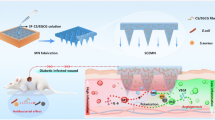
Abstract
Silicate bioceramics have demonstrated great potential in hydrogel dressings for wound healing due to their special origins of promoting endothelial cell angiogenesis and inhibiting apoptosis of cardiomyocyte. However, there are still some deficiencies, such as insufficient biological activity, instability of silicate ion release, and lower wet adhesion on wounds with tissue exudate, limiting their further clinical applications. Herein, inspired by mussels, a multifunctional double-network hydrogel (FS/PAM-Gel-PDA) wound dressing composited gelatin with silicate ceramic powder with satisfactory wet adhesion, stable release of bioactive ions, hemostasis, and the ability of promoting vascular regeneration was engineered through specifically grafting dopamine to gelatin and introducing ferrous silicate ceramic powder into the hydrogel. The comprehensive experimental results substantiate that the FS/PAM-Gel-PDA has wet-adhesion strength of up to 21.78 kPa, and remains stably adherent to porcine myocardial tissues intuitively after bending, twisting, soaking in water, and stretching. The test results of ion release behavior in vitro show that the oxidation and agglomeration of ferrous silicate ceramic powder can be effectively inhibited by using dopamine to form an antioxidant layer on the surface of ceramic powder, and thus, the stable release of Fe2+ and SiO4 4− effective ions can be realized. The animal experiment exhibits that FS/PAM-Gel-PDA can achieve rapid hemostasis in the lethal liver defect model. Meanwhile, the FS/PAM-Gel-PDA reveals the remarkable ability to promote wound healing in a full-thickness skin injury model, which can obviously accelerate skin re-epithelialization. To sum up, the FS/PAM-Gel-PDA has excellent wet adhesion and stable release of active ions to accelerate angiogenesis, which shows great potential in promoting wound healing.
Graphical Abstract

Similar content being viewed by others
1 Introduction
The repair of skin wound tissue involves the processes of blood coagulation, occurrence and progression of inflammation, matrix synthesis, vascular regeneration, fibrous tissue proliferation, re-epithelization, wound contraction, and tissue remodeling [1,2,3]. The conventional wound dressings have some defects, such as poor hemostasis, wound dryness, and adhesion to the wound during replacement, leading to secondary injury and infection [4,5,6,7]. Hydrogels have great potential for tissue engineering applications because they have high water content, can mimic the ECM environment as a carrier for cell transplantation, and promote cell survival, proliferation, differentiation and migration [8,9,10,11,12].
Biological materials, such as bioglass and silicate bioceramics have been proved to affect cell behavior and tissue regeneration, such as promoting stem cell differentiation and angiogenesis [13,14,15], which indicates that silicate biomaterials can play a bioactive role in the tissue engineering processes in-situ by activating cell proliferation, migration, differentiation and extracellular matrix synthesis [16,17,18]. Due to the stable structure and low release of active ions, bioceramics have low biological activity, so that they cannot be directly used for the treatment of tissue defects. However, ion doping can lead to changes in material structure, so as to enhance degradability, increase release of active ions, and augment biological activity [19]. Certain specific metal ions with therapeutic effects, such as strontium, manganese, copper, and zinc ions have been incorporated into the chemical structure of bioceramics, endowing materials with new functions and extending their applications in different biomedical applications [20,21,22,23,24]. Zhang designed a composite hydrogel which contributes to myocardial repair through the release of Zn2+ and SiO4 4− [25]. But it has always been a tough task for researchers to realize the stable release of bioceramic ions. Iron, as the most abundant trace element in human ions, exists in the form of Fe2+ and Fe3+ ions in the human body, which has been proved to have good angiogenic activity in regulating the expression of angiogenesis-related genes, such as VEGF and HIF-1α. However, Fe3+ ions may cause cytotoxicity at higher concentrations, while Fe2+ ions have limitations in practical applications due to their low chemical stability. Therefore, most of the existing researches have adopted antioxidants to inhibit the oxidation of Fe2+ ions. Sheng prepared a novel bioactive photothermal hydrogel with carboxymethyl chitosan to inhibit the oxidation of Fe2+, which synergistically stimulates vascular regeneration through the stable release of Fe2+ and SiO4 4− and photothermal stimulation [26]. In addition, the hydrogel dressings based on ceramic powder have poor wet adhesion, and for the wounds with large amounts of tissue exudate, they are unable to effectively encapsulate the wounds, which cannot meet the application requirements of irregular wounds [27,28,29]. Therefore, designing a hydrogel wound dressing with good wet adhesion and stable release of active ions for accelerating angiogenesis has a very important application prospect.
The selection of hydrogel matrix materials is very crucial in the preparation of hydrogel based on silicate bioceramics. Collagen is one of the most important biofunctional structural proteins in animal connective tissues, and is the earliest widely used natural biomaterial because of its structure similar to extracellular matrix, which promotes cell adhesion, value-added and differentiation [30,31,32,33]. Gelatin (Gel) is a hydrolysed product of collagen, which has excellent biocompatibility, low immunogenicity and biodegradability, facilitates cell adhesion and promotes cell proliferation. Its molecular side chain contains a large number of amino groups, carboxyl groups, amide bonds and other active groups, in the appropriate reaction conditions, these active groups can be chemically reacted with a large number of macromolecules to achieve the effect of modification or crosslinking of Gel, not only to improve the original performance of Gel, give Gel more functions. And one of the unique physicochemical properties of Gel is the reversible solution-gel transition performance, in the temperature close to room temperature, Gel in the medium only swells and not dissolved; with the increase in temperature, Gel dissolved in the medium to form a solution; when the temperature began to decrease, the Gel solution then reformed into a gel. Therefore, gelatin-based hydrogels show great application prospects in the field of tissue repair. However, their poor mechanical properties and wet adhesion properties limit their application. At present, Gel is mostly modified by coupling, cross-linking and grafting to make it functional, such as the introduction of polyacrylamide (PAM) to form a double-network structure, which can greatly improve the mechanical properties of the system [34, 35]. Meanwhile, inspired by mussels, dopamine (DA) has a similar structure to the adhesion proteins of mussels, with high adhesion and excellent cell affinity to various surfaces, so dopamine is widely applied in hydrogels with high wet adhesion [24, 36, 37]. More importantly, dopamine will oxidize and self-polymerize into polydopamine (PDA) under alkaline conditions, which can effectively prevent the oxidation of Fe2+ ions in ceramic powder [38,39,40] so as to further stabilize the release of Fe2+ ions.
In summary, combining the great advantages of silicate bioceramics and gelatin materials in promoting wound healing, in view of the issues of the poor mechanical strength, unstable release of silicate ions, and low wet adhesion properties of the two materials, this paper was inspired by mussels to prepare a double-network structure hydrogel with polyacrylamide by specifically grafting dopamine to gelatin. Then by introducing ferrous silicate ceramic powder (FS) into the hydrogel system, a multifunctional double-network hydrogel (FS/PAM-Gel-PDA) wound dressing composited gelatin with silicate ceramic powder was prepared, which had good biological wet adhesion properties for stable attachment to skin tissues and cardiac tissues, stable release of bioactive ion shown by inhibition of Fe2+ oxidation, mechanical properties, hemostatic properties, biocompatibility and the ability of promoting vascular regeneration. Based on the wet adhesion and strong oxidation of dopamine, the dopamine was grafted onto gelatin and introduced into the ceramic powder prepared in the previous study, and a double-network hydrogel was fabricated, which could stably release Fe2+ and SiO4 4− ions, so as to effectively promote the vascular regeneration at the wounds and accelerate the re-epithelization of skin. Therefore, this study provided a new method for the design and construction of hydrogels with stable release of bioactive ions, which has great potential in the field of promoting wound healing.
2 Materials and methods
2.1 Materials and chemicals
Acrylamide (AM) was purchased from Tianjin Kermel Chemical Reagent Co., Ltd. (Tianjin, China). Gelatin (Gel) and ammonium persulfate (APS) were purchased from Tianjin Tianli Chemical Reagent Co., Ltd. (Tianjin, China). N–N methylene bisacrylamide (MBA) was purchased from Tianjin Damao Chemical Reagent Factory (Tianjin, China). Tetramethylethylenediamine (TEMED), dopamine hydrochloride (DA), and genipin (GP) were purchased from Macklin (Shanghai, China). Ferrous silicate ceramic powder (FS) was synthesized in the previous work.
