Abstract
Background
The association between cocaine- and amphetamine-regulated transcript prepropeptide gene (CARTPT) and obesity-related outcomes has shown in the epidemiological studies. Nevertheless, there is lack of data regarding the CARTPT gene–diet interactions in terms of antioxidant potential of diet. So, this study aimed to test CARTPT gene–dietary non-enzymatic antioxidant capacity (NEAC) interactions on cardio-metabolic risk factors in obese individuals.
Methods and material
The present cross-sectional study was carried out among 288 apparently healthy obese adults within age range of 20–50 years. Antioxidant capacity of diet was estimated by calculating the oxygen radical absorbance capacity (ORAC), ferric reducing antioxidant power (FRAP), total radical-trapping antioxidant parameter (TRAP) and Trolox equivalent antioxidant capacity (TEAC) using a semiquantitative food frequency questionnaire (FFQ). Genotyping for CARTPT rs2239670 polymorphism was conducted by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method.
Results
A significant interaction was revealed between CARTPT rs2239670 and dietary ORAC on BMI (PInteraction = 0.048) and fat mass percent (FM%) (PInteraction = 0.008); in A allele carriers, higher adherence to the dietary ORAC was related to lower level of BMI and FM%. And, the significant interactions were observed between FRAP index and rs2239670 in relation to HOMA (PInteraction = 0.049) and QUICKI (PInteraction = 0.048). Moreover, there were significant interactions of rs2239670 with TRAP (PInteraction = 0.029) and TEAC (PInteraction = 0.034) on the serum glucose level; individuals with AG genotype were more respondent to higher intake of TRAP.
Conclusion
The present study indicated that the relationships between CARTPT rs2239670 and obesity and its-related metabolic parameters depend on adherence to the dietary NEAC. Large prospective studies are needed to confirm our findings.
Similar content being viewed by others
Introduction
Obesity, as a worldwide pandemic, has become a major public health issue, and its prevalence is increasing markedly in many countries [1]. In comparison with 1980, global prevalence of overweight and obesity has nearly doubled in all age–sex groups regardless of race, ethnicity and socioeconomic status [2]. Obesity is a multifactorial and preventable disorder which substantially increases risk of comorbidities such as: cardiovascular diseases (CVDs), stroke, type 2 diabetes, hypertension, fatty liver disease and certain cancers. Thereby, it can affect quality of life, life expectancy, work productivity and healthcare costs [1]. A wealth of evidence has shown that obesity contributes to initiation and progression of these pathological conditions through oxidative stress which is defined as an imbalance between reactive oxygen species (ROS) production and antioxidant defenses [3]. It has been demonstrated that oxidative stress damages or alterations in antioxidant defenses are involved in the pathogenesis and development of obesity-associated consequences such as CVDs and cancer [3,4,5].
Emerging evidence suggests that the development of obesity, as a multifactorial disorder, and its-related comorbidities is determined by interactions between genetic and environmental variables particularly diet [6]. Accumulating evidence suggests that dietary antioxidant intake (e.g., selenium, vitamin C and vitamin E) through non-enzymatic defense mechanisms can protect against cell damage caused by oxidative stress, and its-related inflammatory outcomes [7]. In this regard, the epidemiological research has reported that higher intakes of fruits and vegetables, which are rich sources of antioxidants, are associated with the lower risk of several chronic conditions such as CVDs and cancers [8, 9]. Since various antioxidants are combined in foods and they act in a cumulative and synergistic way in complex matrixes, assessment of single specific antioxidant may not reflect the potential overall antioxidant effect of the diet. Thus, investigation of the dietary total antioxidant capacity (TAC) can provide a better method to examine the favorable influences of dietary antioxidants on chronic diseases prevention [10]. Non-enzymatic antioxidant capacity (NEAC), also recognized as TAC, is a new approach to capture synergistic effects of antioxidants in diet and generally can be estimated through four different methods including: Trolox equivalent antioxidant capacity (TEAC), ferric reducing antioxidant power (FRAP), total radical-trapping antioxidant parameter (TRAP) and oxygen radical absorbance capacity (ORAC) [11]. Recently, evidence from observational investigations has revealed inverse significant associations of dietary NEAC with the risk of stroke, diabetes and different cancers [12,13,14].
