Abstract
Osteoarthritis is a degenerative disease that affects articular cartilage, leading to changes on the macro and micro levels of this multi-component tissue. Understanding the processes underlying this pathology plays an important role in planning the following management tactics. Timely detection of the knee joint degradation at the level of tissue changes can prevent its progressive damage due to the early beginning of appropriate treatment. This study aimed to provide an overview of the current level of knowledge about the composition of cartilage and menisci using a wide range of different diagnostic methods. A systematic review of the literature published from 1978 to 2023 was conducted. Original studies of the knee joint cartilage (articular and meniscus) research, reporting content composition and mechanical properties, were included. Studies of the non-knee joint cartilage, tissue research other than cartilage and meniscus, or reporting treatment outcomes were excluded (n = 111). Thirty-one papers were included in this review, which reported on the composition of animal and human cartilage (articular and meniscus). The most frequently investigated parameters were quantitative proteoglycan determination and hydration level of the cartilage. Cartilage and meniscus degeneration, i.e., reduced collagen and proteoglycan content, reduced mechanical properties, and increased hydration level, was shown in every article about osteoarthritis. Among all diagnostic methods, laboratory methods (biochemical and histological analysis) are the most frequently used, compared to the instrumental ones (spectroscopy, MRI, and CT). At the same time, spectroscopy takes the lead and becomes the most common approach for determining cartilage composition (collagen and proteoglycans content).
Graphical Abstract

Similar content being viewed by others
1 Introduction
Osteoarthritis (OA) is a disease characterized by degenerative changes in the cartilage and other tissues of the joint, including changes in the meniscus, synovial membrane, and subchondral bone [1].
Articular cartilage is a specialized connective tissue that distributes and transfers the load on the underlying bone, reducing friction by providing smooth joint movement. The functions of articular cartilage are performed through unique interaction between the components of the extracellular matrix (ECM), which consists of water (60–80%), collagen (15–20%) and proteoglycans (PGs) (3–10%) [2] (Fig. 1). Biochemically, the two most abundant components of the dry weight (DW) of ECM of articular cartilage, are collagen and glycosaminoglycans (GAGs), which make up approximately 50% to 75% and 15% to 30%, respectively [3].
The meniscus of the knee joint also has 72% water [4]. Three layers of collagen are distinguished in the meniscus tissue. The first is a surface network of fibrils forming a mesh matrix on the areas of the articular surfaces of the femur and tibia; the second is a lamellar layer lying under the fibrillar network and consisting of radially oriented bundles of collagen fibers; and the third is the central main layer of collagen fibrils passing along the circumference from the anterior horn to the posterior [5]. Collagen, a part of the meniscus, is represented by different types. The outer third is dominated by collagen of the first type, and the inner part of the meniscus is dominated by collagen of the second type [6]. Type II collagen makes up 90% to 95% of the collagen content of articular cartilage and forms fibrils intertwined with GAGs that provide structural rigidity, elongation, and strength to articular cartilage [7]. Through characterization studies, it is known that collagen and GAG content, compressive strength, and genetic expression in articular cartilage vary depending on factors such as age, location, and loading area [8, 9].
Various changes in the matrix composition caused by aging or injury lead to the development of such a degenerative disease as OA [10,11,12]. It is currently impossible to restore the affected cartilage by existing treatment methods, and early diagnosis of OA is essential to start adequate treatment aiming at slowing down the progression of degenerative processes [12]. Late stages of OA are characterized by physical (increased permeability and decreased extensibility) and structural (decrease in collagen and PGs concentrations, increased hydration) cartilage changes. At the same time, the composition of cartilage in the early stages of OA differs slightly from the norm, which complicates early diagnostics [13].
Biochemical methods for assessing the composition of cartilage (the concentration of collagen and PGs) are highly accurate, however their use is possible only on the removed cartilage tissue (biopsy), with preliminary chemical treatment, which excludes the use of this method in vivo [14,15,16,17,18]. Histological methods are also not applicable intraoperatively because it is necessary to gather the patient’s biological material for subsequent analysis. Their results can be subjective and depend on the quality of the material, as well as the experience and competence of the diagnostician [2]. This method can be supplemented by subsequent densitometry [19, 20]. Contrast-enhanced computed tomography (CT) seems to be a promising method for quantitative and spatial assessment of the cartilage condition. However, there are certain limitations, such as complex technical equipment, long time of the contrast agent distribution in the thickness of the cartilage, which can take from 2 to 72 h, the need to introduce contrast into the joint cavity, and its possible toxicity [21, 22]. All these problems need to be solved, as so far, they make it difficult to apply this method in vivo or intraoperatively. Magnetic resonance imaging (MRI) is a non-invasive method for diagnosing the state of cartilage. The results are influenced by PGs concentration, collagen orientation, and cartilage hydration level. Intraoperative use is also currently impossible [12, 19, 23].