2.2 Preparation of PAM-Gel-PDA hydrogels
Different amounts of DA (2, 4, 6, 8, DA/AM %) were dissolved in deionized water and the pH was adjusted to 10 by adding 1 mol/L NaOH solution. After oxidation under air for 20 min, the quantitative Gel was added and dissolved at 45 °C, and then DA and Gel were combined with Schiff base through GP. Adding the monomer AM and cross-linking agent MBA, the solution was stirred for 30 min, APS was added as an initiator, and finally the product was placed in an oven at 60 ℃ for 1 h.
2.3 Preparation of FS/PAM-Gel-PDA hydrogels
The quantitative DA was dissolved in distilled water, and the pH was adjusted to 10 by adding 1 mol/L NaOH solution. Different amounts of FS (such as 0.05, 0.1, 0.15, 0.2, Fe2SiO4/AM %) were weighed into the DA solution, and dispersed by ultrasonication for 30 min to obtain the Fe2SiO4/DA suspension. Gel was added, heated and dissolved. Then Step 2.2 was repeated.
2.4 Structural characterization of PAM-Gel-PDA and FS/PAM-GEL-PDA hydrogels
The freeze-dried samples were measured on a Fourier transform infrared spectrometer (FT-IR, Bruker, VECTOR-22, Germany) to obtain the infrared (IR) spectra in the range of 4000–400 cm−1. The chemical composition and state of the freeze-dried samples were analyzed by X-ray photoelectron spectroscop (XPS). The internal network and structure of the samples were observed by cryo-scanning electron microscopy (Cryo-SEM, FEI, SU3500, USA).
2.5 Wet adhesion property test
According to the literature, the wet adhesion properties of the samples on different tissues (such as fresh porcine skin, the porcine skin soaked in PBS for 30 min, and fresh porcine myocardial tissue) were measured using the lap shear test method. An appropriate amount of the sample (10 mm × 10 mm × 2 mm) was placed on the surface of the tissues, covered with another layer of tissues, and pressed for 30 s, and both ends of the tissues were pulled at a speed of 5 mm/min with a universal material testing machine. Each set of experiments was measured three times. The adhesive strength was obtained by using the maximum load divided by the overlapping area.
2.6 Mechanical property test
The hydrogel precursor sol was poured into a dumbbell-shaped groove made by PTFE plate with a length of 16 mm, a width of 4 mm and a thickness of 2 mm to form a hydrogel. Both ends of the samples were pulled at a speed of 10 mm/min using a material testing machine. Each set of experiments was measured three times to obtain the stress–strain curves.
2.7 Rheological behavior
The hydrogel samples were tested by a dynamic rheometer (TA, DHR-, USA). In the strain scanning mode, a planar lamina with a diameter of 40 mm was adopted. The test temperature was 25 °C, the equilibrium time was 120 s, and the strain in the linear viscoelastic area was 5%. There were 64 data acquisition points, and 10 sampling points at the order of magnitudes. When the angular frequency was in the range of 0.1–100 rad/s, the elastic modulus (G') and loss modulus (G'') of each group of samples were collected and recorded.
2.8 Hydrophilicity
A certain mass of freeze-dried sponge samples was weighed, and distilled water was added to prepare a dispersion with a certain concentration. After a glass slide (75 mm × 25 mm) was selected as the substrate, and was dried naturally at room temperature after being dipped in the dispersion, the WCA sizes of different samples were detected using a video contact angle meter (Dataphysics, Germany). The boiled but cooled distilled water was automatically dripped onto the sample surface with 5 μL each time through the instrument, and the average value was obtained after taking multiple points.
2.9 Swellability
A certain mass of freeze-dried hydrogel samples (Wdried) was weighed, immersed in PBS solution, and three samples were collected from each group at different time intervals using filter paper from removing the PBS solution from the surface of the swollen samples and weighed immediately (Wswollen). The swelling ratio was calculated by the following Eq. (1):
2.10 Biodegradability
A certain mass of lyophilised hydrogel samples (WInitial) was weighed and immersed into a mixed solution of PBS and type IV collagenase at 37 ℃ with a collagenase content of 2 mg/ml, taken out at different time intervals, washed with ultrapure water for several times and freeze-dried, and the weight (Wdried) was recorded, three samples were collected from each group. The biodegradation rate was calculated by the following Eq. (2):
2.11 Ion release behavior
A small amount of hydrogel sample was taken into the 50 ml PBS solution, and shaken in an oscillator at 37 ℃ for 24 h. After centrifugation, 30 ml supernatant was taken, and the sample was dissolved with aqua regia. The solution was heated up to 1 ml and diluted with purified water to 30 ml, and then the membrane (0.22 μm) was filtered. The concentrations of Fe and Si ions in the samples were determined with an inductively coupled plasma atomic emission spectrometry (ICP-OES, 710-ES, Varian, USA).
2.12 Biocompatibility
According to Chinese National Standard GB/T16175-1996, the cytocompatibility of hydrogel samples was evaluated by MTT method with China hamster lung cells (CHLs), human umbilical vein endothelial cells (HUVECs) and mouse fibroblasts (L929s) were used as model cells. Before the experiment, the samples were separately placed in the sterile Petri dishes, soaked in 75% alcohol, and sterilized for 2 h. The PBS buffer was added and exchanged twice for 5 min each time (with the purpose of washing away residual alcohol), after which the samples were extracted in RPMI 1640 culture medium according to the Standard of 0.2 g/ml, and placed in an incubator with 5% CO2 at 37 °C for 24 h to obtain the sample extracts.
The cells were seeded into 96-well plates with a density of 5,000 cells/well and incubated in an incubator with 5% CO2 at 37 ℃ for 24 h. After the cells were completely adhered to the wall, the original medium was discarded. In the control group, 200 µL RPMI 1640 medium was added to each well, while in the experimental group, 100 μL sample extract and 100 μL RPMI 1640 medium were added to each well. Six parallel experimental groups were set up in each group. On the 1st, 3rd and 5th days, the absorbance of the plate was measured at 450 nm with a spectrophotometric microplate reader. A confocal laser scanning microscope (CLSM) was used to observe the growth and proliferation of cells on the hydrogel samples.
2.13 In vitro scratch assay
Cells were inoculated into 24-well plates and cultured to achieve a confluent monolayer. A scratch in the cell monolayer was subsequently created in each well by scraping with a p200 pipette tip. The cells were cultured with a low FBS content of 1% (v/v) after being gently washed with culture medium. Then PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Gly), FS/PAM-Gel (Gly) hydrogels were placed into each well. Cells cultured without hydrogel were recorded as a control. Photographs were taken and quantitatively analyzed by Image-J software to determine the migration ratio, which was calculated by the following formula (3):
where M0 and Mt represent the initial scratch area and the healing scratch area, respectively.
2.14 Enzyme-linked immunosorbent assay for VEGF
PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Gly), FS/PAM-Gel (Gly) hydrogels were immersed in six-well plates in PBS, and the plates were incubated in a cell culture incubator for 24 h. The amount of VEGF in the cell supernatant of HUVECs was determined using the VEGF-Elisa kit according to the instructions provided.
2.15 Hemostatic properties in vivo
SD Male rats at SPF-grade with the weight of about 300 g were selected. In order to assess the hemostatic effect of the material, the following hemorrhage model was established by using rats: the hemostatic time and hemorrhagic amount were evaluated by bleeding from cutting livers. The test material was cut into pieces (2.0 cm × 2.0 cm × 3 mm), weighed and sterilized. A piece of clean filter paper was placed under the livers. A quarter of the liver lobe was removed and the wound surface was coated with PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water), and FS/PAM-Gel-PDA (Gly) hydrogels, while the untreated wounds were used as a control. The pressure of 1 N was applied vertically. The wounds were observed approximately every 10 s, and the hemostatic time was recorded immediately after the bleeding completely stopped. Finally, the materials for absorbing the blood were weighed so as to calculate the hemorrhagic amount.