Since obesity is highly heritable, genetic factors substantially play an important role in development of obesity and its serious consequences [15]. Cocaine- and amphetamine-regulated transcript prepropeptide gene (CARTPT), which maps to the chromosome 5q13-14 and expresses the cocaine- and amphetamine-regulated transcript (CART) protein, has been recognized to be a susceptibility locus for obesity (Fig. 1). CART protein, as one of the various neuropeptides in the arcuate nucleus (ARC) of the hypothalamus, has been implicated in modulating feeding behavior and energy balance [16]. In this regard, previous studies have found that CART peptides which are co-expressed with other neurotransmitters such as α-melanocyte-stimulating-hormone (α-MSH) inhibit food intake [17]. The latest scientific studies have indicated that variations in the CARTPT gene might influence obesity, metabolic syndromes (MetS) and its components [18]. However, the results of studies regarding these associations are controversial [19]. These heterogeneous associations may be due to the complicated pathogenesis and etiology of obesity which involve interactions between genetic and environmental factors especially diet. A positive association between CARTPT rs2239670 variant and alcoholism has been reported in the Korean population [20]. Since obesity and substance abuse have a common neurobiological basis, investigation of the association between this polymorphism and obesity and metabolic factors is interesting.
Therefore, since the better understanding of the gene–diet interactions can provide more efficient strategies for personalized treatment, and also as far as we know, no previous evidence is available on the modification effect of diet in terms of dietary antioxidant capacity on the association of CARTPT gene with obesity-related metabolic factors, the aim of the present study was to assess the potential interactions of CARTPT rs2239670 with NEAC intake in relation to cardio-metabolic risk factors among obese population.
Materials and methods
Study participants
This cross-sectional study was carried out in Tabriz, a city in the northwest of Iran, from December 2017 to April 2019 among apparently healthy obese subjects using convenience sampling method. Study participants were 288 apparently healthy obese (body mass index (BMI) ≥ 30 kg/m2) adult aged 20–50 years who were obese. Detailed illustration of recruitment of research participants has been provided elsewhere [21]. Individuals were removed from the study if they met any of the following criteria: being menopausal and pregnant, lactation, prior diagnose of diseases (cardiovascular diseases, hypertension, hyperlipidemia, cancer, type 2 diabetes mellitus and renal diseases), taking any medication affecting the variables studied (hypoglycemic, lipid-lowering, antihypertensive, corticosteroids or antidepressants). All subjects completed a written, informed consent and the ethical committee of Tabriz University of Medical Sciences approved the protocol of this study (Ethics number: IR.TBZMED.REC.1397.266).
Demographic and anthropometric assessments
Information of physical activity and other demographic characteristics (age, sex) was collected by trained interviewer at the beginning of the study. To estimate the level of physical activity, the short form of the international physical activity questionnaire (IPAQ) was used [22]. Weight and height were measured in light clothing using a Seca scale (Seca, Germany) and a tape measure to the nearest 0.1 kg and 0.1 cm, respectively. Waist and hip circumference (WC and HC) was obtained at the narrowest level and largest part, respectively, by a non-elastic measuring tape with accuracy of 0.1 cm, and waist-to-hip ratio (WHR) was then calculated. Assessments of body composition were conducted using bioelectrical impedance analysis (BIA) technology (Tanita, BC-418 MA, Tokyo, Japan). Blood pressure of subjects was determined using a mercury sphygmomanometer after the participants had a 10-min rest in a sitting position. This measurement was carried out two times, and the average of them was considered as subject’s blood pressure.
Biochemical measurements
Blood samples were taken from all study participants after a 12-h fasting period. Plasma and serum were prepared by centrifugation (10 min at 4500 rpm, 4 °C), and their aliquots were frozen at − 80 °C until they were analyzed. Analyses of serum glucose, total cholesterol (TC), triglyceride (TG) and high-density lipoprotein cholesterol (HDL-C) levels were conducted by commercially available kits (Pars Azmoon Inc., Tehran, Iran) [21]. Serum insulin [23] and plasma concentrations of α-MSH and AgRP were measured by enzyme-linked immunosorbent assay kits (Bioassay Technology Laboratory, Shanghai Korean Biotech, Shanghai City, China) [24] based on manufacturer’s protocol. Serum low-density lipoprotein cholesterol (LDL), the homeostasis model of assessment ratio (HOMA-R) and quantitative insulin sensitivity check index (QUICKI) were calculated using the protocols which have been described by Friedewald et al. [25], Matthews et al. [26] and Katz et al. [27], respectively.