Quantitative methods of MRI examination of articular cartilage allow a more accurate assessment of its structure. In most cases, ECM components are assessed, namely PGs, GAGs, and collagen. Among the available methods, the most common, informative, and accessible method is T2 mapping [24]. T2 relaxation time is a noninvasive marker of cartilage degeneration because it is sensitive to the biochemical composition and degree of tissue hydration [25]. The T2 relaxation time depends on the articular cartilage’s water content and the integrity of the ECM. The decrease in T2 relaxation time occurs due to the interaction of collagen fibers (component of the ECM) with water protons [26]. The increase in T2 relaxation time is not associated with a decreased collagen concentration in articular cartilage but is explained by its organization [27]. Thus, T2 relaxation time is a parameter characterizing the hydrophilicity of articular cartilage tissue and the anisotropy of collagen distribution.
Another possible parameter for assessing biochemical changes in cartilage tissue is the relaxation time T1ρ, which characterizes the low-force interaction between low-mobility water molecules and the macromolecular environment [27]. The main component of cartilage that influences the mobility of water molecules is the intercellular substance. The PGs level in the cartilage matrix is mainly responsible for the tissue’s high elasticity and firmness. Thus, a decrease in the concentration of PGs, as large, sedentary molecules, can change the value of the T1ρ parameter, which reflects the initial changes in OA [28]. Another method for MRI diagnosis of OA is the delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) technique, based on studying the loss of GAGs in cartilage tissue. T1p and dGEMRIC are standard methods but require more time to collect data [29].
Thus, the methods are either used retrospectively for the final determination of the stage of OA (biochemical, histological, densitometry) or require refinement of the methodology and technology to be integrated into the practice of an orthopedic doctor (CT, MRI). Each method is unique and is characterized by its physical principle and target in the composition of cartilage tissue.
Modern research is focused on optical and acoustic diagnostic techniques that enable the assessment of the tissue state in real time without damaging it. Near-infrared spectroscopy (NIR spectroscopy) is one of the most promising techniques that has shown great potential for rapidly determining cartilage integrity. NIR spectroscopy determines molecular vibrations, sensitive to certain types of bonds, including C-H, N–H, O–H, and S–H, which form the structural framework of soft biological tissues. In this regard, in recent years, there has been increased interest in this method in the study of the characteristics of plaques, the diagnosis of arthritis, and the assessment of the state of tumors. The use of NIR spectroscopy to assess the state of cartilage requires an understanding of the contribution of individual tissue components to its overall spectral response. Thus, the interpretation of spectroscopy results requires a fundamental understanding of the interaction of certain components of the cartilage matrix with specific areas of the spectrum [11] (Fig. 2). The ECM of articular cartilage consists of networks of collagen fibrils, proteins not associated with collagen, and PGs. Collagen fibrils are responsible for the tissue’s tensile and shear stiffness, providing the structural framework of the tissue. PGs, in turn, play a key role in the tissue's ability to resist and recover from the stress. The spectrum of articular cartilage can be considered as the total value of the interaction of infrared radiation with tissue at the microstructural level. Consequently, various structural changes in the composition of cartilage components can be detected by changes in this spectrum, which reflects the physical and functional characteristics of the tissue [10, 15].
Finding the optimal method for assessing the state of cartilage in OA remains an urgent problem in clinical practice. Therefore, this work aims to understand the current state and possibilities of laboratory and instrumental diagnostic methods (MRI, CT, spectroscopy), namely, their use in intraoperative diagnostics of the cartilage condition.
2 Methods
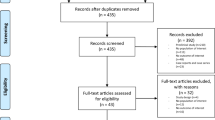
The literature review was performed based on the preferred reporting items for systematic reviews and meta-analysis (PRISMA 2020) statement [30] (Fig. 3). The search was conducted using the following strategy: proteoglycans AND articular AND cartilage AND water AND content NOT ligament NOT treatment OR CH-band. After eliminating 14 articles without DOI, 128 articles were analyzed. After a thorough screening, 97 articles were excluded against the exclusion criteria, and only 31 were eligible for inclusion. Thus, a total of 31 studies were selected for further qualitative analysis.
Inclusion criteria:
-
All original articles and studies in English literature from 1978 to 2023 reporting on the performance of structural contents of knee joint cartilage and meniscus
-
In vitro studies
-
Clinical and experimental studies on animal and human models
Exclusion criteria:
-
Tests were performed on any other joints except for the knee joint
-
Tests were performed on ligaments or tendons
-
Reviews of treatment methods for the cartilage pathology
-
In vivo / in situ / in silico studies
-
Bioengineering studies
-
Literature reviews
-
Other scientific fields except medicine (Physics, Chemistry, Biology)
-
Reviews of research method results only
The collected titles and abstracts were screened using the selection and exclusion criteria outlined above, and eligibility was confirmed after reviewing the full text.
We assessed the risk of bias in randomized trials using the internationally recommended Cochrane Bias Risk Assessment tool (Table 1).
3 Results
3.1 Overview
As shown in Fig. 3, 31 articles out of 142 were selected for a systematic review, and 111 articles were excluded according to the criteria (PRISMA 2020). Among all included articles, 17 of them are experimental studies [1, 2, 13,14,15, 18, 21, 24, 25, 31,32,33,34,35,36,37,38], 8 are designed as controlled experimental studies [12, 23, 39,40,41,42,43,44] and 6 are randomized experimental studies [16, 45,46,47,48,49].