2.16 Hemolytic test
Fresh whole blood was obtained from adult mice using the hepatic blood collection method and then quickly transferred to a centrifuge tube containing sodium heparin as an anticoagulant. Subsequently, 2.5 mL of saline containing anticoagulant was added to the above 2 mL of fresh whole blood and the tube was gently shaken to break it evenly. PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) were placed in a sterilized centrifuge tube containing 2 mL of saline. The system was heated to 37 °C for 30 min in a thermostatic incubator. Next, 15 μL of diluent blood was added to each centrifuge tube and incubated at 37 °C for 1 h. Finally, all tubes with the mixed system were centrifuged at 2500 rpm for 3 min and the absorbance of the supernatant was measured by a UV–Vis spectrophotometer at 545 nm. For comparison, saline and ultrapure water without hydrogel were used as negative and positive controls, respectively. The hemolysis rate (%) was calculated using Eq. (4):
2.17 Wound healing in vivo
2.17.1 Animals and surgery
The conventional rats (at 6–8 weeks old and with the weight of 200–220 g) without characteristic pathogens (SPF) were purchased from SPF (Beijing) Biotechnology Co., Ltd. with a license number of SCXK (Beijing) 2019–0010. The effects of hydrogels on wound healing in vivo of these rats were evaluated using a full-thickness skin defect model. Under the condition of light/dark circulation at 18–26 ℃ for 12 h, these rats were able to freely obtain water and granular food. In addition, animal welfare and experimental procedures were strictly implemented in accordance with the Guidelines for the Care and Use of Laboratory Animals (Department of Science and Technology of Shaanxi Province, China, 2016) and the relevant ethical regulations issued by Hygiene Research Institute of the Ordnance Industry. The hydrogels were sterilized with ultraviolet light for 24 h before implantation. Before surgery, the animals were anaesthetized intraperitoneally with 2% sodium pentobarbital at a dose of 50 mg/kg. Two circular incisions with a diameter of 1 cm were cut with surgical scissors on the left and right sides of the depilated skin. The left side was the self-control group and the right side was the intervention group. In the intervention group, the wounds were covered with the equal areas of PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) hydrogels. After that, the wounds on both sides were evenly smeared with iodophor. Finally, the wounds were successively covered with a layer of cellophane, two layers of medical gauze, and a layer of non-irritating medical adhesive tape for fixation, and then the rats were placed on a thermostatic table at 37 °C until they awoke. After awakening, the rats were raised in single cages.
2.17.2 Wound size measurement
Photographs of the rats were taken by camera on the 0, 3, 7, 9, 12 and 14 days after surgery. The edges and the ranges of the wounds were measured by an analysis software (Image-J software). The amount of the wound closure was calculated by the following formula (5):
where A 0 is the initial wound area (t = 0) and A t is the wound area at each time point.
2.17.3 Histological, immunohistochemical and immunofluorescence analyses
Rats were killed on the 15th day postoperatively. The wound tissue was removed from the surrounding healthy skin, and the samples were fixed with formalin, dehydrated and embedded in paraffin. The 6 μm thick slices were immunohistochemically stained with H&E, Masson and CD31 respectively, and these slices were observed under a microscope to find out the angiogenesis in wound healing.
2.18 Statistical analysis
Data were analyzed using SPSS software, and all data were expressed as mean ± standard deviation (SD), and the experiments were performed at least in triplicate. Statistical analysis of differences between groups was performed using one-way ANOVA. Values of p < 0.05 were determined to indicate statistical significance (*p < 0.05, **p < 0.01, ***p < 0.001).
3 Results and discussion
3.1 Design strategy and structural characterization of FS/PAM-Gel-PDA hydrogels
FS/PAM-Gel-PDA hydrogels were prepared by a simple but environmentally friendly method, and the preparation process is shown in Fig. 1 DA was oxidized to PDA by self-polymerization under alkaline conditions and encapsulated on the surface of FS, which can effectively promote the dispersion and anchoring of the powder while inhibiting the oxidation of the ceramic powder.
The chemical compositions of hydrogel wound dressings are directly determined by FT-IR and XPS, which is a commonly used material analysis technique in the biomedical field. Figure 2b shows the FT-IR spectra of Gel, DA, FS, Gel-DA, PAM-Gel, PAM-Gel-PDA and FS/PAM-Gel-PDA. A broad absorption peak appears at 3287 cm−1 in the Gel spectrum, which is the overlap of the characteristic absorption peaks of -OH and -NH bonds (formed by hydrogen bond and amide bond). As the hydrogen bond strength is increased, the absorption band may migrate towards lower frequencies. The peaks at 1633 cm−1, 1538 cm−1, and 1236 cm−1 correspond to the -C = O stretching vibration peak of amide I band, the -NH bending vibration peak of amide II band, and the -C≡N stretching vibration peak of amide III band, respectively. The peak at 1610 cm−1 in the FT-IR spectrum of DA is the characteristic absorption peak of -C = C in the benzene ring of the DA molecule; The peak appearing at 1280 cm−1 is the characteristic absorption peak of the catechol structure of the DA molecule. After modification, the shapes of the absorption peaks of Gel-DA and Gel are roughly the same. However, after the reaction, the amino groups of Gel molecules will participate in the reaction to generate amide groups, which leads to the decrease of amino group content, but the increase of amide groups. From the infrared images, it is observed that the intensity ratio of the characteristic absorption peak of the amide II band at 1538 cm−1 to the characteristic absorption peak of the amide I band at 1236 cm−1 is decreased, which proves that DA is successfully grafted onto Gel. In the FT-IR spectrum of PAM-Gel, -NH stretching vibration characteristic peaks appear at 3000 cm−1–3500 cm−1, and the -C = O stretching vibration characteristic peak appears at 1645 cm−1. In the FT-IR spectra of FS, PAM-Gel-PDA, and FS/PAM-Gel-PDA, the characteristic absorption peaks of SiO4 2− are at 1050 cm−1. Due to the hygroscopicity of FS, the -OH stretching vibration peak can be observed at 3432 cm−1. From the Schiff base condensation reaction mechanism in Fig. 2a, it can be seen that the amino group of gelatin is bonded with the catechol group by Schiff base, and in the XPS spectra of Gel-DA (Fig. 2c), the appearance of -C = N bond confirms this result. Figure 2d and e show the N spectra of Gel-DA and PAM-Gel-PDA, respectively. Due to the introduction of DA and PAM, the amino and amide bonds are increased obviously, so that the peak areas of C-NH2 and -C = N are obviously increased. The pore structure of hydrogels maintains and controls nutrient and oxygen transport for cellular metabolism. Typically, hydrogel matrices allow several small molecular weight nutrients (e.g. glucose, oxygen) to diffuse easily, but pores smaller than 10 nm may restrict diffusion of certain proteins such as albumin and haemoglobin (with a Stokes radius of 3.1–3.5 nm and 2.4 nm, respectively). The effects of PDA and FS on the hydrogel morphology are investigated by a cryo-scanning electron microscopy. Cryo-SEM images show that PAM-Gel double-network hydrogels have a dense and uniform pore structure with the pore sizes of 20–30 μm (Fig. 2f). The pore size of the system was significantly reduced by the addition of dopamine (Fig. 2g). Due to the introduction of the chemical cross-linker Guanipine (GP) and the modifier DA, they are cross-linked with the Gel molecules and make the pores between the Gel molecules smaller. After the introduction of FS, the surface pores become very dense, but distinct powder particles can be observed (Fig. 2h). Because PDA was coated on the surface of the ceramic powder and crosslinked with Gel molecules, the high crosslinking density becomes higher. After the formation of a double-network structure with PAM, the pore structure becomes dense due to strong hydrogen bonding interactions [28, 33]. Transmission electron microscopy (TEM) shows that the powder morphology was a lumpy structure with uneven size (Fig. 2i). The distribution of elements Fe and Si at the cross-section of FS/PAM-Gel-PDA hydrogels was observed by Energy-dispersive X-ray spectroscopy (EDS) (Fig. 2j and k), and it can be seen that elements Fe and Si are uniformly distributed at the cross-section, which indicates that the powder has good dispersibility.
