Abstract
Background
One of the most common cancers diagnosed worldwide is breast cancer (BC), which is the leading cause of cancer death among women. The radiogenomics method is more accurate for managing and inhibiting this disease, which takes individual diagnosis on genes, environments, and lifestyles of each person. The present study aims to highlight the current state-of-the-art, the current role and limitations, and future directions of radiogenomics in breast cancer.
Method
This systematic review article was searched from databases such as Embase, PubMed, Web of Science, Google Scholar, Scopus, and Cochrane Library without any date or language limitations of databases. Searches were performed using Boolean OR and AND operators between the main terms and keywords of particular topic of the subject under investigation. All retrospective, prospective, cohort, and pilot studies were included, which were provided with more details about the topic. Articles such as letter to the editor, review, and short communications were excluded because of lack of information, discussions, or use of radiogenomics method on other cancers. For quality assessment of articles, STROBE checklist was used.
Result
For the systematic review, 18 articles were approved after assessing the full text of selected articles. In this review, 3614 patients with BC of selected articles were evaluated, and all radiogenomics were associated with more power in classification, differential diagnosis, and prognosis of BC. Among the various modalities to predict genomic indicators and molecular subtypes, DCE-MRI has the higher performance and finally the highest amount of AUC value (0.956) belonged to PI3K gene.
Conclusion
This review shows that radiogenomics can help with the diagnosis and treatment of breast cancer in patients. It has shown that recognizing and specifying radiogenomic phenotypes in the genomic signatures can be helpful in treatment and diagnosis of disease. The molecular methods used in these articles are limited to miRNAs expression, gene expression, Ki67 proliferation index, next-generation RNA sequencing, whole RNA sequencing, and molecular histopathology that can be completed in future studies by other methods such as exosomal miRNAs, specific proteins expression, DNA repair capacity, and other biomarkers that have prognostic and predictive value for cancer treatment response. Studies with control group and large sample size for evaluation of radiogenomics in diagnosis and treatment recommended.
Similar content being viewed by others
Introduction
One of the second most common cancers diagnosed worldwide is breast cancer, which is the leading cause of cancer death among women [1]. The Global Cancer Statistics 2020 report revealed 2.3 million new cases recognized and showed that female breast cancer was the most common [2]. Human breast cancer (BC) shows great interest in exploring the multivariate relationships of cancer [3, 4]. At clinical and molecular levels, BC is defined as a heterogeneous illness which it has distinguished into different subgroups concerning the situation of hormone receptors and altered clinical result. Identifying the biological context associated with tumor progression through imaging features provides additional data that can help in pre-treatment and pre-prognosis, as well as it helps us to know more about the biological features of the tumor [5, 6]. Association of the tumor genome and imaging phenotypes is called radiogenomics [7]. Radiogenomics method is more accurate for managing and inhibiting this disease which takes individual diagnosis in genes, environments, and lifestyles of each person [8]. For genetic examinations, radiogenomics can be achieved by developing an imaging surrogate, and they can be costly, time-consuming, and need invasive tissue sampling. To understand the biology of tumors, radiogenomics recognizes the association between imaging phenotypes and genotypes, and combining them into an integrated sample can enhance the forecast of clinical outcomes [9, 10]. The most common and heterogeneous disease in women is BC. Human epidermal growth factor receptor-2 (HER2), basal BC, luminal A, and luminal B tumors were classified into distinct molecular subtypes (MSTs) to explain differences in the biology of BC by genetic research [11, 12]. Each MST has different subsequent metastatic spread and different patterns of primary disease [13, 14]. Radiation therapy (RT) and rates of response to chemotherapy are different for each MST [15]. BC survival has improved dramatically, with