metab 99(11):E2372–E2376
Lv SS, Wang JY, Wang XQ, Wang Y, Xu Y (2016) Serum vitamin D status and in vitro fertilization outcomes: a systematic review and meta-analysis. Arch Gynecol Obstet 293(6):1339–1345
Ozkan S, Jindal S, Greenseid K et al (2010) Replete vitamin D stores predict reproductive success following in vitro fertilization. Fertil Steril 94(4):1314–1319
Anifandis GM, Dafopoulos K, Messini CI et al (2010) Prognostic value of follicular fluid 25-OH vitamin D and glucose levels in the IVF outcome. Reprod Biol Endocrinol 8:91
Garbedian K, Boggild M, Moody J, Liu KE (2013) Effect of vitamin D status on clinical pregnancy rates following in vitro fertilization. CMAJ Open 1(2):E77–E82
Aleyasin A, Hosseini MA, Mahdavi A et al (2011) Predictive value of the level of vitamin D in follicular fluid on the outcome of assisted reproductive technology. Eur J Obstet Gynecol Reprod Biol 159(1):132–137
Rudick BJ, Ingles SA, Chung K, Stanczyk FZ, Paulson RJ, Bendikson KA (2014) Influence of vitamin D levels on in vitro fertilization outcomes in donor-recipient cycles. Fertil Steril 101(2):447–452
Firouzabadi RD, Rahmani E, Rahsepar M, Firouzabadi MM (2014) Value of follicular fluid vitamin D in predicting the pregnancy rate in an IVF program. Arch Gynecol Obstet 289(1):201–206
Franasiak JM, Molinaro TA, Dubell EK et al (2015) Vitamin D levels do not affect IVF outcomes following the transfer of euploid blastocysts. Am J Obstet Gynecol 212(3):315.e311–315.e316
Acknowledgements
The authors acknowledge the staff department of IVF at Cairo University for the help of this research to be accomplished
Funding
None
Author information
Authors and Affiliations
Contributions
EFO: data collection and analysis, planning of the study protocol, revision of the study. AR: idea of the work, planning of the protocol, revision of the study. AS: idea of the work, planning of the protocol, revision of the study. NF: data collection. MS: analysis and revision of the study. HB: study protocol and data collection. MFS: data collection and revision of the study. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Ethics approval was taken for this study by the Kasralainy Obstetrics and Gynecology Department (20161101). The consent to participate was in a written form.
Consent for publication
The authors confirm giving consent for publication.
Competing interests
The authors confirm the absence of competing interests for this study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omran, E.F., Ramzy, A., Shohayeb, A. et al. Relation of serum vitamin D level in polycystic ovarian syndrome (PCOS) patients to ICSI outcome. Middle East Fertil Soc J 25, 22 (2020). https://doi.org/10.1186/s43043-020-00034-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43043-020-00034-3