Quantitative collagen determination was performed in 20 papers. Primary research methods were Biochemical analysis in 14 papers [14,15,16, 18, 24, 32,33,34, 40, 41, 43, 46, 47, 49], Spectroscopy in 3 papers [13, 24, 36], Histological analysis in 3 papers [21, 33, 38], Immunohistochemical analysis in 1 paper [2] and MRI mathematical calculation in 1 paper [12]. Quantitative PGs determination was performed in 28 papers. The main research methods were Biochemical analysis in 21 papers [14,15,16, 18, 23, 24, 32,33,34, 37, 39,40,41,42,43,44,45,46,47,48,49], Spectroscopy in 4 papers [13, 24, 31, 38], and also MRI [12], Densitometry [21] and Histological analysis [2, 35]. Hydration was performed in 25 papers. Water content was measured by lyophilization and subsequent weighting in 17 papers [2, 13, 15, 16, 18, 21, 23, 24, 32, 33, 36, 40, 43, 44, 46, 47, 49]. Simple weighting was performed in 5 papers [34, 35, 37, 39, 41], and few authors used such methods as MRI [12] and Spectroscopy [1, 48].
At the same time, our area of interest included quantitative components content (concentration), and their spatial distribution in the wall of the cartilage itself, particularly collagen and PGs content in the different layers (superficial, middle, and deep layers). Spatial PGs determination was performed in 9 papers [2, 12, 15, 18, 21, 32, 33, 38, 45], main methods were Spectroscopy in 5 papers [15, 32, 33, 38, 45], CT scan and MRI in 2 papers [12, 21], and Histological analysis [2, 18]. Spatial collagen determination was performed in 8 papers [2, 12, 15, 18, 21, 33, 38, 45]. The main methods were Spectroscopy in 3 papers [15, 38, 45], CT [21, 33], MRI [12], Histological [18] and Immunohistochemical analysis [2].
Among 31 articles, 26 are devoted to the study of the “non-meniscus” cartilage, 4 are about meniscus, and the composition of both meniscus and “non-meniscus’ cartilage was analyzed with their subsequent comparison only in 1 study.
Twelve papers studied the dependence of mechanical properties and cartilage composition on the different stages of OA and several methods of modeling OA [1, 24, 38, 41,42,43,44,45,46,47,48,49]. Mechanical properties have been investigated in 18 papers [1, 13,14,15,16, 18, 23,24,25, 31, 32, 34, 35, 42,43,44, 47, 49], elastic modulus was described in 9 works [1, 13,14,15,16, 18, 31, 43, 49], equilibrium elastic modulus was measured in 3 papers [15, 24, 32], dynamic elastic modulus was presented in 2 papers [23, 24], instantaneous elastic modulus was also in 2 papers [15, 32], relaxation time was detected in 3 papers [1, 25, 34] and permeability was studied in 3 papers [13, 16, 49].
Articles devoted to the methods of modeling OA are experimental studies performed on animals. The most frequently used is a biomechanical method of modeling OA in 4 papers [41, 42, 46, 47], mechanical methods are described in 3 works [43, 44, 48], and chemical methods are applied in 2 papers [45, 49]. The main investigation methods are laboratory methods, such as biochemical and/or histological analysis, performed in all 9 papers about modeling OA [41,42,43,44,45,46,47,48,49]. Some authors used instrumental diagnostic methods (MRI and spectroscopy) [45, 49] (Table S1).
3.2 Methodological quality assessment
All the RCTs included in the present systematic review were analyzed using the “Cochrane risk of bias” tool to detect potential bias. The results of the assessment have been detailed in Table 1. Although a relevant number of RCTs were found (6), it emerged that all studies presented at least two or more domains with “unclear” or “low” risk of bias, thus suggesting that, despite randomization, the overall reliability of the findings should be considered with caution. There is a need for a methodological improvement in future research on this topic.
3.3 Special part
3.3.1 Meniscus cartilage
The highest concentration of the PGs was observed in the middle layer of the meniscus with a subsequent decrease in the deep layer and the lowest concentration in the superficial layer [12, 18, 31]. At the same time, the highest concentration of collagen was noted in the superficial layer [18]. The mechanical properties of the meniscus tissue vary as well, depending on the meniscus zone, so in the anterior part the elastic modulus is 25–30% higher, compared with the middle and posterior parts. At the same time, the permeability of the cartilage and collagen content in it is significantly higher in the posterior zone – permeability of the middle and posterior parts of tissue is 2–3 times higher than one of the anterior part [13, 18, 32]. Comparing “non-meniscus” cartilage and meniscus tissue, it was found that the meniscus contains more collagen but has lower PGs content and hydration level [18, 33].
With the progression of cartilage degeneration, the collagen content decreases (40% difference between 1 and 4 stages of OA), the PGs content decreases slightly, the hydration increases slightly (5% difference between 1 and 4 stages of OA). The permeability increases (50% difference between 1 and 4 stages of OA), and the stretching ability of the meniscus decreases (50% difference between 1 and 4 stages of OA) [13].