the current 10-year survival rate estimated to be over 80%. Issues such as survival and quality of life are very important focuses for cancer research. More than 70% of BC patients undergo RT [16]. The side effects that are caused by RT are a reduction in overall morality and the risk of local recurrence. Radiation has different degrees of toxicity which it can affect the patients. Dosimetry, body habit, and smoking are the clinical factors that depend on the individual radiation sensitivity, and this sensitivity has an important role [17]. To help in treatment decision-making procedures, singular risk forecast model for toxicity of radiation was produced through a combination of patient factor, clinical factors, and predictive biomarkers. Genetic association studies have identified several potential predictors of genetic markers for radiation toxicity [18,19,20]. Although current reference standard is expensive and needs specific equipment as well as technical expertise to classify the MSTs by genetic analysis, it needs alternative tools to classify BCs into distinct MSTs [15]. Early detection of highly malignant BC is important in treatment and diagnosis of BC. Until now, analysis of needle biopsy, immunohistochemistry, or removed surgical specimens (partial tumor tissue) have helped in the discovery of these molecular markers. Due to tumor heterogeneity, this method has particular limitations; in contrast, tumor tissue's general anatomical and functional features can be provided by imaging [21]. Consequently, radiogenomic approaches in BC are still in its early stages and many problems remain in fusion between radiomics features and genomics indexes, to be solved. The present study aims to highlight the current state-of-the-art, the current role and limitations, and future directions of radiogenomics in breast cancer.
Methods
Literature review and search strategy
Articles were searched by two individual researchers. Briefly, the list of collected articles was complemented without any date or language limitations by databases such as Embase, PubMed, Web of Science, Google Scholar, Scopus, and Cochrane Library. The keywords for search were as follows: “radiogenomics,” “estrogen receptor,” “progesterone receptor,” “ER,” “PR,” “HER2,” “ki67,” “molecular subtype,” and “breast cancer.” Searches were performed using Boolean OR and AND operators between the main terms and keywords of particular topic of the subject under investigation. In addition, relevant keywords and Boolean operators were selected to refine the search strategy in each database.
Data extraction and data collection
Independent searches were conducted by two researchers from July 2021 to September 2021. This paper includes all published articles from 2012 to 2020. Studies of patients who have BC and performed radiogenomics analysis on their tumor samples were included. All retrospective, prospective, cohort, pilot studies, and the patients who undergo the radiogenomics method in breast cancer were included. Articles such as letter to the editor, review, short communications, and the patients who undergo the radiogenomics method in other cancers except to breast cancer were excluded. Of 96 full-text articles, 76 were excluded because of irrelevant and without precise quantity information (Fig. 1). From qualified studies, the following data were extracted individually from each study in a standardized way: publication year, authors, study design, method, genomic analysis, as well as outcome (Table 1). Any disagreement was decided by discussion after additional study of the articles.
Assessment of study quality
For methodological validation prior to entry, two independent reviewers evaluated the selected papers. For quality assessment of articles, Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist was used [37]. The STROBE checklist was used to analyze the data. Epidemiological study design that can be conducted as a cross-sectional study, cohort study, case study, is an observational study. When presenting observational studies in the article, the author should know clear information about the work and provide the reader with the suitable information to make a critical assessment of the research. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines are designed to help the author in confirming a high-quality presentation of the observational study. To provide inclusive reporting of descriptive observational articles, 22 questions were used in the STROBE checklist in order to test relation between exposures and health results [38].