Structural characterization of PAM-Gel, PAM-Gel-PDA and FS/PAM-Gel-PDA hydrogels. a Preparation and bonding methods of FS/PAM-Gel-PDA hydrogels; b FT-IR images of Gel, DA, FS, Gel-DA, PAM-Gel, PAM-Gel-PDA, and FS/PAM-Gel-PDA hydrogels; c XPS of Gel; d XPS of Gel-DA; e XPS of PAM-Gel-PDA; f Cryo-SEM of PAM-Gel hydrogel (The scale bar is 5 μm); g Cryo-SEM of PAM-Gel-PDA hydrogel (The scale bar is 5 μm); h Cryo-SEM of FS/PAM-Gel-PDA hydrogel (The scale bar is 5 μm); i TEM of FS ceramic powder (The scale bar is 2 μm); j EDS of element Fe in FS/PAM-Gel-PDA hydrogels (The scale bar is 50 μm); k EDS of element Si in FS/PAM-Gel-PDA hydrogels (The scale bar is 50 μm)
3.2 Analyses of mechanical, hydrophilic and wet adhesion properties of FS/PAM-Gel-PDA hydrogels
The mechanical, rheological, and hydrophilic properties of FS/PAM-Gel-PDA were investigated. Good mechanical properties and flexibility are of great significance for ideal wound dressing applications [41, 42]. The tensile properties of different hydrogels were studied by means of stress–strain curves (Fig. 3c). With the introduction of PDA and FS, the elasticity of hydrogels was decreased, but the toughness was increased. Meanwhile, the rheological results of oscillation frequency conversion (Fig. 3d) show that the energy storage modulus (G′) dominates in the range of strain amplitude from 0.1 to 100. With the addition of DA and FS, the loss modulus (G'') was gradually increased, indicating that its viscosity was increased. The G′ value of FS/PAM-Gel-PDA is the highest, which indicates that its strength is higher. The tensile deformation results also showed that the addition of DA and FS resulted in increased toughness and low deformation. Whereas, the deformation amount of FS/PAM-Gel-PDA (Gly) was the most affected, resulting in a relatively high modulus. The reason for the change in mechanical properties is that the catechol groups form strong hydrogen bonds inside the conjugates and the network becomes dense, which improves its mechanical properties. Genipin with a variety of active groups such as ester group and hydroxyl group [43], a natural chemical cross-linking agent, have been widely used for designing polymer network with sufficient strength, degradability and biocompatibility by cross-linking reaction with biomolecules (such as gelatin, chitosan, etc.) with primary amine structure to realize monomolecular or multimolecular cross-linking. Therefore, the rigidity of the gelatin network was effectively improved by chemical cross-linking of Genipin, giving it good toughness. For wet adhesive materials, hydrophilicity not only helps to destroy the hydration layer between the material and the substrate, and improve the tissue adhesion properties of the material, but also promotes cell adhesion and growth. The contact angles of PAM-Gel, PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) were determined by a dynamic contact angle tester. As can be seen from Fig. 3e, the contact angles of PAM-Gel, PAM-Gel-PDA(Water) and PAM-Gel-PDA(Gly) are 68.0 ± 3°, 69.2 ± 1.5°, and 64.7 ± 1.7°, which are all less than 90°, indicating that PAM-Gel, PAM-Gel-PDA (Water), and PAM-Gel-PDA (Gly) belong to hydrophilic materials. While the contact angles of FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) are 79.5 ± 3.9° and 73.58 ± 4.36°, respectively, which shows that the hydrophilicity of the system was decreased slightly with the introduction of powder materials, but it still has a certain hydrophilicity.
Property characterization of PAM, PAM-Gel, PAM-Gel-PDA and FS/PAM-Gel-PDA hydrogels. a Schematic diagram of adhesion mechanism; b Schematic diagram of a lap shear model; c Tensile curves of PAM, PAM-Gel, PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water), and FS/PAM-Gel-PDA (Gly) hydrogels; (d) G' and G'' of PAM-Gel, PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water), and FS/PAM-Gel-PDA (Gly) hydrogels; e Water contact angles of Gel, Gel-DA, PAM, PAM-Gel, PAM-Gel-PDA and FS/PAM-Gel-PDA hydrogels; f Physical image of PAM-Gel-PDA hydrogel on porcine myocardial tissue after bending, twisting, water immersion and stretching operations; g Wet adhesion properties of PAM-Gel-PDA (Water) hydrogels with different dopamine contents; h Wet adhesion properties of PAM-Gel-PDA hydrogels with different ratios of water to glycerin; i Wet adhesion properties of PAM-Gel-PDA (Gly) hydrogels with different dopamine contents; j Wet adhesion properties of FS/PAM-Gel-PDA (Gly) hydrogels with different FS contents
Good swelling performance is an important parameter to maintain wound wetting properties. As shown in Fig. S1, PAM-Gel, PAM-Gel-PDA (Gly) and FS/PAM-Gel-PDA (Gly) all exhibited a low swelling rate, indicating that their structures were more stable, and water absorption and swelling would not significantly affect the adhesion properties of the hydrogels. Fig. S2 shows the biodegradability of PAM-Gel, PAM-Gel-PDA (Gly) and FS/PAM-Gel-PDA (Gly), where gelatin was broken down under the action of type IV collagenase, which damaged the network structure of the hydrogel. At the same time, the damaged part gradually dissociated, leading to the loss of hydrogel quality. It can be seen that its overall performance is similar to that of dissolution, while FS/PAM-Gel-PDA (Gly) has a suitable biodegradation rate with a 45% mass loss after 14 d.
The wet adhesion of hydrogels plays an important role in wound closure, hemostasis and promotion of wound tissue healing [36, 44]. The wet adhesion properties of FS/PAM-Gel-PDA on porcine skin and porcine heart in the wet state were investigated by constructing a lap shear model (Fig. 3b). As shown in Fig. 3g, the wet adhesion of PAM-Gel-PDA was gradually increased to 15.75 kPa with the increase of the introduced PDA content. After the content of DA/AM reaches 0.8%, the wet adhesion was substantially decreased, because of the higher content of free DA, they will phagocytize the generated active free radicals, resulting in the inability of AM to polymerize and the decline of wet adhesion. In order to further improve the wet adhesion of the hydrogels and increase the storage time of the hydrogels, glycerol (Gly) was introduced into the solvent, and its wet adhesion was detected by changing the ratio of glycerol to water in the solvent. As shown in Fig. 3h, when the ratio of water to glycerol is 1:1, the wet adhesion is optimal, reaching 35.9 kPa. Afterwards, the wet adhesion of the DA gradient was also measured, when the ratio of water to glycerol in the solvent is 1:1, as shown in Fig. 3i. Although the wet adhesion properties of hydrogels with different DA contents are greatly improved, when the content is 0.6%, the wet adhesion property is still the best. The high adhesion strength of FS/PAM-Gel-PDA hydrogels to tissues is attributed to hydrogen bonding and covalent interactions as well as high toughness (Fig. 3a). Direct contact between the material and the tissue is achieved by adding glycerol to the solvent to destroy the hydration layer between the hydrogel dressings and the wet tissues. While the hydrogel contains a large number of amides, hydroxyl groups and carbonyl groups, and the surface of the biological tissue contains -CONH and -OH groups. Therefore, hydrogen bonding interactions can be formed between the hydrogel and the porcine skin. Under the conditions of containing APS radicals and O2, some of the catechol groups of DA were oxidized to active quinones, which easily forms covalent bonds C = N and -S-C with the nucleophilic -NH2 and -SH groups in the tissue via Schiff base and Michael addition reaction, respectively [44]. After introducing FS coated with PDA, it was found that the wet adhesion shows a downward trend with the increase of FS content (Fig. 3j), but when FS/AM mass ratio of 0.15%, the wet adhesion performance was 21.78 kPa, which is still in line with the National Standard for the use of wound dressings. The reason for the decrease of its properties may be due to the fact that the addition of FS leads to a decrease in the cross-linking degree of PAM, and at the same time, the addition of FS will consume a part of PDA, which leads to the decrease of wet adhesion. Figure 3f shows that the PAM-Gel-PDA hydrogel can still be stably adhered to porcine myocardial tissues after a series of operations, such as bending, twisting, soaking in water, and stretching, which suggests that it has the prospect of application in vivo.