Assessment of dietary intakes and NEAC calculation
Dietary intake of participants during the previous year was evaluated using a 147-item semiquantitative food frequency questionnaire (FFQ) which was previously validated for use among Iranian population [28, 29]. Study subjects were asked to report portion size of each food item during the previous year on a daily, weekly, monthly and yearly basis. By using household measurements, portion sizes of consumed food items were converted to grams [30]. Then, Iranian Food Composition Table (FCT) was applied to analyze daily nutrient intake [31]. Information missing from this FCT was completed by the United States Department of Agriculture FCT [32].
NEAC is an indicator of diet quality which describes the ability of different antioxidants in food to remove free radicals, and its value was measured using the following indices: FRAP [33] which estimates the reducing potency of dietary antioxidants, TRAP [34] that evaluates the chain-breaking antioxidant potential for scavenging peroxyl radicals, ORAC [35] which measures the antioxidant capacity against peroxyl radicals using an area under curve (AUC) technique and TEAC [34] which is based on scavenging ability of antioxidants against a radical cation in both lipophilic and hydrophilic environments. Since Maillard products from the coffee roasting process are the main contributors to the high in vitro antioxidant capacity of coffee [36] and also due to high molecular weight of these products, the proportion absorbed through the intestinal mucosa and whether they display an antioxidant effect in vivo is still unknown [37], we decided to exclude the contribution of coffee to NEAC. Dietary NEAC values for 64, 63, 59 and 65 food items in the FFQ were assigned by ORAC, FRAP TRAP and TEAC, respectively. Finally, to obtain total dietary NEAC for each participant, corresponding NEAC values of single foods were multiplied by the daily intake of each food consumed and then summed up.
DNA extraction and genotyping
The genomic DNA was extracted from blood samples by phenol/chloroform extraction method. Nano Drop 2000C spectrophotometer was applied to determine the quality and quantity of the DNA extracted from each sample. Genotyping the CARTPT rs2239670 variant was carried out by polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) method. The following primers were used for PCR amplification: forward 5′-CCTGCTGCTGATGCTACCTCT-3′ and reverse 5′-GCGCTTCGATCTGCAACACAC-3′. The cycling conditions in DNA thermocycler were as follows: 94 °C for 5 min (initial denaturation), 35 cycles of denaturation at 94 °C for 30 s, annealing at 60 °C for 30 s and extension at 72 °C for 20 s. At the end of the final cycle, an additional extension step occurred at 72 °C for 10 min. PCR amplification was optimized in a total volume of 25 μl containing 2 µl genomic DNA, 12.5 µl distilled water, 0.5 µl of each primer and 10 µl Taq DNA Polymerase Master Mix (Ampliqon, Denmark). PCR product was digested with ApaI restriction enzyme (Takara, Japan), and then, digested product was subjected to electrophoresis on 3% agarose gel. After visualizing by electrophoresis, the A allele appeared as fragment with length of 552 bp, while G allele was distinguished as 340 and 212 bp fragments.
Statistical analyses
All variables were checked for normality of distribution by Kolmogorov–Smirnov test. The comparison of qualitative and quantitative variables was conducted by chi- square test and analysis of variance (ANOVA), respectively. Data on continuous and categorical variables were presented as the mean ± SD and the frequencies or percentages, respectively. ANCOVA multivariate interaction model with adjustment for confounders was applied to assess the interactions between CARTPT rs2239670 polymorphism and dietary NEAC on cardio-metabolic risk factors. Statistical Package for Social Science (SPSS Inc., Chicago IL, USA) version 22.0 was employed for data analyses. A p value less than 0.05 was considered significant.