Result
Research finding
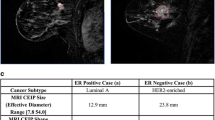
Out of 1118 articles, 962 were individually assessed after being identified through database searching. Duplicate articles were removed, and 163 articles were reviewed. Then, 76 articles were excluded, including irrelevant studies and articles with inadequate information that did not match inclusion criteria. For the systematic review, 18 articles were approved after assessing the full text of selected articles. The evaluation process is shown in Fig. 1. In this review, 3614 patients with BC of selected articles were evaluated, and all radiogenomics were associated with more power in classification, differential diagnosis, and prognosis of BC. Also, the studies showed the relationship between imaging features, molecular subgroups, and tumor molecular biomarkers. In this study, it was observed that radiomic features of magnetic resonance imaging (MRI), dynamic contrast-enhanced MRI, diffusion-weighted MRI, contrast-enhanced ultrasonography (CEUS), and computed tomography (CT) can differentiate the essential molecular features of BC patients. These molecular biomarkers include mRNAs, microRNAs, lncRNAs, ER, PR, HER2, Myc, p53, RTK/RAS, PI3K and Ki-67 expression, human epidermis, and status of growth factor signaling pathways. Imaging phenotypes have related to basic genes, expression patterns, and mutation as the radiogenomics' goals. In radiogenomics of breast cancer, it mostly concentrates on primarily contrast-enhanced MR imaging and the association of its features with molecular subtypes, individual genomic signatures, or recurrence scores. The limited radiogenomic goal is to create imaging biomarkers using phenotypic and genotypic criteria that can predict risk and outcomes and thus better classify patients for accurate therapeutic care. The CT and MRI imaging features were texture, morphologies, and dynamic features. US features included size, orientation, shape, echo pattern, margin, and calcifications. Also, vascular features were assessed by using contrast agent-enhanced US and microvascular US: vessel morphological features, vascular index, penetrating vessels, distribution, margin, internal homogeneity, enhancement degree, and perfusion defect (Fig. 2). Mammography features were mass with or without calcifications, breast composition or margin, the density of mass, the morphology of calcifications, mass density, mass margin, mass shape, and asymmetry or architectural distortion (Fig. 2). The accuracy of random forest model was higher by approximately 13% on average than accuracy of logistic regression model. In order to compare between the predictive power of different radiomics methods, AUC values have been extracted. As shown in Fig. 3, DCE-MRI modality has the higher performance to predict genomic biomarkers (mean = 0.91%), including cell cycle check points, expression of genes such as Mye, PI3K, RTK/RAS, P53, and finally ER + /ER−, PR + /PR−, HER2 + /HER2, and triple-negative indicators. Among the indicators, the highest amount of AUC value (0.956) belonged PI3K gene.
Discussion
This study showed that recognizing and specifying radiogenomic phenotypes in the genomic signatures can be helpful. In several studies, clinical phenotypes such as triple-negative status (TN), estrogen receptor (ER), human epidermal growth factor 2 (HER2), and progesterone normalization methods' ability have been evaluated. [28, 30,31,32, 39]. They showed that radiomic features differentiate the essential molecular features of BC as well as may provide potential biomarkers for the development of precision medicine [29].
Associating radiomics with genomics is a developing area of research usually discussed as “radiogenomics” or more specifically “imaging-genomics.” This developing field addresses novel high-throughput methods of relating information-rich radiographic images with genomic data as well as other clinically related information. Radiogenomics has the potential to influence therapeutic and diagnostic approaches by creating more personalized real-time measurements in response to therapy and prognostic signatures, without having to rely on biopsy to represent cancer lesions within a patient [40].
Radiomics
The idea of radiomics was first discussed by the Dutch researcher Lambin in 2012. This idea presented tumor heterogeneity [41]. Compared to traditional proteomics and genomic methods, radiomics can be a noninvasive method for evaluating tumors and their microenvironment and predicting genetic heterogeneity of the tumor. The use of extracted semiautomatic imaging features has several benefits. The human eye cannot easily recognize the analyzed features. Repeatable and devoid of intra-observable variability, the analysis can be done by computer algorithms when the lesion is annotated by the radiologist. As the radiologist has to study and report, this analysis can be done in the context, so it only requires an extra burden and time for the radiologist to sketch a box circa the lesion. Considering the situation of future, the workflow of radiologist is minimally affected, but the radiologist can provide added value by adding information from the molecular subgroup to his/her dictation report [15]. The main limitation of radiomics is the lack of reproducibility due to the inconsistent radiomic methods and the lack of optimization of the acquisition parameters. Nevertheless, the field of machine vision for image identification has been given potential by the recent advancement of deep learning. In this context, Anderson et al. recently showed that the performance of the computer-aided diagnosis was significantly better than the convolutional neural network feature extraction for contrast-enhanced magnetic resonance imaging (MRI) in the BC diagnosis [42].