3.3.2 “Non-meniscus” cartilage
In healthy cartilage, most PGs are contained in the deep layer, while the highest collagen concentration was observed in the superficial and middle layers [2, 15, 21, 45]. The increase in PGs and collagen increases the elastic modulus [14, 34]. The amount of collagen is the same in places with different physiological loads due to the natural biomechanics of movements (higher loaded, so-called, high weight bearing zones – the lower part of the medial and lateral condyle of the femur and lower loaded, so-called, low weight bearing zones – the anterior part of the condyles of the femur and the femoral groove) [15, 39]. At the same time, the amount of PGs differs – cartilage of the high weight bearing zones (femur’s condyles) contains 20% more PGs, compared with the cartilage of the low weight bearing zones (femoral groove) [15, 39]. Also, PGs affect water binding – with a decrease in PGs, the water output from the cartilage increases at pressure [23, 35] (Fig. 4). The average value of the cartilage hydration is 82%. Most of the water – 84%, is contained in the superficial layer with a gradual decrease in the middle layer to 40–60% hydration in the deep layer [21, 36, 37]. At the same time, cartilage of the low weight bearing zones (femoral groove) was remarkable for higher hydration level, compared with high weight bearing zones (femoral condyles) [15]. Comparing cartilage from different parts of the knee joint within the same bone (tibia), such as "free" cartilage (normally not covered by the meniscus) and cartilage directly underlying the meniscus, it was found PGs and collagen content, as well as hydration level were higher in cartilage not covered by the meniscus, compared to cartilage lining the base of the meniscus. Thus, the concentration of PGs and collagen is 17–20% higher, and the hydration is 15% higher in "free" cartilage [40].
What should be mentioned is that the cartilage of the high weight bearing zones (femoral condyles) has higher short-term and equilibrium elastic modulus but its stress-relaxation time is lower, compared with the one of low weight bearing zones (femoral groove) [15, 25, 34]. Cartilage from the patella contains 20% more PGs, its permeability is 66% higher, its elastic modulus is 30% lower, and its thickness is 23% greater than the femur cartilage. The collagen content of patella cartilage and femoral cartilage is the same [16].
3.3.3 OA modeling
We analyzed literature data on changes in cartilage composition in different types of OA modeling (reproduction by various processes inherent to natural changes occurring in OA). Different approaches were used in modeling:
-
1.
Biomechanical modeling
-
Valgus osteotomy (redistribution of cartilage load in the knee joint).
-
Increasing the load on cartilage by running (to study the effect of excessive loading on cartilage composition at habitually loaded sites).
-
Cruciate ligament crossing and meniscectomy (to model knee instability and study the changes it will lead to).
-
Immobilization of the limb (to study the composition of cartilage in conditions of complete absence of load on the knee joint).
-
-
2.
Chemical modeling
-
Interleukin and trypsin treatment (to reproduce the loss of PGs and collagen, mimicking their natural loss in OA).
-
As a result of valgus osteotomy, an increase in collagen and PGs content was observed in the cartilage with a lower load (medial femoral condyle). While in the cartilage with higher load (lateral femoral condyle), there was a decrease (both collagen and PGs), first in the upper layers of cartilage and then in all layers. The water content of cartilage was also found to increase with greater loading [46].
When OA was modeled using physical activity, namely running, it was found that the collagen content in the cartilage of the lateral femoral condyle decreased by 14–20%. The PGs content was virtually unchanged except at the posterior edge of the medial condyle, where a 30% decrease was observed. The hydration of the cartilage of the sulcus block and the anterior and middle portions of the lateral femoral condyle increased by 5–17% [41]. As a result of instability caused by cruciate ligament crossing, a 10% decrease in PGs content and a 30% increase in shear modulus were observed in the cartilage of the operated knee joint, compared to the control group [42].
In the modeling of OA using lateral meniscectomy, a 20% decrease in collagen content, a 52% increase in PGs content, an increase in hydration, a 26–135% increase in cartilage thickness, and a 70% decrease in shear modulus were found in the cartilage of the lateral zone of the tibial plateau. At the same time, in the cartilage of the medial zone, a 10% decrease in collagen content, a 14–19% increase in PGs content, a 19–30% increase in cartilage thickness, and no change in hydration and shear modulus were found [47].
When the lower limb was immobilized for 4 weeks, a decrease in PGs content and a 6% increase in cartilage hydration were observed. Mechanical parameters also changed—elastic modulus increased by 140%, and shear modulus decreased by 75%, compared to cartilage from the control group. When immobilized for 8 weeks, tensile modulus increased by 88%. The ratio of PGs to collagen in the cartilage of the immobilized group did not change compared to the control group [43, 44, 48].
When cartilage was treated with interleukin-1β to reproduce the processes of OA, the following results were obtained: the content of PGs in cartilage decreased by 30%, and the elastic modulus decreased by 22%. The decrease in collagen was insignificant: a 5–10% decrease in concentration was observed [49]. Modeling OA with trypsin (causing cartilage degradation mainly due to lysis of the GAGs) was performed to evaluate Raman microspectroscopy profits and disadvantages,which proved the positive significant correlation between histological analysis results and spectroscopy. High spatial resolution and section-free technique for cartilage condition assessment make this method promising for implementation into standard clinical practice [45].