Results
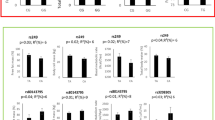
The mean (SD) age and BMI of the study subjects were 38.04 (7.47) years and 34.72 (3.88) kg/m2, respectively. Distribution of study subjects in terms of general characteristics across CARTPT rs2239670 genotypes is shown in Table 1. A statistically significant difference was found in the mean WHR (P = 0.025) according to different genotypes. The analysis did not reveal any significant differences for other general characteristics. Table 2 summarizes the dietary macro- and micronutrients intakes of the study participants. The mean (± SD) values of energy, protein, carbohydrate and fats intakes were 3042.91 (1077.89), 97.86 (34.67), 440.02 (164.76) and 108.04 (46.85), respectively. The genotype and allele frequencies for the CARTPT rs2239670 polymorphism among dietary NEAC tertiles are presented in Table 3. The results of the comparison showed that participants carrying the heterozygote genotype were more likely to have lower adherence to dietary FRAP, TRAP and TEAC; however, there was no statistically significant difference. Besides, in spite of non-significant associations, minor allele carriers were assigned to the second tertile of ORAC. On the other hand, as indicated in Table 3, the frequency of mutant allele increased when following a healthy diet rich in antioxidants, while differences were not significant (P > 0.05). The total frequencies of genotypes among population studied were as follows: AA (10.76%), AG (20.13%) and GG (69.9%). And, the minor allele frequency observed in this study was 20.79%. There was no significant difference regarding the mean values of biochemical parameters of subjects across different genotypes of CARTPT rs2239670 variant (Table 4). As shown in Table 5, there were significant differences regarding WC, BMR, TG, LDL-C and glucose between various tertile of dietary NEAC (P < 0.05). Although the participants in the highest tertile of TEAC had higher means of WC (P = 0.031) and TG (P = 0.037), mean of serum concentration of LDL-C was partially lower in this category than the first tertile (P = 0.053). Moreover, those in the highest tertile of the NEAC (ORAC, FRAP, TRAP and TEAC) had higher level of BMR (P < 0.05) than those in the lowest category. Similarly, higher means of glucose level (P = 0.018) were seen among those in third tertile of the ORAC. In addition, participants who assigned to the second tertile of FRAP had higher WC (P = 0.04) compared to other tertiles. As elucidated in Fig. 2, after adjusting for sex and age, significant interactions were revealed between CARTPT rs2239670 variant and dietary ORAC on BMI (PInteraction = 0.048) and fat mass percent (FM%) (PInteraction = 0.008); the lowest means of BMI and FM% were observed in A allele carriers in the third tertile of dietary ORAC. Moreover, significant interactions were observed between FRAP indicator and CARTPT rs2239670 polymorphism in relation to HOMA (PInteraction = 0.049) (Fig. 2) and QUICKI (PInteraction = 0.048) (Fig. 3), such that adherence to the dietary FRAP modified positively the association of the CARTPT rs2239670 variant with HOMA and QUICKI in G allele carriers. In other words, dietary NEAC could not modulate detrimental effects of CARTPT rs2239670 polymorphism on insulin resistance indices (HOMA and QUICKI) in participants carrying AA genotype. Additionally, significant interactions were observed between CARTPT rs2239670 variant and TRAP (PInteraction = 0.029) and TEAC (PInteraction = 0.034) in relation to serum glucose level (Fig. 3); the highest serum glucose concentration was found in the AA homozygote carriers assigned to the second tertile of TRAP and TEAC.
Discussion
As far as we know, the current research is the first attempt to examine the interactions of CARTPT rs2239670 polymorphism with dietary NEAC in relation to cardio-metabolic risk factors. Our findings suggest that the rs2239670 on chromosome 5q13-14 interacts with dietary ORAC, FRAP and TEAC to influence obesity and obesity-related metabolic phenotypes. Specifically, when compliance with ORAC was high, minor allele carriers were less susceptible to the development of obesity-related traits such as BMI and FM%. On the other hand, improving the adherence to dietary FRAP might reduce the genetic association with insulin resistance indices (HOMA-IR and QUICKI) only among G allele carriers (AG and GG). However, positive interactions of CARTPT rs2239670 on serum glucose level were found even in high compliance with TRAP and TEAC. Thus, the results of modification effect of diet on the associations of CARTPT with obesity and metabolic factors were not homogenous. According to our results, frequency of the rare allele (20.79%) was nearly similar to that of the other population or ethnic groups such as Korean (17%) [20] and Malaysian (30%) [19]. This discrepancy in the minor allele frequency reported might be due to variations in sample size, demographic characteristics of population like age, ethnicity and gender and also various lifestyles.