From radiomics to radiogenomics
Tumors' biological details are essential in choosing suitable therapy plan and achieving effective therapy results. As the result of cancer, promising genes were identified from the development of genetic research in BC. Also, development of confirmed genomic signatures allows the classification of BCs to differentiate molecular subgroups, predicting the cancer recurrence risk, and predicting response to the treatment. In general, the profiles and pathways of gene expression modification induced by ionizing radiation are cell-related. The information shows that HR condition can be related to the certain genes and pathway's signatures. Genomic biomarkers and gene signatures of specific tumor subtypes, certain subtypes of tumor gene signatures, and genomic biomarkers depending on molecular characteristics and HR condition can simplify RT biomarkers using personal biomarkers itself or with association of selected treatments. Therefore, to enhance the forecast of clinical outcomes, the synergistic power and integrated radiomics to radiogenomics models are needed. Finally, detection of significant features of the tumor tissue by noninvasive molecular, anatomical, and functional methods can provide potential biomarkers for the development of personalized medicine. The typical workflow of radiogenomic studies that was discussed in this article is shown in Fig. 2.
Current status of radiogenomics in precision RT
Current radiogenomics research has been conducted toward understanding tumor biology, heuristic analysis of individual genes, and the development of imaging substitutes for genetic analysis with the aim of developing term clinical care tools [9]. With significant advances in genetics and genomics over the last 30 years, it has been hypothesized that genomic alterations may affect radiation-related adverse events [43]. Regardless of the technological developments produced in past few years that make RT highly targeted to the selected tumor, for each tumor, the RT programs are yet to be considered as the equal total number of dose given biological alterations to different types of tumor. Nevertheless, significant changes in the response to radiation were caused by the diversity of molecular illustration of certain cancer subtypes. Hence, to select a suitable therapies plan, tumors' biological details are essential, consequently achieving effective RT results. Broad scientific confirmation showed that RT is an important treatment for different types of cancer containing BC for different cancer types either alone or by combination with other therapies. For patients who are diagnosed with BC, they have two choices: mastectomy or partial protection (partial care after surgery) followed by RT. These choices will be affected by numerous factors such as medical, psychology, and sociology factors. The important factor of RT is the side effects of high-dose RT which has toxicities radiation. It may result in poor cosmesis or pain that can affect the BC patients' quality of life. Its effect can lead to negative psychological consequences. Therefore, an experiment that can predict the likelihood of radiation damage is helpful because it helps patients with BC and their physicians to find the most appropriate individualized treatment. This information finally allows physicians to prescribe initialed overall doses or certain subtypes of tumor treatment; therefore, it increases the radio-sensitivity [21]. BC is highly heterogeneous, and image performance varies in size, shape, brightness, and lesion values [44]. Studies have shown that personal medicine depends on the combination and contemplating patient characteristics such as tumor phenotypes and genotypic profiles [22]. Therefore, early monitoring and anticipation of the patient's response to treatment are particularly important, especially due to the toxic or expensive drugs in response's heterogeneity as well as the loss of chances for adequate early replacement treatment. Although many drugs have special procedures, they are not targeted for genetic lesions. Therefore, it will face difficulty in selecting patients based on basic genetic features [45]. This has led to the creation of a radiogenomic field that aims to identify genetic factors that form a wide spectrum of responses observed in radiation-treated patients. Although genomic characteristics are likely to influence tumor radiation response, the focus of radiogenomics research is to identify biomarkers that influence sensitivity to normal tissue and radiation-induced tissue. The general purpose of radiogenomics in radiotherapy is to develop assays to predict that patients will likely exhibit RT complications.