3.3 Analyses of ion release behavior in vitro and biocompatibility of FS/PAM-Gel-PDA hydrogels
Bioactive ceramics, due to their good biocompatibility, are absorbed and dissolved by the organism upon contact with body fluids, allowing the release of bioactive ions within them into the wound area. Thus, stable and reliable ion release plays an important role in promoting wound healing [22]. The ion release behavior of FS/PAM-Gel-PDA hydrogel was determined by inductively coupled plasma mass spectrometry (ICP). Figure 4b partially summarizes the recent studies on the wet adhesion strength of gelatin-based hydrogels and the ion release behavior of ion-doped ceramic powders [45, 46], and compares the adhesion strength requirements for hydrogel wound dressings in the national standard (above 10 kPa) and the optimal range of bioactive concentration for ion release (0.5–12 ppm), and finds that most of the hydrogel dressings can meet the minimum requirements, but some of the hydrogel dressings with excessively high adhesion strength and ion release may cause secondary injury to the wound and residual excessive metal ionic appendages, so it is very necessary to achieve the optimal adhesion strength and stable and sustainable ion release [47,48,49,50]. The results in Fig. 4c show that the Fe and Si ion content of the FS/PAM-Gel-PDA hydrogel was 0.154 and 0.608 ppm, respectively, after soaking in PBS solution for 24 h, which is in accordance with the national standard for heavy metal ions contained in wound dressings. By measuring the release of Fe and Si ions at 1, 3, 5, 7, 9, 12 and 14 days, it can be seen that FS/PAM-Gel-PDA hydrogel can achieve stable and sustainable ion release, and the wound has basically healed after 14 days, at which time the release of iron ions and silicon ions were 4.473 and 10.595 ppm, respectively, which is a level that does not lead to heavy metal poisoning. And studies have shown that Si ions, due to their favorable biological activity, were released in living organisms and do not lead to their toxicity [13]. Owing to the different biological activities of Fe2+ and Fe3+, the types of iron ions in the solution were determined by KMnO4 chromogenic reaction. The discoloration of the KMnO4 solution indicates that the released ions are Fe2+. Because DA oxidizes and self-polymerizes into PDA nanoparticles under alkaline conditions, DA was coated on the surface of the ceramic powder to produce an antioxidant layer which can inhibit the oxidation of Fe2+. At the same time, the PDA nanoparticle layer slows down the penetration of the organism's body fluids, which slows down the dissolution of FS and achieves a stable release of Fe2+ and SiO4 4− (Fig. 4a).
Ion release behavior and biocompatibility of PAM-gel, PAM-Gel-PDA and FS/PAM-Gel-PDA hydrogels. a Schematic diagram of ion release mechanism; b Comparative chart of bibliographic data; c Ion release rates of Fe and Si in FS/PAM-Gel-PDA; The optical density (OD) values at 450 nm (d) and cell viability (e) of HUVECs were assessed by MTT assay; The optical density (OD) values at 450 nm (f) and cell viability (g) of L929s were assessed by MTT assay; The confocal laser scanning microscope of HUVECs (h) and L929s (i) cells cultured with hydrogels for the 1st, 3rd, and 5th days. The scale bar is 100 μm. n = 5, *p < 0.05, **p < 0.01, ***p < 0.001
The good biocompatibility of hydrogel wound dressings is the basic property for biological applications [51,52,53]. The co-culture experiment of methyl thiazolyl tetrazole (MTT) and fluorescent staining for living/dead cells recorded by a confocal laser scanning microscope was adopted to prove the biocompatibility of FS/PAM-Gel-PDA, where HUVECs and L929s represent the levels of vascular growth capacity and immunotoxicity, respectively. The number of living cells was judged by the measured absorbance value (OD). The higher the OD value, the greater the number of cells or the stronger the activity. As shown in Fig. S3a, Fig. 4d and f, China hamster lung cells (CHLs) were co-cultured with PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water), and FS/PAM-Gel-PDA (Gly) extracts for 5 days, respectively, according to the MTT assay. Compared with the control group, there is no significant difference in the OD values, indicating that their cellular activities are similar. In contrast, the OD values of FS/PAM-Gel (Gly) were found to be significantly lower than those of the control group by co-culturing Human umbilical vein endothelial cells (HUVECs) and L929 mouse fibroblast cells (L929s) cells with PAM-Gel (Gly), FS/PAM-Gel-PDA (Gly), and FS/PAM-Gel (Gly) for five days, which indicated that there might be cytotoxicity of FS/PAM-Gel (Gly). According to Fig. S3b, Fig. 4e and g, after 5 days of culture, the cell survival rates of PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly) and FS/PAM-Gel-PDA (Gly) groups all exceed 100%, which proves that PAM-Gel-PDA and FS/PAM-Gel-PDA have excellent biocompatibility, and both of them have the properties to promote cell growth and propagation. In contrast, the cell activity plots of HUVECs and L929s cells with PAM-Gel (Gly), FS/PAM-Gel-PDA (Gly), and FS/PAM-Gel (Gly) demonstrated that FS/PAM-Gel (Gly) was significantly toxic, suggesting that Fe2+ released from FS without PDA coating during cell culture does oxidize to Fe3+ producing toxicity and leading to a significant decrease in cell activity. Fluorescence double staining for living and dead cells was performed using calcein and propidium iodide (PI), respectively. After co-cultured with PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water), FS/PAM-Gel-PDA (Gly) and FS/PAM-Gel (Gly) extracts, CHLs, HUVECs and L929s were stained with calcein/PI, and the fluorescence images of the co-cultured living/dead cells were observed by confocal laser scanning microscope. As can be seen in Fig. S4, Fig. 4h and i, a small number of red dead cells occur after 5 days of co-culture, but the number of green living cells is still much higher than that of the control group (the living cells show green fluorescence while the dead cells show red fluorescence), proving that PAM-Gel-PDA and FS/PAM-Gel-PDA have excellent biocompatibility, which is consistent with the results obtained by the MTT assay.
4 Pro-cell migration ability of FS/PAM-Gel-PDA hydrogels
It is commonly believed that stimulation of cell migration during the wound healing process contributes to wound healing, and it was found by cell migration assay that hydrogels coated with FS by PDA significantly promoted the cell migration of HUVECs (Fig. 5a, b) and L929s (Fig. 5c, d), whereas FS without PDA coating had an inhibitory effect, which suggests that active factors released from PDA-coated FS can effectively stimulate the cell migration and do not produce toxic components. The concentration of VEGF in the cells was determined by enzyme-linked immunosorbent assay (Elisa) (Fig. 5e, f), and the concentration of human vascular endothelial growth factor (VEGF) in the cell supernatant of the FS/PAM-Gel-PDA (Gly) group was much higher than that of the control group after 5 days of incubation, which indicated that FS/PAM-Gel-PDA (Gly) could promote the expression of VEGF. Immunofluorescence detection also confirmed the experimental results of Elisa, and the Elisa standard curve is shown in Fig. S9.
Effect of hydrogels on cell migration capacity. Cell mobility (a) and images (b) of PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Gly) and FS/PAM-Gel (Gly) hydrogels on HUVECs; Cell mobility (c) of PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Gly) and FS/PAM-Gel ( Gly) hydrogels for cell mobility (c) and images (d) of L929s (The scale bar is 100 μm); VEGF secretion from HUVECs after 24 h co-culture with hydrogels (e); Immunofluorescence staining images of vascular endothelial growth factor after 24 h co-culture with hydrogel (f) (The scale bar is 25 μm). n = 5, *p < 0.05, **p < 0.01, ***p < 0.001
5 Hemostatic analyses in vivo of FS/PAM-Gel-PDA hydrogels
Hydrogels have excellent adhesion properties and can effectively adsorb red blood cells and platelets, so they can be used as hemostatic dressings. The hemostatic effects of hydrogels were evaluated on the animal models of induced liver injury (Fig. 6a, b, c). In this model, the hemostasis time of all wounds varied according to the use of different hydrogel dressings: control (210 s) > FS/PAM-Gel-PDA (Water) (98 s) > FS/PAM-Gel-PDA (Gly) (70 s) > PAM-Gel-PDA (Water) (55 s) > PAM-Gel-PDA ( Gly) (47 s); moreover, the magnitude of blood loss caused by each group of samples was: control (2.41 g) > FS/PAM-Gel-PDA (Water) (0.69 g) > FS/PAM-Gel-PDA (Gly) (0.63 g) > PAM-Gel-PDA (Water) (0.59 g) > PAM-Gel- PDA (Gly) (0.48 g). Firstly, the excellent wet adhesion of the hydrogel ensures that it adheres firmly to the bleeding site and rapidly absorbs moisture from the blood into the hydrophilic network, thereby promoting blood coagulation. Secondly, the catechol of PDA promotes the aggregation and adhesion of red blood cells (RBC) by interacting with nucleophilic reagents on blood proteins [27, 44, 54]. Thirdly, GP contained in the hydrogel can significantly inhibit thrombin-induced exocytosis of human umbilical vein endothelial cells and the expression of adhesion molecules, and markedly inhibit the adhesion of endothelial cells and monocytes [55]. To sum up, the hemostatic properties of PAM-Gel-PDA(Gly) were made optimal (Fig. 6d). However, the addition of powder reduces the wet adhesion and hydrophilic properties of the system, which results in the poor hemostatic effect of FS/PAM-Gel-PDA (Gly), but it is still much better than that of the control group. By analyzing the hemolysis rate of the prepared hydrogels (Fig. 6e), the hemolysis rate of all hydrogels was less than 5%, indicating that they have good vascular compatibility.