Previous evidence has revealed that polymorphisms in the CARTPT gene are linked to human obesity [16]. In this regard, leu34Phe missense mutation in CARTPT gene was detected in Italian subjects with early-onset obesity [16]. Likewise, it was reported that the A-156G polymorphism in the promoter region of CARTPT was related to adiposity among Japanese subjects [38]. Moreover, the studies have shown that genetic polymorphisms in the CARTPT gene might affect susceptibility to MetS and its components such as dyslipidemia, high blood pressure and hyperglycemia [18]. It should be taken into account that all variants in CARTPT gene have not been related to obesity phenotypes and findings in this regard are conflicting [39]. For example, in accordance with the results of Walder study, C1442G polymorphism of CARTPT gene was not related to obesity among Pima Indians [39]. Other studies which have specifically investigated the association of the CARTPT rs2239670 polymorphism with addictive behaviors such as alcohol dependence have confirmed a positive relation [20]. However, the studies which have evaluated this variant in relation to obesity are scarce. According to our knowledge, only one study has assessed the CARTPT rs2239670–obesity relation and showed no association between this variant and obesity among the Malaysian subjects [19]. All of these heterogeneities in findings warrant further research efforts among different populations. Since the rs2239670 variant located in the intron 1 of CARTPT gene and its strong effects on obesity and related metabolic factors may be removed during splicing process of mRNA encoding CART proteins, effects of this variant on CART function are still unknown [19]. However, genetic polymorphisms in the CART locus may affect the expression of the CART peptide, which is related to hypothalamic anorectic and orexigenic neuropeptides [40]. Totally, it seems that CART peptides have a modulatory role in feeding behavior and exert anorexigenic effect, although the biological mechanism of this function remains unclear [41].
As far as we are aware, no previous study has investigated the gene–diet interactions of CARTPT with adherence to the dietary NEAC on metabolic profile in obesity to compare our finding. However, there has been a lot of research investigating the interaction between genetic variations and diet or dietary ingredients on obesity and its related complications [42]. For example, Mirzababaei et al. examined the interaction of the rs1333048 variant on 9p21 genetic region with TAC on the risk of MetS and they revealed that high ORAC intake may improve the increased risk of MetS in homozygous subjects for the minor allele (AA genotype) [42]. Subsequent study by Mahmoudi-Nezhad et al. has documented the significant interactions between healthy dietary patterns (healthy eating index and diet quality index-international (DQI-I)) and CARTPT rs2239670 genotypes affecting metabolic parameters [43]; higher compliance with DQI-I decreased the metabolic risk parameters in AA homozygote carriers.
Noticeably, the main finding of the present research was that the association of the CARTPT rs2239670 polymorphism with cardio-metabolic factors depended on the dietary antioxidant intakes; a good compliance with NEAC blunted the relationship between the CARTPT gene and cardio-metabolic risk factors. While the underlying mechanisms behind this interactions are not still clarified, these favorable effects of the total antioxidant capacity may be mediated by vitamin C, vitamin E and its isomers, selenium, carotenoids, isoflavones, flavonoids and proanthocyanidins [44, 45]. In this regard, there are numerous studies which have indicated the beneficial effects of high-antioxidant foods (for example, fruits, vegetables, olive oil, nuts and tea) on the obesity, insulin resistance, glucose homeostasis and lipid profiles [46, 47]. These beneficial effects of antioxidants on the metabolic profile may be partly attributed to other activities of antioxidants such as regulation of metabolic pathways in brown adipose tissue and increase thermogenesis, suppression of adipogenesis and induction of catabolism in adipose tissue [48].
Strengths and weaknesses
As far as we know, this is the first study to examine the interaction of CARTPT rs2239670 genotypes with dietary NEAC on the metabolic factors in obese subjects and identifying these gene–diet interactions may provide the best personalized dietary advice for high-risk participants according to their genetic makeup to decrease the heavy burden of obesity and its-related chronic diseases. However, the present study has certain limitations that need to be noted. Firstly, since this is a cross-sectional study, ascertained causality cannot be argued but it helps to generate hypotheses that can be examined by prospective cohort or other studies. Secondly, a rather small sample size of our study may not cover statistical power for analyzing the interaction effect. So, the results of our study must be interpreted warily and require replication and confirmation in larger and different populations. Thirdly, the present study was limited to the assessment of only single polymorphism from a single gene, while there are multiple well-known genes that have been implicated in the pathogenesis of obesity and its related consequences. Fourthly, our results may not necessarily be extrapolated to the general population as this study was carried out among a population from Tabriz with different cultures and lifestyle factors. Fifthly, despite adjustment for several confounders in the analyses, residual confounding by other unmeasured factors was inevitable. Lastly, under-reporting of dietary intake, as a potential bias, is common among obese individuals which may cause underestimation of the true effect [49]. Thus, we excluded the extreme-energy reporters from analysis.