Limitations and future directions
All reviewed radiogenomic research indicated potential interest in introducing the new candidate biomarkers. In BC, these markers have established possible value for diagnostic/prognostic. However, insufficient number of patients is the most important limitation of studies. Because of inadequate data and overestimated detection accuracy, exterior confirmation cannot be performed. It should take into consideration that even the classification of radiomics is useful for few results, but large samples should be confirmed before clinical use. Finally, it seems that future research is needed to evaluate the worthiness, radiomics biomarkers reproducibility, and confirmation of prospective cohort by using large sample sizes [46]. Anatomic location of the MRI methods like complete pathology is correlated with multiple tumors by biopsy as the more precise approach. Also, standardizing imaging features is a challengeable. Capturing images from a scanner and protocol type has increased the reliability of the radiomics dataset. Researchers admit which protocols of image acquisition are diverse through associations; also, their results are broadened with greater validity. Larger sample sizes and rigorous studies are already required for computer diagnostics [21]. Due to the high prevalence and importance of diagnosis and treatment of breast cancer, health promotion and increased survival of cancer patients are essential. This study shows that radiogenomic protocol can help to recognize the tumors, classification, differential diagnosis, maps before surgery of BC, relationship between imaging features and tumor molecular biomarkers, and forecast the recurrence and benefit of chemotherapy. Because of the heterogeneity of the studies and information in Table 1 using different methods of imaging and genetic assay, meta-analysis was not able to implement on this information.
Conclusion
The general purpose of diagnostic radiogenomics is to discover new imaging features that reflect genomic alterations associated with tumor phenotypes, lymph node status, HR status, HER2, Ki67, and MSTs. Different imaging modalities and molecular biomarkers have been used for this purpose. In imaging modalities, the features of MRI images are mainly used. However, recently published articles show that the features extracted from the US and CT can be applied to the classification of different BC subtypes. The molecular methods used in these articles are limited to miRNAs expression, gene expression, Ki67 proliferation index, next-generation RNA sequencing, whole RNA sequencing, and molecular histopathology that can be completed in future studies by other methods such as exosomal miRNAs, specific proteins expression, DNA repair capacity, and other biomarkers that have prognostic and predictive value for cancer treatment response. The role of radiogenomics in BC and potential applications in radiotherapy were reviewed in this study. This review study shows that radiogenomics can improve diagnosis and treatment of breast cancer in patients. All radiogenomic studies were associated with more power in classification, differential diagnosis, and prognosis of BC. It has shown that recognizing and specifying radiogenomic phenotypes in the genomic signatures can be helpful. Studies with control group and large sample size for evaluation of radiogenomics in diagnosis and treatment recommended.
Availability of data and materials
Not applicable.
References
Darvish L, Ghorbani M, Teshnizi SH, Roozbeh N, Seif F, Bayatiani MR et al (2018) Evaluation of thyroid gland as an organ at risk after breast cancer radiotherapy: a systematic review and meta-analysis. Clin Transl Oncol 20(11):1430–1438. https://doi.org/10.1007/s12094-018-1875-7