3.3.4 Changes at different stages of OA
In the early stages of OA, PGs content decreases in the superficial and middle layers of cartilage, while in late OA, it decreases in all layers. Also, in early OA, the collagen content does not change, while in the late stages, a decrease was observed mainly in the middle layers [38]. In contrast to cartilage in late OA, healthy cartilage contains 30% more collagen and 60% more PGs [24]. Total water content observed by the NIR spectroscopy tends to decrease with the progression of OA, with a 25% difference between stages 1 and 4 according to ICRS, which is associated with a decrease in cartilage thickness and an overall decrease in the proportion of water in detected volume. Cartilage degeneration is also accompanied by changes in mechanical properties, such as elastic modulus decreases with OA progression (80% difference between stages 1 and 4 according to ICRS), relaxation time decreases (40% difference between stages 1 and 4 according to ICRS) [1] (Fig. 5).
4 Discussion
This systematic review analyzes more than 30 articles devoted to the study of the composition of cartilage in osteoarthritis published over the past 40 years.
For a long time, the ideas about the cartilage structure were based only on histological tissue analysis, but advances in radiology diagnostic methods changed all conceptions dramatically. Now, it is possible to examine the condition of the cartilage without using traumatic material sampling from patients. Diagnostics include MRI and CT, as well as spectroscopy. The analysis of such a large number of articles demonstrates a growing interest in such a diagnostic method as spectroscopy—more than a third of the works (13) conducted a study of cartilage using spectroscopy, while less than ten studies using CT and MRI (9). It is worth noting that the peak of works about using spectroscopy has been in the last 8 years (since 2015).
One of the actual non-invasive methods adequate for diagnosing intraarticular damage to the articular cartilage is MRI. However, the accuracy of determining the degree of cartilage tissue hydration is debatable since the results significantly depend on the concentration of PGs and collagen in the cartilage [2, 12]. Using specific techniques (measurement of mcDESPOT at 3.0 T) is still possible to non-invasively determine the content of PGs. However, these techniques require further development [11]. CT is an effective way to diagnose the state of cartilage tissue, the results of which correlate with an accurate histological picture. This method provides quantitative results on matrix components such as PGs and collagen and can also be used to study the spatial arrangement of these components [22, 33]. The main disadvantages of this method are the high cost and long timing of the procedure, since the minimum time for the penetration of contrast into the cartilage and its distribution takes 2 h, and the highest correlation between the results of CT and histological examination is observed 48 h after the injection of contrast, which makes this method applicable in a hospital setting only [21]. The histological method, supplemented by densitometry, is considered the golden standard for diagnosing the stage of OA. Still, as the sampling of cartilage tissue along with the underlying bone is needed for its implementation, this sampling must be carried out from several places since the cartilage condition may differ in different areas. This makes it impossible for intraoperative use due to the inevitable traumatization of the cartilage [19,20,21, 33]. Mechanical properties of cartilage significantly correlate with its condition, but, unfortunately, with the use of a mechanical diagnostic method, whether it is the study of the elastic modulus, shear modulus or dynamic pressure modulus, it is impossible to diagnose the early stage of OA as in this period the changes are insignificant and occur at the microstructural level only [11, 13, 14, 20, 24].
As can be seen from the results obtained, the research methods used cannot be applied to intraoperative diagnostics. Fourier transform infrared spectroscopy allows us to determine changes in the content of collagen and proteoglycans for unstained cartilage samples. In particular, to identify a decrease in the content of proteoglycans at early stages and collagen at later OA stages [13, 38]. Polarizing microscopy also makes it possible to detect changes in collagen orientation in the early stages [38]. Therefore, optical methods can provide important diagnostic information. However, techniques implemented in these studies require prior sample preparation and are unsuitable in an intraoperative context. Near-infrared spectroscopy can be used to observe diffuse reflectance spectra and determine changes in the content of the main components during in situ measurements. This is possible due to lower water absorption index values, as discussed in [1]. Therefore, the advancement of this technique is of considerable interest.
The developed and applied spectroscopy method makes it possible to assess the condition of the patient's cartilage during surgery, making measurement more objective and helping doctors to plan subsequent treatment strategies more precisely.
It should also be noted that some papers lack information about the methodology of the study, as a result of which the reader may have a skeptical attitude to the results of these articles, as in the works of [2, 12] and [36], there is no information on the number of animals involved in the experiment, and in the works of [36] and [35], there is no data on both the number of animals participating in the experiment and the number of samples of knee joint cartilage taken.
5 Conclusion
According to our findings, spectroscopy is the primary instrumental method for determining cartilage’s quantitative and spatial structure, and it has a wide range of usage—from experimental animal studies to clinical practice applications. A progressively developing topic is assessing the cartilage composition with ray methods, such as MRI, CT, and Spectroscopy. The increased interest in spectroscopy sets the direction of the search and the subject of subsequent research in this field for a better and more accurate understanding of the cartilage composition. Timely detection of the knee joint degradation at the level of tissue changes can prevent its progressive damage due to the early beginning of appropriate treatment.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
References
Rovnyagina NR, Budylin GS, Dyakonov PV, Efremov YM, Lipina MM, Goncharuk YR, Murdalov EE, Pogosyan DA, Davydov DA, Korneev AA, Serejnikova NB, Mikaelyan KA, Evlashin SA, Lazarev VA, Lychagin AV, Timashev PS, Shirshin EA. Grading cartilage damage with diffuse reflectance spectroscopy: Optical markers and mechanical properties. J Biophotonics. 2023;16(3): e202200149. https://doi.org/10.1002/jbio.202200149.