Conclusion
In conclusion, our finding showed a statistically significant gene–diet interaction between the CARTPT rs2239670 and compliance with healthy and good quality diet rich in antioxidants in relation to obesity and related metabolic phenotypes; high intake of NEAC by minor allele carriers attenuated genetic association with BMI and FM%; however, high compliance with these indices could not affect genetic predisposition to blood sugar abnormalities. Further studies are warranted to confirm our results, which may be of important in public health.
Availability of data and materials
Data of the current research will be available with a reasonable request from the corresponding author.
Abbreviations
- NEAC:
-
Non-enzymatic antioxidant capacity
- CARTPT:
-
Cocaine- and amphetamine-regulated transcript prepropeptide
- FFQ:
-
Food frequency questionnaire
- ORAC:
-
Oxygen radical absorbance capacity
- FRAP:
-
Ferric reducing antioxidant power
- TRAP:
-
Total radical-trapping antioxidant parameter
- TEAC:
-
Trolox equivalent antioxidant capacity
- CVDs:
-
Cardiovascular diseases
- ROS:
-
Reactive oxygen species
- MetS:
-
Metabolic syndromes
References
Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol 15(5):288–298
Chooi YC, Ding C, Magkos F (2019) The epidemiology of obesity. Metabolism 1(92):6–10
Bondia-Pons I, Ryan L, Martinez JA (2012) Oxidative stress and inflammation interactions in human obesity. J Physiol Biochem 68(4):701–711
Sosa V, Moliné T, Somoza R, Paciucci R, Kondoh H, Leonart ME (2013) Oxidative stress and cancer: an overview. Ageing Res Rev 12(1):376–390
Csányi G, Miller FJ Jr (2014) Oxidative stress in cardiovascular disease. Int J Mol Sci 15(4):6002–6008
Herrera BM, Lindgren CM (2010) The genetics of obesity. Curr Diab Rep 10(6):498–505
Frei B (2004) Efficacy of dietary antioxidants to prevent oxidative damage and inhibit chronic disease. J Nutr 134(11):3196s-s3198
Vieira AR, Abar L, Vingeliene S, Chan DS, Aune D, Navarro-Rosenblatt D et al (2016) Fruits, vegetables and lung cancer risk: a systematic review and meta-analysis. Ann Oncol 27(1):81–96
Spence JD (2006) Nutrition and stroke prevention. Stroke 37(9):2430–2435
Serafini M, Del Rio D (2004) Understanding the association between dietary antioxidants, redox status and disease: is the Total Antioxidant Capacity the right tool? Redox Rep 9(3):145–152
Pellegrini N, Serafini M, Colombi B, Del Rio D, Salvatore S, Bianchi M et al (2003) Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J Nutr 133(9):2812–2819
Colarusso L, Serafini M, Lagerros YT, Nyren O, La Vecchia C, Rossi M et al (2017) Dietary antioxidant capacity and risk for stroke in a prospective cohort study of Swedish men and women. Nutrition 33:234–239
Parohan M, Sadeghi A, Khatibi SR, Nasiri M, Milajerdi A, Khodadost M et al (2019) Dietary total antioxidant capacity and risk of cancer: a systematic review and meta-analysis on observational studies. Crit Rev Oncol Hematol 138:70–86
Mancini FR, Affret A, Dow C, Balkau B, Bonnet F, Boutron-Ruault MC et al (2018) Dietary antioxidant capacity and risk of type 2 diabetes in the large prospective E3N-EPIC cohort. Diabetologia 61(2):308–316
Yu Z, Han S, Cao X, Zhu C, Wang X, Guo X (2012) Genetic polymorphisms in adipokine genes and the risk of obesity: a systematic review and meta-analysis. Obesity (Silver Spring) 20(2):396–406
del Giudice EM, Santoro N, Cirillo G, D’Urso L, Di Toro R, Perrone L (2001) Mutational screening of the CART gene in obese children: identifying a mutation (Leu34Phe) associated with reduced resting energy expenditure and cosegregating with obesity phenotype in a large family. Diabetes 50(9):2157–2160
Murphy KG (2005) Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief Funct Genomic Proteomic 4(2):95–111
Vasseur F, Guérardel A, Barat-Houari M, Cottel D, Amouyel P, Froguel P et al (2007) Impact of a CART promoter genetic variation on plasma lipid profile in a general population. Mol Genet Metab 90(2):199–204