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A et al (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71(3):209–249. https://doi.org/10.3322/caac.21660
Yamamoto S, Han W, Kim Y, Du L, Jamshidi N, Huang D et al (2015) Breast cancer: radiogenomic biomarker reveals associations among dynamic contrast-enhanced MR imaging, long noncoding RNA, and metastasis. Radiology 275(2):384–392. https://doi.org/10.1148/radiol.15142698
Zhu Y, Li H, Guo W, Drukker K, Lan L, Giger ML et al (2015) Deciphering genomic underpinnings of quantitative MRI-based radiomic phenotypes of invasive breast carcinoma. Sci Rep 5(1):1–10
Wang X, Chao L, Chen L, Tian B, Ma G, Zang Y et al (2008) Correlation of mammographic calcifications with Her-2/neu overexpression in primary breast carcinomas. J Digit Imag 21(2):170–176. https://doi.org/10.1007/s10278-008-9105-4
Wang Y, Ikeda DM, Narasimhan B, Longacre TA, Bleicher RJ, Pal S et al (2008) Estrogen receptor–negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression. Radiology 246(2):367–375. https://doi.org/10.1148/radiol.2462070169
Mazurowski MA (2015) Radiogenomics: what it is and why it is important. J Am Coll Radiol 12(8):862–866. https://doi.org/10.1016/j.jacr.2015.04.019
Pinker K, Chin J, Melsaether AN, Morris EA, Moy L (2018) Precision medicine and radiogenomics in breast cancer: new approaches toward diagnosis and treatment. Radiology 287(3):732–747. https://doi.org/10.1148/radiol.2018172171
Grimm LJ, Mazurowski MA (2020) Breast cancer radiogenomics: current status and future directions. Acad Radiol 27(1):39–46. https://doi.org/10.1016/j.acra.2019.09.012
Wu J, Tha KK, Xing L, Li R (2018) Radiomics and radiogenomics for precision radiotherapy. J Radiat Res 59(suppl_1):i25–i31. https://doi.org/10.1093/jrr/rrx102
Perou CM, Sørlie T, Eisen MB, Van De Rijn M, Jeffrey SS, Rees CA et al (2000) Molecular portraits of human breast tumours. Nature 406(6797):747–752. https://doi.org/10.1038/35021093
Sørlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H et al (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A 98(19):10869–10874. https://doi.org/10.1073/pnas.191367098
Huber KE, Carey LA, Wazer DE (eds) (2009) Breast cancer molecular subtypes in patients with locally advanced disease: impact on prognosis, patterns of recurrence, and response to therapy. Semin Radiat Oncol, Elsevier
Wiechmann L, Sampson M, Stempel M, Jacks LM, Patil SM, King T et al (2009) Presenting features of breast cancer differ by molecular subtype. Ann Surg Oncol 16(10):2705–2710. https://doi.org/10.1245/s10434-009-0606-2
Grimm LJ, Zhang J, Mazurowski MA (2015) Computational approach to radiogenomics of breast cancer: luminal A and luminal B molecular subtypes are associated with imaging features on routine breast MRI extracted using computer vision algorithms. J Magn Reson Imag 42(4):902–907. https://doi.org/10.1002/jmri.24879
Rowland JH, Hewitt M, Ganz PA (2006) Cancer survivorship: a new challenge in delivering quality cancer care. J Clin Oncol 24(32):5101–5104. https://doi.org/10.1200/JCO.2006.09.2700
Rattay T, Symonds R, Shokuhi S, Talbot C, Schnur J (2018) The patient perspective on radiogenomics testing for breast radiation toxicity. Clin Oncol 30(3):151–157. https://doi.org/10.1016/j.clon.2017.12.001
Bahreyni-Toossi M-T, Azimian H, Aghaee-Bakhtiari SH, Mahmoudi M, Sadat-Darbandi M, Zafari N (2021) Radiation-induced DNA damage and altered expression of p21, cyclin D1 and Mre11 genes in human fibroblast cell lines with different radiosensitivity. Mutat Res 823:111760. https://doi.org/10.1016/j.mrfmmm.2021.111760