Kobayashi-Miura M, Miura T, Osago H, Yamaguchi Y, Aoyama T, Tanabe T, Matsumoto KI, Fujita Y. Rat articular cartilages change their tissue and protein compositions during perinatal period. Anat Histol Embryol. 2016;45(1):9–18. https://doi.org/10.1111/ahe.12165.
Athanasiou KA, Darling EM, Hu JC, DuRaine GD, Reddi AH. Articular cartilage. Boca Raton (FL): CRC Press; 2016.
Fox AJ, Bedi A, Rodeo SA. The basic science of human knee menisci: structure, composition, and function. Sports Health. 2012;4(4):340–51. https://doi.org/10.1177/1941738111429419.
Petersen W, Tillmann B. Collagenous fibril texture of the human knee joint menisci. Anat Embryol (Berl). 1998;197(4):317–24. https://doi.org/10.1007/s004290050141.
Guo U, Liu S, Zhu Y, Yu S, Lu S, Yuan M, Gao Y, Huang J, Yuan Z, Peng J, Wang A, Wang Y, Chen J, Zhang L, Sui H, Xu V, Guo K. Achievements and prospects in the field of tissue-engineered meniscus scaffolds for meniscus regeneration. Stem Cells Int. 2015;517520. https://doi.org/10.1155/2015/517520.
Bielajew BJ, Hu JC, Athanasiou KA. Collagen: quantification, biomechanics, and role of minor subtypes in cartilage. Nat Rev Mater. 2020;5(10):730–47.
Pritzker KPH, Gahunia HK. Articular cartilage: homeostasis, aging and degeneration. In: Gahunia HK, Gross AE, Pritzker KPH, editors. Articular cartilage of the knee: health, disease and therapy. New York: Springer; 2020. p. 99–122.
Guo JB, Liang T, Che YJ, Yang HL, Luo ZP. Structure and mechanical properties of high-weight-bearing and low-weight-bearing areas of hip cartilage at the micro- And nano-levels. BMC Musculoskelet Disord. 2020;21(1):1–9.
Afara IO, Oloyede A. Resolving the near-infrared spectrum of articular cartilage. Cartilage. 2021;13(1_suppl):729S-737S. https://doi.org/10.1177/19476035211035417.
Grondin MM, Liu F, Vignos MF, Samsonov A, Li WJ, Kijowski R, Henak CR. Bi-component T2 mapping correlates with articular cartilage material properties. J Biomech. 2021;116: 110215. https://doi.org/10.1016/j.jbiomech.2020.110215.
Fleck AKM, Kruger U, Carlson K, Waltz C, McCallum SA, Lucas LuX, Wan LQ. Zonal variation of MRI-measurable parameters classifies cartilage degradation. J Biomech. 2017;65:176–84. https://doi.org/10.1016/j.jbiomech.2017.10.011.
Warnecke D, Balko J, Haas J, Bieger R, Leucht F, Wolf N, Schild NB, Stein SEC, Seitz AM, Ignatius A, Reichel H, Mizaikoff B, Dürselen L. Degeneration alters the biomechanical properties and structural composition of lateral human menisci. Osteoarthritis Cartilage. 2020;28(11):1482–91. https://doi.org/10.1016/j.joca.2020.07.004.
Keenan KE, Besier TF, Pauly JM, Smith RL, Delp SL, Beaupre GS, Gold GE. T1ρ dispersion in articular cartilage: Relationship to material properties and macromolecular content. Cartilage. 2015;6(2):113–22. https://doi.org/10.1177/1947603515569529.
Karchner JP, Yousefi F, Bitman SR, Darvish K, Pleshko N. Non-destructive spectroscopic assessment of high and low weight bearing articular cartilage correlates with mechanical properties. Cartilage. 2019;10(4):480–90. https://doi.org/10.1177/1947603518764269.
Froimson MI, Ratcliffe A, Gardner TR, Mow VC. Differences in patellofemoral joint cartilage material properties and their significance to the etiology of cartilage surface fibrillation. Osteoarthritis Cartilage. 1997;5(6):377–86. https://doi.org/10.1016/s1063-4584(97)80042-8.
Julkunen P, Wilson W, Jurvelin JS, Rieppo J, Qu CJ, Lammi MJ, Korhonen RK. Stress-relaxation of human patellar articular cartilage in unconfined compression: prediction of mechanical response by tissue composition and structure. J Biomech. 2008;41(9):1978–86. https://doi.org/10.1016/j.jbiomech.2008.03.026.
Párraga Quiroga JM, Emans P, Wilson W, Ito K, van Donkelaar CC. Should a native depth-dependent distribution of human meniscus constitutive components be considered in FEA-models of the knee joint? J Mech Behav Biomed Mater. 2014;38:242–50. https://doi.org/10.1016/j.jmbbm.2014.03.005.