Hemostasis experiments in the rat liver model. a Blood loss images of the lethal liver defect model; b Hemostatic time of different materials under the lethal liver defect model; c Blood loss volume of different materials under the lethal liver defect model; d Schematic diagram of hemostatic mechanism; e Hemolysis rate of different materials. n = 5, *p < 0.05, **p < 0.01
6 Wound healing in vivo with FS/PAM-Gel-PDA hydrogels
Wound healing is a complex process, including several overlapping stages: hemostasis, inflammation, angiogenesis, fibrous tissue proliferation, re-epithelization, wound contraction and tissue reconstruction. The hydrogel designed by us can effectively adhere to the wet and irregular wound surface and effectively promote wound healing through stable ion release. The effects of the induction and promotion of PAM-Gel-PDA (Water), PAM-Gel-PDA (Gly), FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) on tissue wound healing were evaluated by establishing a model for evaluating full-thickness skin defects of rats with specific pathogens free (SPF). Figure 7 shows the images of the full-thickness skin defect wounds in the rats with SPF treated with different remedy methods after the 0, 3, 7, 9, 12 and 14 days. During the whole postoperative period, no adverse reactions occur in all animal groups. Figure 7a shows a schematic diagram of the healing process of the hydrogel-treated wounds, and the wound areas of all experimental groups become progressively smaller over time in all test groups. In addition, the wound healing rate of rats in FS/PAM-Gel-PDA (Water) and FS/PAM-Gel-PDA (Gly) groups is much higher, and the order of the healing rates in each group is FS/PAM-Gel-PDA (Gly) group > FS/PAM-Gel-PDA (Water) group > PAM-Gel-PDA (Gly) group > PAM- Gel-PDA (Water) group (Fig. 7b). The results show that hydrogel can promote wound healing, and the FS/PAM-Gel-PDA (Gly) hydrogel can achieve the most satisfactory wound healing, with only 2.0% of the wound area remaining to be essentially healed after 14 days. Because the glycerol added to the FS/PAM-Gel-PDA (Gly) hydrogel ensures that the water content of the hydrogel dressing does not obviously decrease over time, so that FS can stably release the ions promoting blood vessel regeneration.
Effects of hydrogels on full-thickness wound healing in the rat model in vivo. a The wound healing is roughly observed with different treatment methods on the 0, 3, 7, 9, 12 and 14 days; b Wound area closure rates for different methods of wound management; Wound slices treated with different hydrogels were stained with H&E (c) (The scale bar is 50 μm), Masson (d) (The scale bar is 50 μm), CD31 (e) (The scale bar is 200 μm) after 3、7、14 days. n = 6, *p < 0.05, **p < 0.01
In order to further assess the ability of PAM-Gel-PDA and FS/PAM-Gel-PDA to promote healing, tissue slices of the wounds were analyzed by H&E and Masson staining, and CD31 immunohistochemistry on the 15th day (Fig. 7c, d, e). Different expression intensities of blood vessels (red arrows), fibroblasts (yellow arrows) and collagen deposition (orange arrows) in the wound area were evaluated. After wound healing, the basic structures of epithelium and dermis were formed in the control group. Moreover, the number of cells is large, and the collagen fiber bundles are wavy with a slender morphology, and were arranged parallel to the epidermis. There is an inflammatory response, minimal collagen formation and deposition, and a smaller number of hair follicles and angiogenesis. However, in the intervention group, the cell content in the tissue is decreased, the collagen fiber bundles are thick, the morphology is curly, and the overall morphology is close to that of normal skin tissue. The wounds treated with FS/PAM-Gel-PDA have thicker epithelial and dermal tissues, less inflammation, and more hair follicles and blood vessels. In addition, they also exhibit higher collagen deposition and compact collagen growth, indicating that FS/PAM-Gel-PDA group can significantly promote wound healing by promoting the proliferation of fibroblasts and keratinocytes, the formation of granulation tissues, and re-epithelization. The results are consistent with those of H&E, Masson, CD31 staining. Among them, CD31 immunohistochemical staining show the number of mature blood vessels in each group (Fig. S10), and by statistically analyzing them, the number of CD31 positives was higher in all sample groups compared with the control group, and the highest number of positives was found in the FS/PAM-Gel-PDA (Gly) group, which was attributed to the release of Fe2+ stimulating the formation of mature blood vessels. According to the comprehensive hemostatic results in rats, the results of whole cortex wound healing experiments and cell migration assay in rats, FS/PAM-Gel-PDA has an effective hemostatic effect, significant angiogenesis, collagen fibrillogenesis, and good cell migration promotion, which can effectively promote the wound healing process [44, 56].
7 Conclusion
In order to improve the insufficient bioactivity of bioactive ceramic hydrogel, the instability of silicate ion release, and the lower wet adhesion on the wound surface with tissue exudate, a novel double-network hydrogel wound dressing composited gelatin with silicate ceramic powder was prepared by combining Gel, PAM, PDA and ferrous silicate ceramic powder through Schiff base bond and hydrogen bond in this paper. By cross-linking gelatin, which contains a large number of active groups with good cell affinity and adhesion, with dopamine, it not only improves the biocompatibility of the system, wet adhesion properties, but also inhibits the oxidation of Fe2+ in the ceramic powder and realizes the stable release of Fe2+ and SiO4 4− in the system, which gives it a good pro-vascular regeneration ability. Meanwhile, the introduction of glycerol destroys the hydration layer between the material and the tissue, so that the catechol groups in the system are in direct contact with the tissue, which greatly improves the wet adhesion properties of the material. In vivo experiments confirm that the composite hydrogel can adhere to wet skin tissues, promote the formation of blood vessels, and accelerate the wound healing through the stable release of bioactive ions, such as Fe2+ and SiO4 4−. The results of various experiments verify that dopamine can inhibit the oxidation of divalent iron ions in ceramic powders and endow the hydrogels with strong wet adhesion properties to achieve the stable release of bioactive ions, and that the prepared double-network hydrogel wound dressing composited gelatin with silicate ceramic powder has a promising clinical application in the field of wound repair.
Availability of data and materials
All the data supporting the findings of this study are included in this manuscript.
Abbreviations
- Gel:
-
Gelatin
- AM:
-
Acrylamide
- DA:
-
Dopamine
- PDA:
-
Polydopamine
- FS:
-
Ferrous silicate ceramic powder
- APS:
-
Ammonium persulfate
- MBA:
-
N-N methylene bisacrylamide
- TEMED:
-
Tetramethylethylenediamine
- GP:
-
Genipin
- FT-IR:
-
Fourier transform infrared spectrometer
- XPS:
-
X-ray photoelectron spectroscop
- Cryo-SEM:
-
Cryo-scanning electron microscopy
- ICP-OES:
-
Inductively coupled plasma atomic emission spectrometry
- CHLs:
-
China hamster lung cells
- HUVECs:
-
Human umbilical vein endothelial cells
- L929s:
-
Mouse fibroblasts
- CLSM:
-
Confocal laser scanning microscope
- Gly:
-
Glycerol
- TEM:
-
Transmission electron microscopy
- MTT:
-
Methyl thiazolyl tetrazole
References
Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration, Pharmaceutics. 2020;12:2080735.