Bahreyni-Toossi M-T, Zafari N, Azimian H, Mehrad-Majd H, Farhadi J, Vaziri NF (2020) Alteration in expression of Trim29, TRIM37, TRIM44, and β-catenin genes after irradiation in human cells with different radiosensitivity. Cancer Biother Radiopharm. https://doi.org/10.1089/cbr.2020.3915
Bahreyni-Toossi MT, Vosoughi H, Azimian H, Rezaei AR, Momennezhad M (2018) In vivo exposure effects of 99mTc-methoxyisobutylisonitrile on the FDXR and XPA genes expression in human peripheral blood lymphocytes. Asia Ocean J Nucl Med Biol 6(1):32
Zhang Y, Zhu Y, Zhang K, Liu Y, Cui J, Tao J et al (2020) Invasive ductal breast cancer: preoperative predict Ki-67 index based on radiomics of ADC maps. Radiol Med 125(2):109–116. https://doi.org/10.1007/s11547-019-01100-1
Gallivanone F, Cava C, Corsi F, Bertoli G, Castiglioni I (2019) In Silico approach for the definition of radiomiRNomic signatures for breast Cancer differential diagnosis. Int J Mol Sci 20(23):5825. https://doi.org/10.3390/ijms20235825
Park AY, Han M-R, Park KH, Kim JS, Son GS, Lee HY et al (2020) Radiogenomic analysis of breast cancer by using B-mode and vascular US and RNA sequencing. Radiology. https://doi.org/10.1148/radiol.2020191368
Yeh AC, Li H, Zhu Y, Zhang J, Khramtsova G, Drukker K et al (2019) Radiogenomics of breast cancer using dynamic contrast enhanced MRI and gene expression profiling. Cancer Imag 19(1):48. https://doi.org/10.1186/s40644-019-0233-5
Saha A, Harowicz MR, Grimm LJ, Kim CE, Ghate SV, Walsh R et al (2018) A machine learning approach to radiogenomics of breast cancer: a study of 922 subjects and 529 DCE-MRI features. Br J Cancer 119(4):508–516. https://doi.org/10.1038/s41416-018-0185-8
Shin SU, Lee J, Kim JH, Kim WH, Song SE, Chu A et al (2017) Gene expression profiling of calcifications in breast cancer. Sci Rep 7(1):1–11. https://doi.org/10.1038/s41598-017-11331-9
Lin P, Liu W, Li X, Wan D, Qin H, Li Q et al (2020) MRI-based radiogenomics analysis for predicting genetic alterations in oncogenic signalling pathways in invasive breast carcinoma. Clin Radiol. https://doi.org/10.1016/j.crad.2020.02.011
Park EK, Lee K-s, Seo BK, Cho KR, Woo OH, Son GS et al (2019) Machine learning approaches to radiogenomics of breast cancer using low-dose perfusion computed tomography: predicting prognostic biomarkers and molecular subtypes. Sci Rep 9(1):1–11. https://doi.org/10.1038/s41598-019-54371-z
Castaldo R, Pane K, Nicolai E, Salvatore M, Franzese M (2020) The Impact of normalization approaches to automatically detect radiogenomic phenotypes characterizing breast cancer receptors status. Cancers 12(2):518. https://doi.org/10.3390/cancers12020518
Sutton EJ, Oh JH, Dashevsky BZ, Veeraraghavan H, Apte AP, Thakur SB et al (2015) Breast cancer subtype intertumor heterogeneity: MRI-based features predict results of a genomic assay. J Magn Reson Imag 42(5):1398–1406. https://doi.org/10.1002/jmri.24890
Juan MW, Yu J, Peng GX, Jun LJ, Feng SP, Fang LP (2018) Correlation between DCE-MRI radiomics features and Ki-67 expression in invasive breast cancer. Oncol Lett 16(4):5084–5090. https://doi.org/10.3892/ol.2018.9271
Mazurowski MA, Zhang J, Grimm LJ, Yoon SC, Silber JI (2014) Radiogenomic analysis of breast cancer: luminal B molecular subtype is associated with enhancement dynamics at MR imaging. Radiology 273(2):365–372. https://doi.org/10.1148/radiol.14132641
Woodard GA, Ray KM, Joe BN, Price ER (2018) Qualitative radiogenomics: association between Oncotype DX test recurrence score and BI-RADS mammographic and breast MR imaging features. Radiology 286(1):60–70. https://doi.org/10.1148/radiol.2017162333
Wan T, Bloch BN, Plecha D, Thompson CL, Gilmore H, Jaffe C et al (2016) A radio-genomics approach for identifying high risk estrogen receptor-positive breast cancers on DCE-MRI: preliminary results in predicting OncotypeDX risk scores. Sci Rep 6:21394. https://doi.org/10.1038/srep21394