Berberat JE, Nissi MJ, Jurvelin JS, Nieminen MT. Assessment of interstitial water content of articular cartilage with T1 relaxation. Magn Reson Imaging. 2009;27(5):727–32. https://doi.org/10.1016/j.mri.2008.09.005.
Kiviranta P, Lammentausta E, Töyräs J, Nieminen HJ, Julkunen P, Kiviranta I, Jurvelin JS. Differences in acoustic properties of intact and degenerated human patellar cartilage during compression. Ultrasound Med Biol. 2009;35(8):1367–75. https://doi.org/10.1016/j.ultrasmedbio.2009.03.003.
Bhattarai A, Mäkelä JTA, Pouran B, Kröger H, Weinans H, Grinstaff MW, Töyräs J, Turunen MJ. Effects of human articular cartilage constituents on simultaneous diffusion of cationic and nonionic contrast agents. J Orthop Res. 2021;39(4):771–9. https://doi.org/10.1002/jor.24824.
Kallioniemi AS, Jurvelin JS, Nieminen MT, Lammi MJ, Töyräs J. Contrast agent enhanced pQCT of articular cartilage. Phys Med Biol. 2007;52(4):1209–19. https://doi.org/10.1088/0031-9155/52/4/024.
Milentijevic D, Torzilli PA. Influence of stress rate on water loss, matrix deformation and chondrocyte viability in impacted articular cartilage. J Biomech. 2005;38(3):493–502. https://doi.org/10.1016/j.jbiomech.2004.04.016. PMID: 15652547.
Kiviranta P, Töyräs J, Nieminen MT, Laasanen MS, Saarakkala S, Nieminen HJ, Nissi MJ, Jurvelin JS. Comparison of novel clinically applicable methodology for sensitive diagnostics of cartilage degeneration. Eur Cell Mater. 2007;13:46–55. https://doi.org/10.22203/ecm.v013a05.
Aoki T, Watanabe A, Nitta N, Numano T, Fukushi M, Niitsu M. Correlation between apparent diffusion coefficient and viscoelasticity of articular cartilage in a porcine model. Skeletal Radiol. 2012;41(9):1087–92. https://doi.org/10.1007/s00256-011-1340-y.
Kijowski R, Blankenbaker DG, Munoz Del Rio A, Baer GS, Graf BK. Evaluation of the articular cartilage of the knee joint: value of adding a T2 mapping sequence to a routine MR imaging protocol. Radiology. 2013;267(2):503–13. https://doi.org/10.1148/radiol.12121413.
Emanuel KS, Kellner LJ, Peters MJM, Haartmans MJJ, Hooijmans MT, Emans PJ. The relation between the biochemical composition of knee articular cartilage and quantitative MRI: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2022;30(5):650–62. https://doi.org/10.1016/j.joca.2021.10.016.
Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in OA. Eur Cells Mater. 2007;13(5):75–86.
Gao J, Xu X, Yu X, Fu Y, Zhang H, Gu S, Cao D, Guo Q, Xu L, Ding J. Quantitatively relating magnetic resonance T1 and T2 to glycosaminoglycan and collagen concentrations mediated by penetrated contrast agents and biomacromolecule-bound water. Regen Biomater. 2023;10:rbad035. https://doi.org/10.1093/rb/rbad035.
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372: n71. https://doi.org/10.1136/bmj.n71.
Mahmood MF, Clarke MJ, Riches DP. Proteoglycans exert a significant effect on human meniscal stiffness through ionic effects. Clin Biomech (Bristol, Avon). 2020;77: 105028. https://doi.org/10.1016/j.clinbiomech.2020.105028.
Honkanen JT, Danso EK, Suomalainen JS, Tiitu V, Korhonen RK, Jurvelin JS, Töyräs J. Contrast enhanced imaging of human meniscus using cone beam CT. Osteoarthritis Cartilage. 2015;23(8):1367–76. https://doi.org/10.1016/j.joca.2015.03.037.
Honkanen JTJ, Turunen MJ, Freedman JD, Saarakkala S, Grinstaff MW, Ylärinne JH, Jurvelin JS, Töyräs J. Cationic contrast agent diffusion differs between cartilage and meniscus. Ann Biomed Eng. 2016;44(10):2913–21. https://doi.org/10.1007/s10439-016-1629-z.
Akizuki S, Mow VC, Muller F, Pita JC, Howell DS. Tensile properties of human knee joint cartilage. II. Correlations between weight bearing and tissue pathology and the kinetics of swelling. J Orthop Res. 1987;5(2):173–86. https://doi.org/10.1002/jor.1100050204.
Naka MH, Morita Y, Ikeuchi K. Influence of proteoglycan contents and of tissue hydration on the frictional characteristics of articular cartilage. Proc Inst Mech Eng H. 2005;219(3):175–82. https://doi.org/10.1243/095441105X34220.
Zernia G, Huster D. Collagen dynamics in articular cartilage under osmotic pressure. NMR Biomed. 2006;19(8):1010–9. https://doi.org/10.1002/nbm.1061.