Luo X, Liu Y, Qin R, Ao F, Wang X, Zhang H, Yang M, Liu X. Tissue-nanoengineered hyperbranched polymer based multifunctional hydrogels as flexible “wounded treatment-health monitoring” bioelectronic implant. Applied Materials Today. 2022;29:101576.
Qi L, Zhang C, Wang B, Yin J, Yan S. Progress in Hydrogels for Skin Wound Repair. Macromol Biosci. 2022;22:e2100475.
Hu S, Yang Z, Zhai Q, Li D, Zhu X, He Q, Li L, Cannon RD, Wang H, Tang H, Ji P, Chen T. An All-in-One “4A Hydrogel”: through First-Aid Hemostatic, Antibacterial, Antioxidant, and Angiogenic to Promoting Infected Wound Healing. Small. 2023;19:e2207437.
Luo X, Yin C, Ji L, Feng J, Zhang P, Wang X, Ma Y, Liu X. An Antimicrobial Polymer Brush Coating to Fabricate High-Performance, Durable, Self-Sterilization, and Recyclable Face Masks. Chemistry of Materials. 2023;(21):35.
Zeng Q, Qi X, Shi G, Zhang M, Haick H. Wound Dressing: From Nanomaterials to Diagnostic Dressings and Healing Evaluations. ACS Nano. 2022;16:1708–33.
Ghosal K, Agatemor C, Špitálsky Z, Thomas S, Kny E. Electrospinning tissue engineering and wound dressing scaffolds from polymer-titanium dioxide nanocomposites. Chem Eng J. 2019;358:1262–78.
Xu X, Jerca VV, Hoogenboom R. Bioinspired double network hydrogels: from covalent double network hydrogels via hybrid double network hydrogels to physical double network hydrogels. Mater Horiz. 2021;8:1173–88.
Cao X, Liu H, Yang X, Tian J, Luo B, Liu M. Halloysite nanotubes@polydopamine reinforced polyacrylamide-gelatin hydrogels with NIR light triggered shape memory and self-healing capability. Composites Science and Technology. 2020;191:108071.
Dong H, Wang L, Du L, Wang X, Li Q, Wang X, Zhang J, Nie J, Ma G. Smart Polycationic Hydrogel Dressing for Dynamic Wound Healing. Small. 2022;18:e2201620.
X. Liu, B. Cui, X. Wang, M. Zheng, Z. Bai, O. Yue, Y. Fei, H. Jiang, Nature-Skin-Derived e-Skin as Versatile “Wound Therapy-Health Monitoring” Bioelectronic Skin-Scaffolds: Skin to Bio-e-Skin, Adv Healthc Mater, (2023) e2202971.
Yuen HY, Bei HP, Zhao X. Underwater and wet adhesion strategies for hydrogels in biomedical applications. Chemical Engineering Journal. 2022;431:133372.
Li H, Chang J. Stimulation of pro-angiogenesis by calcium silicate bioactive ceramic. Acta Biomater. 2013;9:5379–89.
Gong F, Yang N, Xu J, Yang X, Wei K, Hou L, Liu B, Zhao H, Liu Z, Cheng L. Calcium Hydride-Based Dressing to Promote Wound Healing. Adv Healthc Mater. 2023;12:e2201771.
Fan C, Xu Q, Hao R, Wang C, Que Y, Chen Y, Yang C, Chang J. Multi-functional wound dressings based on silicate bioactive materials. Biomaterials. 2022;287:121652.
Wang X, Ye M, Shen J, Li J, Li Y, Bao Z, Chen H, Wu T, Shen M, Zhong C, Yang X, Gou Z, Zhao S, Xu S. Core-shell-typed selective-area ion doping wollastonite bioceramic fibers enhancing bone regeneration and repair in situ. Applied Materials Today. 2023;32:101849.
Han Y, Li Y, Zeng Q, Li H, Peng J, Xu Y, Chang J. Injectable bioactive akermanite/alginate composite hydrogels for in situ skin tissue engineering. J Mater Chem B. 2017;5:3315–26.
Macha IJ, Sufi S. Novel slow drug release bioceramic composite materials for wound dressing applications: potential of natural materials. SN Applied Sciences. 2020;2:1977.
Yu Q, Sun H, Yue Z, Yu C, Jiang L, Dong X, Yao M, Shi M, Liang L, Wan Y, Zhang H, Yao F, Li J. Zwitterionic Polysaccharide-Based Hydrogel Dressing as a Stem Cell Carrier to Accelerate Burn Wound Healing. Adv Healthc Mater. 2023;12:e2202309.
Saghiri MA, Asatourian A, Orangi J, Sorenson CM, Sheibani N. Functional role of inorganic trace elements in angiogenesis-Part II: Cr, Si, Zn, Cu, and S. Crit Rev Oncol Hematol. 2015;96:143–55.
Luo X, Wang J, Zhang P, Feng J, Meng X, Li K, Yin C, Wang P, Ji L, Liu Y, Qiao Z. “Rooting” engineering strategy for construction of highly stable, scalable, size-customized oxidized graphene/PA6 ultrafine fiber composite nanofiltration membranes for water purification. Surfaces and Interfaces. 2024;45:103879.
Tanwar A, Date P, Ottoor D. ZnO NPs incorporated gelatin grafted polyacrylamide hydrogel nanocomposite for controlled release of ciprofloxacin. Colloid and Interface Science Communications. 2021;42:100413.
Zhang Z, Li W, Liu Y, Yang Z, Ma L, Zhuang H, Wang E, Wu C, Huan Z, Guo F, Chang J. Design of a biofluid-absorbing bioactive sandwich-structured Zn-Si bioceramic composite wound dressing for hair follicle regeneration and skin burn wound healing. Bioact Mater. 2021;6:1910–20.
Zong S, Lv H, Liu C, Zhu L, Duan J, Jiang J. Mussel inspired Cu-tannic autocatalytic strategy for rapid self-polymerization of conductive and adhesive hydrogel sensors with extreme environmental tolerance, Chemical Engineering Journal. 2023;465:142831.
Y. Zhang, X. Li, Z. Zhang, H. Li, D. Chen, Y. Jiao, C. Fan, Z. Zeng, J. Chang, Y. Xu, B. Peng, C. Yang, Y. Que, Zn2SiO4 Bioceramic Attenuates Cardiac Remodeling after Myocardial Infarction, Adv Healthc Mater, (2023) e2203365.
Sheng L, Zhang Z, Zhang Y, Wang E, Ma B, Xu Q, Ma L, Zhang M, Pei G, Chang J. A novel “hot spring”-mimetic hydrogel with excellent angiogenic properties for chronic wound healing. Biomaterials. 2021;264:120414.
Lv Y, Cai F, He Y, Li L, Huang Y, Yang J, Zheng Y, Shi X. Multi-crosslinked hydrogels with strong wet adhesion, self-healing, antibacterial property, reactive oxygen species scavenging activity, and on-demand removability for seawater-immersed wound healing. Acta Biomater. 2023;159:95–110.
Han L, Yan L, Wang K, Fang L, Zhang H, Tang Y, Ding Y, Weng L, Xu J, Weng J, Liu Y, Ren F, Lu X. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Materials. 2017;9:e372–e372.
Gao Y, Chen J, Han X, Pan Y, Wang P, Wang T, Lu T. A Universal Strategy for Tough Adhesion of Wet Soft Material. Advanced Functional Materials. 2020;30:03207.
Xiang L, Cui W. Biomedical application of photo-crosslinked gelatin hydrogels. Journal of Leather Science and Engineering. 2021;3:43.
Shi S, Wang L, Song C, Yao L, Xiao J. Recent progresses of collagen dressings for chronic skin wound healing. Collagen and Leather. 2023;5:1364.
Deng G, Huang K, Jiang X, Wang K, Song Z, Su Y, Li C, Zhang S, Wang S, Huang Y. Developments for collagen hydrolysates as a multifunctional antioxidant in biomedical domains. Collagen and Leather. 2023;5:1319.
Chen Q, Pei Y, Tang K, Albu-Kaya MG. Structure, extraction, processing, and applications of collagen as an ideal component for biomaterials - a review. Collagen and Leather. 2023;5:1275.
Perez V, Felix M, Romero A, Guerrero A. Influence of the processing variables on the microstructure and properties of gelatin‐based scaffolds by freeze‐drying. Journal of Applied Polymer Science. 2019;136:47671.