Yamamoto S, Maki DD, Korn RL, Kuo MD (2012) Radiogenomic analysis of breast cancer using MRI: a preliminary study to define the landscape. AJR Am J Roentgenol 199(3):654–663. https://doi.org/10.2214/AJR.11.7824
Tamez-Pena J-G, Rodriguez-Rojas J-A, Gomez-Rueda H, Celaya-Padilla J-M, Rivera-Prieto R-A, Palacios-Corona R et al (2018) Radiogenomics analysis identifies correlations of digital mammography with clinical molecular signatures in breast cancer. PLoS ONE. https://doi.org/10.1371/journal.pone.0193871
Abdi F, Kazemi F, Tehrani FR, Roozbeh N (2016) Protocol for systematic review and meta-analysis: hop (Humulus lupulus L.) for menopausal vasomotor symptoms. BMJ Open 6(4):e010734. https://doi.org/10.1136/bmjopen-2015-010734
Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med 85:867–872. https://doi.org/10.1371/journal.pmed.0040296
Grimm LJ (2016) Breast MRI radiogenomics: current status and research implications. J Magn Reson Imag 43(6):1269–1278. https://doi.org/10.1002/jmri.25116
Yeh AC, Li H, Zhu Y, Zhang J, Khramtsova G, Drukker K et al (2019) Radiogenomics of breast cancer using dynamic contrast enhanced MRI and gene expression profiling. Cancer Imag 19(1):1–11
Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, Van Stiphout RG, Granton P et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48(4):441–446. https://doi.org/10.1016/j.ejca.2011.11.036
Anderson R, Li H, Ji Y, Liu P, Giger ML (eds) (2019) Evaluating deep learning techniques for dynamic contrast-enhanced MRI in the diagnosis of breast cancer. Medical Imaging 2019: Computer-Aided Diagnosis. SPIE
Rosenstein BS (ed) (2017) Radiogenomics: identification of genomic predictors for radiation toxicity. Semin Radiat Oncol, Elsevier
Joseph C, Papadaki A, Althobiti M, Alsaleem M, Aleskandarany MA, Rakha EA (2018) Breast cancer intratumour heterogeneity: current status and clinical implications. Histopathology 73(5):717–731. https://doi.org/10.1111/his.13642
Mehta S, Hughes NP, Li S, Jubb A, Adams R, Lord S et al (2016) Radiogenomics monitoring in breast cancer identifies metabolism and immune checkpoints as early actionable mechanisms of resistance to anti-angiogenic treatment. EBioMedicine 10:109–116. https://doi.org/10.1016/j.ebiom.2016.07.017
Fusco R, Sansone M, Filice S, Carone G, Amato DM, Sansone C et al (2016) Pattern recognition approaches for breast cancer DCE-MRI classification: a systematic review. J Med Biol Eng 36(4):449–459. https://doi.org/10.1007/s40846-016-0163-7
Acknowledgements
Not applicable.
Funding
None.
Author information
Authors and Affiliations
Contributions
LD and HA conceived the study and participated in design and conduct of study including article collection, statistical analysis, and manuscript preparation. MT-BT and NR contributed to manuscript review and preparation. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
I, Hosein Azimian, hereby declare that I participated in the study and in the development of the manuscript titled “The role of radiogenomics in the diagnosis of breast cancer: a systematic review.” I have read the final version and give my consent for the article to be published in Egyptian Journal of Medical Human Genetics.
Competing interests
The authors declared that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Darvish, L., Bahreyni-Toossi, MT., Roozbeh, N. et al. The role of radiogenomics in the diagnosis of breast cancer: a systematic review. Egypt J Med Hum Genet 23, 99 (2022). https://doi.org/10.1186/s43042-022-00310-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43042-022-00310-z