Lisa Y, Sook HF, Yee HS (2012) Association of the cocaine- and amphetamine-regulated transcript prepropeptide gene (CARTPT) rs2239670 variant with obesity among kampar health clinic patrons. Malaysia Malays J Med Sci 19(1):43–51
Jung SK, Hong MS, Suh GJ, Jin SY, Lee HJ, Kim BS et al (2004) Association between polymorphism in intron 1 of cocaine- and amphetamine-regulated transcript gene with alcoholism, but not with bipolar disorder and schizophrenia in Korean population. Neurosci Lett 365(1):54–57
Khodarahmi M, Asghari-Jafarabadi M, Abbasalizad FM (2019) A structural equation modeling approach for the association of a healthy eating index with metabolic syndrome and cardio-metabolic risk factors among obese individuals. PLoS ONE 14(7):e0219193
Booth M (2000) Assessment of physical activity: an international perspective. Res Q Exerc Sport 71(Suppl 2):114–120
Kheirollahi A, Teimouri M, Karimi M, Vatannejad A, Moradi N, Borumandnia N, Sadeghi A (2020) Evaluation of lipid ratios and triglyceride-glucose index as risk markers of insulin resistance in Iranian polycystic ovary syndrome women. Lipids Health Dis 19(1):235
Kravchychyn ACP, Campos RMDS, Corgosinho FC, Masquio DCL, Vicente SECF, Ferreira YAM, Silva PL, Ganen AP, Oyama LM, Tock L, de Mello MT, Tufik S, Dâmaso AR (2018) The long-term impact of high levels of alpha-melanocyte-stimulating hormone in energy balance among obese adolescents. Ann Nutr Metab 72(4):279–286
Friedewald WT, Levy RI, Fredrickson DS (1972) Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 18(6):499–502
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419
Katz A, Nambi SS, Mather K, Baron AD, Follmann DA, Sullivan G et al (2000) Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. J Clin Endocrinol Metab 85(7):2402–2410
Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F (2010) Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr 13(5):654–662
Esfahani FH, Asghari G, Mirmiran P, Azizi F (2010) Reproducibility and relative validity of food group intake in a food frequency questionnaire developed for the Tehran Lipid and Glucose Study. J Epidemiol 20(2):150–158
Ghaffarpour M, Houshiar-Rad M, Kianfar H (1999) The manual for household measures, cooking yields factors and edible portion of foods. Tehran Nashre Olume Keshavarzy 7(213):42–58
Khodarahmi M, Kahroba H, Jafarabadi MA, Mesgari-Abbasi M, Farhangi MA (2020) Dietary quality indices modifies the effects of melanocortin-4 receptor (MC4R) rs17782313 polymorphism on cardio-metabolic risk factors and hypothalamic hormones in obese adults. BMC Cardiovasc Disord 20(1):57
Asghari G, Yuzbashian E, Mirmiran P, Azizi F (2017) The association between Dietary Approaches to Stop Hypertension and incidence of chronic kidney disease in adults: the Tehran Lipid and Glucose Study. Nephrol Dial Transplant 32(suppl_2):ii224–ii30
Carlsen MH, Halvorsen BL, Holte K, Bøhn SK, Dragland S, Sampson L et al (2010) The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J 9:3
Pellegrini N, Serafini M, Salvatore S, Del Rio D, Bianchi M, Brighenti F (2006) Total antioxidant capacity of spices, dried fruits, nuts, pulses, cereals and sweets consumed in Italy assessed by three different in vitro assays. Mol Nutr Food Res 50(11):1030–1038
Wu X, Beecher GR, Holden JM, Haytowitz DB, Gebhardt SE, Prior RL (2004) Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J Agric Food Chem 52(12):4026–4037
Delgado-Andrade C, Morales FJ (2005) Unraveling the contribution of melanoidins to the antioxidant activity of coffee brews. J Agric Food Chem 53(5):1403–1407
Morales FJ, Somoza V, Fogliano V (2012) Physiological relevance of dietary melanoidins. Amino Acids 42(4):1097–1109
Yamada K, Yuan X, Otabe S, Koyanagi A, Koyama W, Makita Z (2002) Sequencing of the putative promoter region of the cocaine- and amphetamine-regulated-transcript gene and identification of polymorphic sites associated with obesity. Int J Obes Relat Metab Disord 26(1):132–136