Mushtaq F, Raza ZA, Batool SR, Zahid M, Onder OC, Rafique A, Nazeer MA. Preparation, properties, and applications of gelatin-based hydrogels (GHs) in the environmental, technological, and biomedical sectors. Int J Biol Macromol. 2022;218:601–33.
Wu T, Cui C, Huang Y, Liu Y, Fan C, Han X, Yang Y, Xu Z, Liu B, Fan G, Liu W. Coadministration of an Adhesive Conductive Hydrogel Patch and an Injectable Hydrogel to Treat Myocardial Infarction. ACS Appl Mater Interfaces. 2020;12:2039–48.
An H, Gu Z, Zhou L, Liu S, Li C, Zhang M, Xu Y, Zhang P, Wen Y. Janus mucosal dressing with a tough and adhesive hydrogel based on synergistic effects of gelatin, polydopamine, and nano-clay. Acta Biomater. 2022;149:126–38.
Han L, Lu X, Liu K, Wang K, Fang L, Weng LT, Zhang H, Tang Y, Ren F, Zhao C, Sun G, Liang R, Li Z. Mussel-Inspired Adhesive and Tough Hydrogel Based on Nanoclay Confined Dopamine Polymerization. ACS Nano. 2017;11:2561–74.
Hou Y, Li Y, Li Y, Li D, Guo T, Deng X, Zhang H, Xie C, Lu X. Tuning Water-Resistant Networks in Mussel-Inspired Hydrogels for Robust Wet Tissue and Bioelectronic Adhesion. ACS Nano. 2023;17:2745–60.
Lee SS, Kim HD, Kim SHL, Kim I, Kim IG, Choi JS, Jeong J, Kim JH, Kwon SK, Hwang NS. Self-Healing and Adhesive Artificial Tissue Implant for Voice Recovery. ACS Appl Bio Mater. 2018;1:1134–46.
Qiao Z, Mieles M, Ji HF. Injectable and moldable hydrogels for use in sensitive and wide range strain sensing applications. Biopolymers. 2020;111:e23355.
Yang K, Zhou X, Li Z, Wang Z, Luo Y, Deng L, He D. Ultrastretchable, Self-Healable, and Tissue-Adhesive Hydrogel Dressings Involving Nanoscale Tannic Acid/Ferric Ion Complexes for Combating Bacterial Infection and Promoting Wound Healing. ACS Appl Mater Interfaces. 2022;14:43010–25.
Muzzarelli RAA. Genipin-crosslinked chitosan hydrogels as biomedical and pharmaceutical aids. Carbohyd Polym. 2009;77:1–9.
Fan X, Wang S, Fang Y, Li P, Zhou W, Wang Z, Chen M, Liu H. Tough polyacrylamide-tannic acid-kaolin adhesive hydrogels for quick hemostatic application. Mater Sci Eng C Mater Biol Appl. 2020;109:110649.
Ahmed A, Nath J, Baruah K, Rather MA, Mandal M, Dolui SK. Development of mussel mimetic gelatin based adhesive hydrogel for wet surfaces with self-healing and reversible properties. Int J Biol Macromol. 2023;228:68–77.
Zandi N, Dolatyar B, Lotfi R, Shallageh Y, Shokrgozar MA, Tamjid E, Annabi N, Simchi A. Biomimetic nanoengineered scaffold for enhanced full-thickness cutaneous wound healing. Acta Biomater. 2021;124:191–204.
Yuan Y, Zhang Z, Mo F, Yang C, Jiao Y, Wang E, Zhang Y, Lin P, Hu C, Fu W, Chang J, Wang L. A biomaterial-based therapy for lower limb ischemia using Sr/Si bioactive hydrogel that inhibits skeletal muscle necrosis and enhances angiogenesis. Bioact Mater. 2023;26:264–78.
Y. Qiu, J. Tian, S. Kong, Y. Feng, Y. Lu, L. Su, Y. Cai, M. Li, J. Chang, C. Yang, X. Wei, SrCuSi4O10/GelMA Composite Hydrogel-Mediated Vital Pulp Therapy: Integrating Antibacterial Property and Enhanced Pulp Regeneration Activity, Adv Healthc Mater, (2023) e2300546.
Yang Z, Zhao F, Zhang W, Yang Z, Luo M, Liu L, Cao X, Chen D, Chen X. Degradable photothermal bioactive glass composite hydrogel for the sequential treatment of tumor-related bone defects: From anti-tumor to repairing bone defects. Chemical Engineering Journal. 2021;419:129520.
Chen Y, Udduttula A, Xie X, Zhou M, Sheng W, Yu F, Weng J, Wang D, Teng B, Manivasagam G, Zhang JV, Ren P-G, Kang B, Zeng H. A novel photocrosslinked phosphate functionalized Chitosan-Sr5(PO4)2SiO4 composite hydrogels and in vitro biomineralization, osteogenesis, angiogenesis for bone regeneration application. Composites Part B: Engineering. 2021;222:109057.
Pokhrel LR, Williams F, Cook PP, O’Rourke D, Murray G, Akula SM. Preclinical efficacy and safety of novel SNAT against SARS-CoV-2 using a hamster model. Drug Deliv Transl Res. 2022;12:3007–16.
Zhang B, He Y, Liu J, Shang J, Chen C, Wang T, Chen M, Li Y, Gong G, Fang J, Zhao Z, Guo J. Advancing collagen-based biomaterials for oral and craniofacial tissue regeneration. Collagen and Leather. 2023;5:120.
Li Y, Xiong J, Hu Y, Miao W, Huang H. Wrapping collagen-based nanoparticle with macrophage membrane for treating multidrug-resistant bacterial infection. Journal of Leather Science and Engineering. 2022;4:1062.
Song F, Kong Y, Shao C, Cheng Y, Lu J, Tao Y, Du J, Wang H. Chitosan-based multifunctional flexible hemostatic bio-hydrogel. Acta Biomater. 2021;136:170–83.
Luo X, Ao F, Huo Q, Liu Y, Wang X, Zhang H, Yang M, Ma Y, Liu X. Skin-inspired injectable adhesive gelatin/HA biocomposite hydrogel for hemostasis and full-thickness dermal wound healing. Biomater Adv. 2022;139:212983.
Wang Y, Xiao D, Quan L, Chai H, Sui X, Wang B, Xu H, Mao Z. Mussel-inspired adhesive gelatin-polyacrylamide hydrogel wound dressing loaded with tetracycline hydrochloride to enhance complete skin regeneration. Soft Matter. 2022;18:662–74.
Acknowledgements
We sincerely acknowledge the funding and generous support of National Natural Science Foundation of China (22278259 and 22278257), The Key R&D Program of Shaanxi Province (2022GY-272), The Scientific Research Program Funded by Shaanxi Provincial Education Department (22JY013), The Chinese Postdoctoral Science Foundation (2021M692000) and Wenzhou Science and Technology Major Project (ZY2022028).
Funding
National Natural Science Foundation of China, 22278259, Xiaomin Luo, 22278257, Xinhua Liu, The Scientific Research Program Funded by Shaanxi Provincial Education Department, 22JY013, Xinhua Liu, The Chinese Postdoctoral Science Foundation, 2021M692000, Xinhua Liu, Wenzhou Science and Technology Major Project, ZY2022028, Chen Yang.
.
Author information
Authors and Affiliations
Contributions
XML: Conceptualization, Supervision, Funding acquisition; LFJ: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Resources, Data Curation, Writing—Original Draft, Project administration; FA: Conceptualization; CY: Resources; JC: Resources; CYY: Formal analysis; HJR: Writing—Review & Editing; MT: Visualization; LYL: Visualization; XHL: Funding acquisition, Supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
All procedures in this study were carried out in accordance with the provisions of the Guidelines for the Care and Use of Laboratory Animals (Department of Science and Technology of Shaanxi Province, China, 2016, SYXK2022-006) and the relevant ethical regulations implemented by the Institute of Hygiene in the Arms Industry.
Competing interests
The authors declare no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Luo, X., Ji, L., Ao, F. et al. On-demand engineered double-network gelatin/silicate composited hydrogels with enhanced wet adhesion and stable release of bioactive ion for promoting wound healing. Collagen & Leather 6, 18 (2024). https://doi.org/10.1186/s42825-024-00161-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-024-00161-x