Torzilli PA. Influence of cartilage conformation on its equilibrium water partition. J Orthop Res. 1985;3(4):473–83. https://doi.org/10.1002/jor.1100030410.
Saarakkala S, Julkunen P, Kiviranta P, Mäkitalo J, Jurvelin JS, Korhonen RK. Depth-wise progression of osteoarthritis in human articular cartilage: investigation of composition, structure and biomechanics. Osteoarthritis Cartilage. 2010;18(1):73–81. https://doi.org/10.1016/j.joca.2009.08.003.
Slowman SD, Brandt KD. Composition and glycosaminoglycan metabolism of articular cartilage from habitually loaded and habitually unloaded sites. Arthritis Rheum. 1986;29(1):88–94. https://doi.org/10.1002/art.1780290112.
Bullough PG, Yawitz PS, Tafra L, Boskey AL. Topographical variations in the morphology and biochemistry of adult canine tibial plateau articular cartilage. J Orthop Res. 1985;3(1):1–16. https://doi.org/10.1002/jor.1100030101.
Säämämen AM, Kiviranta I, Jurvelin J, Helminen HJ, Tammi M. Proteoglycan and collagen alterations in canine knee articular cartilage following 20 km daily running exercise for 15 weeks. Connect Tissue Res. 1994;30(3):191–201. https://doi.org/10.3109/03008209409061971.
Altman RD, Tenenbaum J, Latta L, Riskin W, Blanco LN, Howell DS. Biomechanical and biochemical properties of dog cartilage in experimentally induced osteoarthritis. Ann Rheum Dis. 1984;43(1):83–90. https://doi.org/10.1136/ard.43.1.83.
Setton LA, Mow VC, Müller FJ, Pita JC, Howell DS. Mechanical behavior and biochemical composition of canine knee cartilage following periods of joint disuse and disuse with remobilization. Osteoarthritis Cartilage. 1997;5(1):1–16. https://doi.org/10.1016/s1063-4584(97)80027-1.
Leroux MA, Cheung HS, Bau JL, Wang JY, Howell DS, Setton LA. Altered mechanics and histomorphometry of canine tibial cartilage following joint immobilization. Osteoarthritis Cartilage. 2001;9(7):633–40. https://doi.org/10.1053/joca.2001.0432.
Gao T, Boys AJ, Zhao C, Chan K, Estroff LA, Bonassar LJ. Non-destructive spatial mapping of glycosaminoglycan loss in native and degraded articular cartilage using confocal Raman microspectroscopy. Front Bioeng Biotechnol. 2021;9:744197. https://doi.org/10.3389/fbioe.2021.744197.
Wei L, Hjerpe A, Brismar BH, Svensson O. Effect of load on articular cartilage matrix and the development of guinea-pig osteoarthritis. Osteoarthritis Cartilage. 2001;9(5):447–53. https://doi.org/10.1053/joca.2000.0411.
Appleyard RC, Burkhardt D, Ghosh P, Read R, Cake M, Swain MV, Murrell GA. Topographical analysis of the structural, biochemical and dynamic biomechanical properties of cartilage in an ovine model of osteoarthritis. Osteoarthritis Cartilage. 2003;11(1):65–77. https://doi.org/10.1053/joca.2002.0867.
Palmoski MJ, Brandt KD. Running inhibits the reversal of atrophic changes in canine knee cartilage after removal of a leg cast. Arthritis Rheum. 1981;24(11):1329–37. https://doi.org/10.1002/art.1780241101. PMID: 7317111.
Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, Reddy R. Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med. 2005;54(5):1087–93. https://doi.org/10.1002/mrm.20678.
Acknowledgements
The authors are grateful to Elena Gnedovskaya for providing the services of a professional manuscript proofreader.
Funding
This study was supported by the Russian Science Foundation (grant no. 21–79-10325); by the academic leadership program Priority 2030 proposed by Federal State Autonomous Educational Institution of Higher Education I. M. Sechenov First Moscow State Medical University of the Ministry of Health of the Russian Federation (Sechenov University).
Author information
Authors and Affiliations
Contributions
Bogdan Raikov, Marina Lipina, Alexey Lychagin, Nikita Belov, David Pogosyan, Kirill Azarkin, and Emirkhan Murdalov were responsible for the literature research, collecting the data, and preparing the figures and tables included in the manuscript; Bogdan Raikov, Marina Lipina, Alexey Lychagin, Eugene Kalinsky, Yuliya Goncharuk, Tagir Kudrachev, Ivan Vyazankin, Eugene Nagornov, Vadim Cherepanov wrote the paper; Bogdan Raikov, Marina Lipina, Alexey Lychagin, Vladimir Telpukhov, Andrey Gritsyuk, Andrey Garkavi, Gennadiy Kavalerskiy, Gleb Budylin, Evgeny Shirshin, Nataliya Rovnyagina, Anton Kurpyakov were responsible for the critical revision of the entire manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Raikov, B., Lipina, M., Azarkin, K. et al. Methods for determining the molecular composition of knee joint structures in osteoarthritis: collagen, proteoglycans and water content: a systematic review. Collagen & Leather 6, 30 (2024). https://doi.org/10.1186/s42825-024-00173-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-024-00173-7