Walder K, Morris C, Ravussin E (2000) A polymorphism in the gene encoding CART is not associated with obesity in Pima Indians. Int J Obes Relat Metab Disord 24(4):520–521
Hunter RG, Philpot K, Vicentic A, Dominguez G, Hubert GW, Kuhar MJ (2004) CART in feeding and obesity. Trends Endocrinol Metab 15(9):454–459
Ahmadian-Moghadam H, Sadat-Shirazi MS, Zarrindast MR (2018) Cocaine- and amphetamine-regulated transcript (CART): a multifaceted neuropeptide. Peptides 110:56–77
Mirzababaei A, Mollahosseini M, Rahimi MH, Yekaninejad MS, Maghbooli Z, Sobhani R et al (2018) Interaction between a variant of chromosome 9p21.3 locus and diet antioxidant capacity on metabolic syndrome in Tehrani adults. Diabetol Metab Syndr 10:76
Mahmoudi-Nezhad M, Farhangi MA, Kahroba H (2020) Cocaine and amphetamine-regulated transcript prepropeptide gene (CARTPT) polymorphism interacts with Diet Quality Index-International (DQI-I) and Healthy Eating Index (HEI) to affect hypothalamic hormones and cardio-metabolic risk factors among obese individuals. J Transl Med 18(1):16
Dakhale GN, Chaudhari HV, Shrivastava M (2011) Supplementation of vitamin C reduces blood glucose and improves glycosylated hemoglobin in type 2 diabetes mellitus: a randomized, double-blind study. Adv Pharmacol Sci 2011:195271
Manning PJ, Sutherland WH, Walker RJ, Williams SM, De Jong SA, Ryalls AR et al (2004) Effect of high-dose vitamin E on insulin resistance and associated parameters in overweight subjects. Diabetes Care 27(9):2166–2171
Li M, Fan Y, Zhang X, Hou W, Tang Z (2014) Fruit and vegetable intake and risk of type 2 diabetes mellitus: meta-analysis of prospective cohort studies. BMJ Open 4(11):e005497
Maritim AC, Sanders RA, Watkins JB 3rd (2003) Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol 17(1):24–38
Lee MS, Kim CT, Kim Y (2009) Green tea (-)-epigallocatechin-3-gallate reduces body weight with regulation of multiple genes expression in adipose tissue of diet-induced obese mice. Ann Nutr Metab 54(2):151–157
Fisher JO, Johnson RK, Lindquist C, Birch LL, Goran MI (2000) Influence of body composition on the accuracy of reported energy intake in children. Obes Res 8(8):597–603
Acknowledgements
The authors thank all the participants of the current study. We also thank from Research Undersecretary of Tabriz University of Medical Sciences for financial support of the current work with grant number of 65305.
Funding
This study was supported by funding from Tabriz University of Medical Sciences (Grant number: 65305 and registration number: IR.TBZMED.REC.1399.207).
Author information
Authors and Affiliations
Contributions
MAF and GS designed the study and served as supervisors for this research. They also generated the study’s first hypothesis. GS and AS were involved in data collection and patients’ recruitment. MKH was involved in manuscript writing and revision. She also performed the statistical analysis. HK performed the genotyping analysis and was involved in revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (Ethic number: IR.TBZMED.REC.1399.207). All study participants completed written informed consent at the beginning of the study.
Consent for publication
This is formally to submit the article entitled “Hypothalamic-pituitary hormones will be affected by the interaction between 5q13-14-rs2239670 (CARTPT) gene variants and diet in different obesity phenotypes” prepared by the Tabriz University of Medical Sciences for review and, hopefully, publication in your prestigious journal. The authors would like to advise that all authors listed have contributed to the work.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khodarahmi, M., Sobhrakhshan Khah, A., Farhangi, M.A. et al. Dietary total antioxidant capacity interacts with a variant of chromosome 5q13-14 locus to influence cardio-metabolic risk factors among obese adults. Egypt J Med Hum Genet 23, 117 (2022). https://doi.org/10.1186/s43042-022-00328-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00328-3